Abstract
Multidrug resistant (MDR) cancer cells overexpressing P‐glycoprotein (P‐gp) display variations in invasive and metastatic behavior. We aimed to clarify the mechanism(s) underlying this observation and transfected vectors carrying CD147, a glycoprotein enriched on the surface of tumor cells that stimulates the production of matrix metalloproteinases (MMPs), and specific shCD147 into MCF7 and MCF7/Adr cells, respectively. Using quantitative real‐time polymerase chain reaction and Western blot, we found that overexpression of CD147 in MCF7 cells up‐regulated MDR1, MMP2, and MMP9 on both transcription and expression levels, which promoted tumor cells metastasis and conferred them multidrug resistance to P‐gp substrate drugs, as determined by in vitro invasion assay and 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay. On the other hand, silencing of CD147 in MCF7/Adr cells led to the opposite effect. Moreover, Erk1/2 in CD147‐overexpressing clones were observed to be highly activate and after treatment with U0126, an Erk1/2‐specific inhibitor, the expression of MDR1, MMP2 and MMP9 were decreased significantly. Thus, CD147 may assume a dual role, since it had intrinsic stimulative effects on tumor invasion in vitro as well as increasing resistance to P‐gp substrate drugs. (Cancer Sci 2007; 98: 1064–1069)
Multidrug resistance (MDR) and tumor metastasis are the two main causes of treatment failure and mortality in cancer patients. These two properties of malignant tumors have been studied extensively and there are evidences suggesting functional linkage between the two phenotypes.( 1 , 2 , 3 ) Up‐regulation of CD147 has been reported in many MDR cancers.( 4 ) CD147 (EMMPRIN or extracellular matrix metallo‐proteinase inducer) is the major stimulator of MMPs. They congregate on the surfaces of most tumor cells to produce elevated levels of several MMPs.( 5 , 6 , 7 ) The greatly increased expression or activity of distinct MMPs observed in MDR cancer cells could be attributed to the elevated expression of CD147.( 4 ) Morever, Misra et al.( 8 ) have demonstrated that CD147 is also involved in resistance of cancer cells to some chemotherapeutic agents. Inhibition of CD147 gene expression via RNAi could increase chemosensitivity to paclitaxel in the human ovarian cancer cell line.( 9 ) As we know, more than one mechanism participates in MDR to chemotherapeutic drugs, the detailed mechanisms underlying these observations have not been clarified yet. MDR is often associated with overexpression of P‐glycoprotein (P‐gp), a transmembrane, adenosine triphosphate (ATP)‐dependent transporter encoded by the MDR1 gene.( 10 , 11 ) Our main purpose in the current research was to explore whether CD147 participated in the regulation of both MDR1 and MMPs. Additional, tyrosine kinases have been confirmed to be required not only for CD147 induction of MMPs,( 12 , 13 , 14 , 15 ) but for mediation of the expression of MDR1 as well.( 16 ) Since tyrosine kinases are integrally involved in MAP kinase (MAPK) signaling pathways, we attempted to further clarify whether MMPs and MDR1 expression could simultaneously be mediated by CD147 via MAPK pathways. Our results suggest that expression of CD147 is the reason for the promoted tumor cells metastasis and MDR to P‐gp substrate drugs through the regulation of MDR1, MMP2 and MMP9 expression in breast cancer cell lines
Materials and Methods
Cell culture. Human mammary carcinoma cell line MCF7 was established from pleural effusion.( 17 ) Its MDR counterpart, MCF7/Adr, was further derived for high P‐gp expression.( 18 ) Cells were cultured in RPMI 1640 (Gibco) containing 10% fetal bovine serum (FBS) (PAA), 100 units/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. For consistent MDR‐1 gene expression, MCF7/Adr cells were maintained in the presence of adriamycin (Sigma, USA).
Plasmid transfection. MCF7 cells were transfected with eukaryotic expression vector pcDNA3.0‐CD147 (kindly provided by Professor Kenji Kadomatsu), while MCF7/Adr cells were transfected with pSUPER‐shCD147 (generously gifted by Professor Xiang Chen), respectively. Transfection was carried out according to Lipofectamine 2000 (Invitrogen) manufacturer's instructions. For MCF7 cells, three stable transfectants named MT1, MT2, MT3, were selected for neomycin resistance in the medium containing 800 µg/mL G418 and were then maintained in the medium with 500 µg/mL G418 (Sigma). For MCF7/Adr cells, three stable transfectants named AT1, AT2, AT3, were selected for puromycin resistance in the medium containing 100 ng/mL puromycin and then maintained in the medium with 70 ng/mL puromycin (Alexis Biochemicals).
Reverse transcription and quantitative real‐time polymerase chain reaction. Total RNA of transfected cells and their control cells was extracted using Tripure isolation reagent (Sangon, China). The RNA samples were subjected to reverse transcription (RT) with 2 µg RNA, Oligo (dT)18_dNTP, and reaction buffer supplied with M‐MLV reverse transcriptase (Promega). Real‐time polymerase chain reaction (PCR) reactions were carried out in 20 µL solution with 2 µg cDNA and 1 mM of each forward and reverse primer and 2 × SYBR green Mix (Takara). Changes in mRNA expression level were calculated following normalization with glyceraldehyde phosphate dehydrogenase (GAPDH). Relative gene expression was determined by the fluorescence intensity ratio of the target gene to GAPDH. The primers used in the real‐time PCR reactions were designed based on information from the human genomic data base. The following are the primers used for the specific amplification of GAPDH, MMP2, MMP9 and MDR1: GAPDH forward primer: 5′‐CATCAAGAAGGTGGTGAAG C‐3′, and reverse primer: 5′‐GGAAATT GTGA GG GAGATGC‐3′; MMP2 forward primer: 5′‐GGCCTCTCCT GACATTGA CCT T‐3′, and reverse primer: 5′‐GGCC TCGTA TACCGCATCAAT C‐3′; MMP9 forward primer: 5′‐TTTGACAGCGACAAGAAGTGG‐3′, and reverse primer: 5′‐A GGGCGAGGACCATAGAGG‐3′; MDR1 forward primer: 5′‐CCCATCATTGCAATAG CAGG‐3′, and reverse primer: 5′‐GTTCAAACTTCTGCTCCTGA‐3′.
Western blotting. Cells were harvested and lyzed in lysis buffer (0.5% NonidetP‐40, 10 mM Tris, pH 7.4, 150 mM NaCl, 1mMEDTA, 1 mM Na3VO4) containing protease inhibitors (1 mM phenylmethyl sulfonyl fluoride [PMSF]). Proteins (50 µg/well) were then subjected to standard sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE). For Western blotting analysis, proteins were transferred to polyvinylidene fluoride (PVDF) membranes, then blocked in 8% non‐fat milk in 10 mM Tris, pH 7.5, 100 mMNaCl, 0.1% (w/v) Tween 20 for 1 h. The membranes were first incubated with antibodies against β‐actin (Sigma), CD147 (Santa Cruz), P‐gp (Chemicon), MMP2 (Neomarkers, CA, USA), MMP9 (Santa Cruz), phosphospecific and total Erk1/2, p38, JNK (Cell Signaling), respectively, overnight at 4°C, followed by 1 h incubation with the appropriate secondary antibody. For quantification of protein expression levels, AlexaFluor‐700/800 nm secondary conjugates were used and PVDF membranes were analyzed using the Odyssey Infra‐Red Imaging System and software (Li‐Cor BioSciences) according to the manufacturer's instructions.
Drug sensitivity assay. To assess their multidrug chemosensitivity, transfected cells and their corresponding control cells were plated in 96‐well plates at a density of 104 cells/well and further incubated for 24 h. The medium was then removed and replaced with fresh medium containing paclitaxel (Sigma), vincristine (Sigma), bleomycin (Alexis Biochemicals) and adriamycin, respectively, with varying PPC (plasma peak concentrations, 0.1 PPC, 1.0 PPC and 10.0 PPC) for another 48 h. After that, cells were stained with 20 µL sterile MTT dye (3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide, 5 mg/mL; Sigma) at 37°C for 4 h followed by removing the culture medium and mixing 150 µL of dimethylsulfoxide (DMSO) thoroughly for 10 min. Spectrometric absorbance at 490 nm was measured with a microplate reader. Each group contained three wells and was repeated three times. The IC50 value was determined by the dose of drug that caused 50% cell viability.
In vitro invasion assay. Transwell plates (Corning Costar) were coated with basement membrane matrigel (20 mg/mL, Becton Dickinson) for 4 h at 37°C. Transfected and control cells were detached with trypsin and washed with serum‐containing medium. A total of 1 × 104 cells were added to the upper chamber in a total volume of 100 µL of serum‐free medium supplemented with 0.5 mg/mL bovine serum albumin (BSA). The lower chamber contained 600 µL conditioned medium (incubating NIH3T3 cells in serum‐free RPMI 1640 medium for 24 h) as a chemoattractant. After 18 h, cells that migrated through the permeable membrane were fixed in methanol, stained with hematoxylin and eosin, and counted. Each assay was carried out in triplicate and repeated three times.
Statistical analysis. Statistics were conducted by SPSS software. The results are presented as mean ± standard errors (SEM). anova, Student's t‐test analysis and Dunnett's multiple comparison tests were used to compare mean values. A P‐value of less than 0.05 was defined as statistical significance.
Results
Vectors stably expressing CD147 and CD147 shRNA caused specific and effective up‐ or down‐regulation of CD147 expression, respectively. As shown in Figure 1, protein and mRNA levels of CD147 in MCF7/Adr cells were much higher than those in MCF7 cells. The transfection efficiencies of individual stable transfectants were first evaluated using RT‐PCR. Relative CD147 mRNA levels in each transfectant were normalized against mRNA levels of an internal control gene, GAPDH, carried out in different runs. As shown in Figure 1a, transfectants named MT1, MT2, MT3 and AT1, AT2, AT3, were transfected with pcDNA3.0‐CD147 and pSUPER‐shCD147, respectively. We demonstrated a significant promotion or reduction of transcription of CD147 mRNA when compared with negative controls. In addition, Western blot analysis (Fig. 1b) showed the responsive changes in corresponding stable transfectants. The results above showed that the expression of CD147 could be up‐ or down‐regulated effectively by vectors expressing CD147 and CD147 shRNA, respectively.
Figure 1.
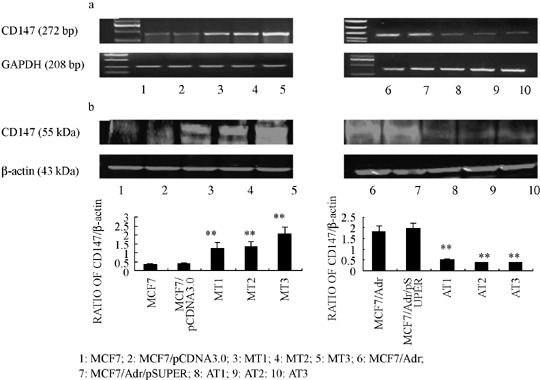
Production of CD147 mRNA and protein in MCF7, MCF7/Adr cells were up‐ or down‐regulated after transfecting vectors expressing CD147 and CD147 shRNA, respectively. (a) CD147 mRNA assessed by real time polymerase chain reaction (PCR). (b) CD147 protein assessed by Western blot. The expression of GAPDH mRNA and β‐actin protein were also examined and served as controls for sample loading. Bar graphs quantified and compared the immunoblots signal intensity. **P < 0.05 vs control cells.
Regulation of MDR1, MMP2, MMP9 mRNA and protein levels by CD147 in MCF7 cells. To investigate whether CD147 affects MDR1, MMP2, MMP9 gene expression, total RNA was obtained from untreated MCF7 and MT1, MT2, MT3 cells, MDR1, MMP2, MMP9 and GAPDH mRNA levels were measured by quantitative real‐time PCR. As seen in Figure 2a, endogenous MDR1, MMP2, MMP9 mRNA levels were remarkably higher in MCF7/CD147 transfectants than in MCF7 cells and their vector control transfectant (P < 0.05). To assess whether the CD147‐induced increase in MDR1, MMP2, MMP9, and mRNA levels were associated with a corresponding elevation in protein levels, lysates from the above cell lines were prepared and Western blot was carried out. Results of multiple Western blot analyses showed that P‐gp, MMP2 and MMP9 levels were significantly higher in MT1, 2, 3 than in their negative control transfectants (Fig. 2b).
Figure 2.
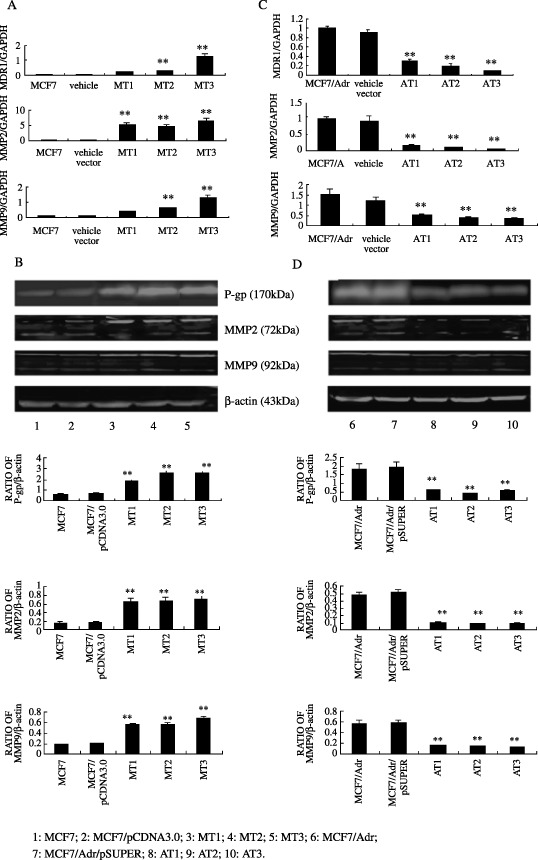
Effects of CD147 on the regulation of MDR1, MMP2 and MMP9 gene expression. MCF7, MCF7/pCDNA3.0, MT1, 2, 3 cells and MCF7/Adr, MCF7/Adr/pSUPER, AT1, 2, 3 cells were harvested, total RNA were extracted, and lysates were electrophoresed through 8% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Endogenous MDR1, MMP2 and MMP9 mRNA (a, c) and protein levels (b, d) were measured by real‐time polymerase chain reaction (PCR) and Western blot, respectively. Bar graphs represent mean ± SEM of three independent experiments. **P < 0.05 cs control cells.
Effects of CD147 specific shRNA on transcription and expression of MDR1, MMP2, 9 in MCF7/Adr cells. Either transcription or expression of MMP2 and MMP9 in MCF7/Adr cells were apparently higher than those in MCF7 cells. To further confirm the participation of CD147 in regulation of MDR1, MMP2 and MMP9 expression, we used a MCF7/Adr‐derived cell line in which overexpression of CD147 was suppressed by stable transfection of CD147‐RNAi vector (pSUPER‐CD147). Quantitative real‐time PCR revealed distinct changes in MDR1, MMP2, MMP9 mRNA levels between CD147‐silencing transfectants and their negative controls (Fig. 2c). Western blot analysis showed the corresponding reductions in protein levels (Fig. 2d).
Knockdown of CD147 in MCF7/Adr cells results in reduced resistance to P‐gp substrate drugs, whereas elevated expression of CD147 in MCF7 cells leads to the opposite effects. CD147 has been reported to be overexpressed in human MDR carcinoma cell lines. Our previous results also demonstrated that CD147 participated in regulation of MDR1 gene expression. Therefore, we hypothesized that CD147 would effect the sensitivity of P‐gp substrate drugs and P‐gp non‐substrate drugs. To test this hypothesis, we used a CD147‐overexpressing clone (MT3) and a CD147‐silencing clone (AT3) previously derived from the MCF7 and MCF7/Adr line, respectively.
As reported in Table 1, CD147 overexpression had varying effects on drug sensitivity, depending on the drug used. Interestingly, the CD147 transfectant showed decreased sensitivity to P‐gp substrate drugs, Toxel, vincristine (VCR) and adriamycin (ADR) (P < 0.05), while knockdown of CD147 in MCF7/Adr cells leaded to the opposite effects (P < 0.05) when compared with their negative controls. The resistance to P‐gp non‐substrate drugs, bleomycin (BLM) of both clones, remained unchanged (P > 0.05).
Table 1.
IC50 of Toxel, VCR, ADR, BLM in CD147 transfectants (MT3), shCD147 transfectants (AT3) and their negative controls
| Drugs | MCF7 | Vehicle vector | MT3 | MCF7/Adr | Vehicle vector | AT3 |
|---|---|---|---|---|---|---|
| Toxel | 0.0140 ± 0.0210 | 0.0187 ± 0.0050 | *0.7562 ± 0.0238 | 0.4018 ± 0.1322 | 0.3857 ± 0.0966 | *0.0698 ± 0.0025 |
| VCR | 0.4658 ± 0.0946 | 0.5021 ± 0.0359 | *9.2321 ± 0.3561 | 13.7932 ± 0.4546 | 12.9825 ± 0.4879 | *2.3164 ± 0.1598 |
| ADR | 0.4126 ± 0.1201 | 0.5980 ± 0.0200 | *43.1548 ± 3.0068 | 30.0355 ± 6.2413 | 28.6307 ± 6.4592 | *8.8400 ± 0.1014 |
| BLM | 0.5386 ± 0.0231 | 0.6013 ± 0.0021 | 0.5932 ± 0.1026 | 0.4769 ± 0.0863 | 0.5567 ± 0.0987 | 0.5031 ± 0.1372 |
ADR, adriamycin; BLM, bleomycin; Toxel, paclitoxel; VCR, vincristine. IC50 values are expressed in µM and were evaluated as reported in Materials and Methods. Standard deviations for all of the experiments carried outin triplicate were less than 5%. *P < 0.05 vs control cells.
In vitro invasion assay. Invasion activity of the most effective CD147 (MT3) and CD147 specific shRNA transfectants (AT3) were assayed in vitro in transwell chambers. MT3 showed much higher invasion activities while AT3 showed much lower when compared with corresponding controls (P < 0.05) (Fig. 3).
Figure 3.
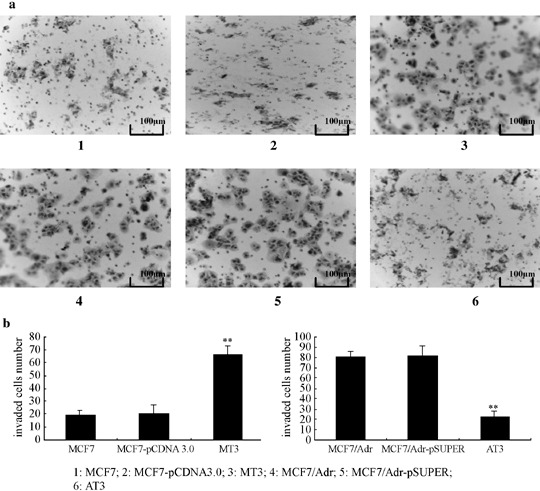
Effects of CD147 and its specific shCD147 on Matrigel invasion of MCF7 and MCF7/Adr cells. Matrigel invasion was evaluated by using transwell chambers as described in Materials and Methods. (a) 1: MCF7; 2:MCF7/pCDNA3.0; 3:MT3; 4:MCF7/Adr; 5.MCF7/Adr/pSUPER; 6:AT3. (b) Number of cells migrated was evaluated in three fields for each experimental group and averaged. Statistical analysis was carried out with Dunnett’s‐test. **P < 0.05 vs control cells.
Involvement of ERK1/2 in regulation of MDR1, MMP2, MMP9 by CD147. Since previous studies have established that MDR1, MMP2, and MMP9 work in conjunction with the MAPK pathway, we investigated the role of it in the regulation of MDR1, MMP2, and MMP9 by CD147. Phosphorylated Erk1/2, p38 and JNK in the MCF7‐derived transfectants were compared to their control lines by Western blot. The blots were then reprobed for total Erk1/2, p38, c‐Jun N‐terminal kinase (JNK) (Fig. 4a) and revealed that only distinct changes in endogenous Erk1/2 phosphorylation level were observed between CD147‐overexpressing lines and their control cells. We then tested the effects of Erk1/2 inhibitor on MDR1, MMP 2, MMP9 expression. As seen in Figure 4b, U0126, an inhibitor of MAPK/extracellular signal regulated kinase (Erk), led to significant dose‐dependent (1–10 µM) inhibition of expression of P‐gp, MMP2, MMP9 in the MT3 clone (the most effective CD147 transfectant).
Figure 4.
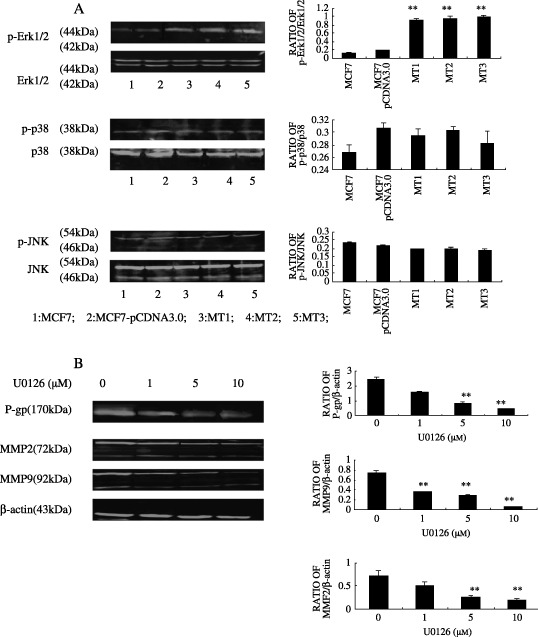
Erk1/2 was involved in regulation of MDR1, MMP2,9 by CD147. Activation state of three pathways of MAP kinase (MAPK) (a) in tumor cells were first examined by immunoblot analysis. To determine the effects of U0126, MT3 cells were treated with various concentrations of u‐0126 or vehicle for 24 h in serum‐free media. At the end of incubation, the expression of MDR1, MMP2, and MMP9 were analyzed by Western blot. (b) Bar graphs quantified and compared the immunoblots signal intensity. **P < 0.05 vs control cells.
Discussion
CD147, also known as EMMPRIN, basigin, M6 and tumor cell‐derived collagenase stimulatory factor, is a highly glycosylated transmembrane protein that belongs to the immunoglobulin superfamily.( 19 ) Previous studies demonstrated that CD147 promotes invasion of tumor cells by stimulating stromal cells to produce elevated levels of MMPs. MMPs play crucial roles in several aspects of tumor progression, including growth, invasion, metastasis, and angiogenesis. In additional support for its key role in the processes of tumorigenesis and metastasis is that CD147 has been reported as one of the most constantly up‐regulated mRNA in metastatic cells.( 5 , 6 , 7 ) Recently, CD147 has been proposed as a marker of poor prognosis in some carcinomas.( 20 )
In the current study, we detected that transfecting the vectors encoding CD147 into MCF7 cells significantly promoted the expression of both MMP2 and MMP9. However, silencing CD147 expression in MCF7/Adr cells caused an attenuation in MMP2 and MMP9 expression. Furthermore, silencing of CD147 in MCF7/Adr cells greatly inhibited the cell invasion activity in vitro, whereas the promotion of CD147 in MCF7 cells resulted in the opposite effect. We anticipate this explanation is due to MMPs induced by CD147, since the MMPs activity in cancerous tissues is primarily due to tumor cell production of the enzymes needed for the invasion and metastatic spread.( 21 )
The MDR phenotype acquired by initially drug responsive patients remains a major hindrance to efficient chemotherapy. Previously, we have shown that during the development of drug resistance to adriamycin in MCF7 cells, the promoted expression of MDR1 coincided with upregualtion of CD147. Overexpressed CD147 and MMPs were also reported in several MDR cancer cell lines compared to the parental controls,( 4 ) which were consistent with what we observed in the current research. MDR1 overexpression is one form of MDR; it encodes the 170 kDa P‐gp, a transmembrane pump that enables efflux of drugs from cells.( 22 ) Thus far, the relationship between MDR and tumor metastasis was still unclear. We have now discovered that transcription and expression levels of MDR1 in MCF7 cells were largely promoted after pcDNA3.0‐CD147 was transfected, while silencing CD147 in MCF7/Adr cells caused the suppression of MDR1 in similar fashion. We also evaluated the sensitivity of both stable transfectants to P‐gp substrate drugs and to P‐glycoprotein non‐substrate drug. The MTT results revealed that supression of CD147 significantly increased the chemosensitivity to P‐gp substrate drugs, while MDR to these drugs was enhanced in CD147‐overexpressed clones compared with their respective controls. However, chemosensitivity to P‐gp non‐substrate, bleomycin, remained unchanged in both kinds of transfectants. This interesting phenomenon is consistent with the increased expression of P‐gp induced by CD147, indicating that CD147 is responsible for the altered multidrug resistance to P‐gp substrate drugs through the regulation of MDR1 expression in breast cancer cells.
Hemler et al.( 23 ) discovered that CD147 associates with α3β1 integrin, a cell receptor that plays an important role in cell adhesion. Receptor binding and clustering of integrins at the cell surface appear to be triggering events that initiate interactions with cytoskeleton components and stimulate intracellular signaling.( 24 , 25 ) The 125 kDa tyrosine kinase focal adhesion kinase (FAK) associates with integrins in focal contacts and initiates a cascade of intracellular signals in response to adhesion, including MAPK activation.( 26 ) MAPK pathway plays an important role in regulating many fundamental processes, such as cell growth, mobility, differentiation and multidrug resistance.( 27 ) MAPK transmits the signal it receives from Ras to the nucleus, where regulation of gene transcription determines the fate of cells.( 28 ) Activation of MAPK pathways could result in alterations in the expression levels of either MDR1,( 16 ) or MMP2,9( 4 , 12 , 13 , 14 ) that, are responsible for resistance to chemotherapeutic drugs, degradation of ECM, and, are required for cell mobility.( 29 , 30 ) Whether CD147 uses distinct signaling pathways in the regulation of downstream genes remains to be determined. It is possible however, that several distinct pathways can be stimulated by CD147 depending on the cell system.( 31 ) Thus, we assessed phosphorylation levels of MAPK in CD147‐overexpressed clones and found that only Erk1/2 phosphorylation was constitutively up‐regulated in MCF7 cells treated with pcDNA3.0‐CD147. These findings suggest that the Erk1/2‐mediated MDR1, MMP2, MMP9 expression might be directly regulated by CD147 in MCF7 cells, and CD147 may play an important role in regulating Erk activity. Furthermore, U0126, Erk1/2 specific inhibitor, significantly inhibited MDR1, MMP2 and MMP9 expression in these transfectants in a dose‐dependent manner, demonstrating that CD147 regulated P‐gp, MMP2 and MMP9 production through the Erk1/2 pathway. Yang et al.( 4 ) have found that inhibition of MAPK/Erk by U‐0126 decreases the activity and expression of MMP‐9, MMP‐2, and MMP‐1, our results in this study further demonstrated that MAPK/Erk is the shared pathway involved in regulation of both MDR1 and MMP2 and MMP9 expression.
In summary, our work confirms that CD147 regulates the expression of MDR1, MMP2, and MMP9 via an Erk1/2‐dependent signaling pathway in breast cancer cells, which is consistent with their alterations in cellular invasiveness in vitro and multidrug resistance. This provides further insight into the mechanism underlying the functional linkage between drug resistance and tumor metastasis, pinpoints the inhibitive points that could effectively down‐regulate multidrug resistance, tumor invasiveness in vitro in a simultaneous manner, and enlightens the appropriate choice of chemotherapy drugs in clinic treatments.
Acknowledgments
Studies in our laboratory were supported by Funds of the Shanghai Board of Health (No. 044082), Funds of the Shanghai Scientific Association (No. 05ZR14023) and Funds of the National Institutes of Health/National Cancer Institute (No. CA 66077 and No. CA109371). We thank members of our laboratory for helpful discussions.
References
- 1. Kerbel RS, Waghorne C, Korczak B, Lagarde A, Breitman ML. Clonal dominance of primary tumours by metastatic cells: genetic analysis and biological implications. Cancer Surv 1988; 7: 597–629. [PubMed] [Google Scholar]
- 2. Kerbel R, Korczak S, Lagarde B. A Growth dominance of the metastatic cancer cell: cellular and molecular aspects. Adv Cancer Res 1990; 5: 87–132. [DOI] [PubMed] [Google Scholar]
- 3. Su ZZ, Austin VN, Zimmer SG. Fisher, PB, Defining the critical gene expression changes associated with expression and suppression of the tumorigenic and metastatic phenotype in Ha‐ras‐transformed cloned rat embryo fibroblast cells. Oncogene 1993; 8: 1211–19. [PubMed] [Google Scholar]
- 4. Yang JM, Xu Z, Wu H, Zhu H, Wu X, Hait WN. Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol Cancer Res 2003; 1: 420–7. [PubMed] [Google Scholar]
- 5. Yan LS, Zucker BP. Toole. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost 2005; 93: 199–204. [DOI] [PubMed] [Google Scholar]
- 6. Kanekura T, Chen X, Kanzaki T. Basigin (CD147) is system with an improved hairpin and its significant expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer 2002; 99: 520–8. [DOI] [PubMed] [Google Scholar]
- 7. Bordador LC, Li X, Toole B et al. Expression of emmprin by oral squamous cell carcinoma. Int J Cancer 2000; 85: 347–52. [PubMed] [Google Scholar]
- 8. Misra S, Ghatak S, Zoltan‐Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem 2003; 278: 25 285–8. [DOI] [PubMed] [Google Scholar]
- 9. Zou W, Zhu H, Wu X. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO‐8910pm cells. Cancer Lett 2006; 248: 211–18. [DOI] [PubMed] [Google Scholar]
- 10. Yang JM, Yang GY, Medina DJ, Vassil AD, Liao J, Hait WN. Treatment of multidrug resistant (MDR1) murine leukemia with P‐glycoprotein substrates accelerates the course of the disease. Biochem Biophys Res Commun 1999; 266: 167–73. [DOI] [PubMed] [Google Scholar]
- 11. Cillo C, Dick JE, Ling V, Hill RP. Generation of drug‐resistant variants in metastatic B16 mouse melanoma cell lines. Cancer Res 1987; 47: 2604–8. [PubMed] [Google Scholar]
- 12. Ding S, Chamberlain M, McLaren A, Goh L, Duncan I, Wolf CR. Cross‐talk between signalling pathways and the multidrug resistant protein MDR‐1. Br J Cancer 2001; 85: 1175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osborn MT, Chambers TC. Role of the stress‐activated/c‐Jun NH2‐terminal protein kinase pathway in the cellular response to Adriamycin and other chemotherapeutic drugs. J Biol Chem 1996; 271: 30 950–5. [DOI] [PubMed] [Google Scholar]
- 14. Yang JM, Vassil AD, Hait WN. Activation of phospholipase C induces the expression of the multidrug resistance (MDR1) gene through the Raf‐MAPK pathway. Mol Pharmacol 2001; 60: 674–80. [PubMed] [Google Scholar]
- 15. Davidson B, Givant‐Horwitz V, Laranovici P et al. Matrix rnetalloproteinases (MMP), EMMPRIN (extracellular matrix metalloproteinase inducer) and mitogen‐activated protein kinases (MAPK): co‐expression in metastatic serous ovarian carcinoma. Clin Exp Metastasis 2003; 20: 621–31. [DOI] [PubMed] [Google Scholar]
- 16. Lim M, Martinez T, Jablons D et al. Tumor‐derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett 1998; 441: 88–92. [DOI] [PubMed] [Google Scholar]
- 17. Soule HD, Vazguez J, Long A et al. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst 1973; 51: 1409–16. [DOI] [PubMed] [Google Scholar]
- 18. Davies R, Budworth J, Riley J, Snowden R, Gescher A, Gant TW. Regulation of P‐glycoprotein 1 and 2 gene expression in two MCF‐7/ADR cell line subclones. Br J Cancer 1996; 73: 307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suzuki S, Sato M, Senoo H, Ishikawa K. Direct cell–cell interaction enhances pro‐MMP‐2 production and activation in co‐culture of laryngeal cancer cells and Broblasts: involvement EMMPRIN Mt1‐MMP. Exp Cell Res 2004; 293: 259–66. [DOI] [PubMed] [Google Scholar]
- 20. Davidson B, Goldberg I, Berner A, Kristensen GB, Reich R. EMMPRIN (extracellular matrix metalloproteinase inducer) is a novel marker of poor outcome in serous ovarian carcinoma. Clin Exp Metastasis 2003; 20: 161–9. [DOI] [PubMed] [Google Scholar]
- 21. Gabison EE, Hoang‐Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie 2005; 87: 361–8. [DOI] [PubMed] [Google Scholar]
- 22. Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976; 455: 152–62. [DOI] [PubMed] [Google Scholar]
- 23. Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin‐associated proteins. J Biol Chem 1997; 272: 29 174–80. [DOI] [PubMed] [Google Scholar]
- 24. Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev 2005; 24: 195–222. [DOI] [PubMed] [Google Scholar]
- 25. Filippo G. Giancotti FG. Integrin signaling: pecificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 1997; 9: 691–700. [DOI] [PubMed] [Google Scholar]
- 26. Li W, Duzgun A, Sumpio BE, Basson MD. Integrin and FAK‐mediated MAPK activation is required for cyclic strain mitogenic effects in Caco‐2 cells. Am J Physiol Gastrointest Liver Physiol 2005; 280: 75–87. [DOI] [PubMed] [Google Scholar]
- 27. Huang C, Jacobson K, Schaller MD. MAP kinases and cell mobility. J Cell Sci 2004; 117: 4619–28. [DOI] [PubMed] [Google Scholar]
- 28. Brauchle M, Gluck D, Padova FD, Han JH, Gram H. Independent role of p38 and ERK1/2 mitogen‐activated kinases in the upregulation of matrix metalloproteinase‐1. Exp Cell Res 2000; 258: 135–44. [DOI] [PubMed] [Google Scholar]
- 29. Iyer V, Pumiglia K, DiPersio CM. a3b1 integrin regulates MMP‐9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin‐mediated MMP gene expression. J Cell Sci 2005; 118: 1185–95. [DOI] [PubMed] [Google Scholar]
- 30. Hofmann UB, Westphal JR, Van Kraats AA, Ruiter DJ, Van Muijen GN. Expression of integrin alpha (v) beta (3) correlates with activation of membrane‐type matrix metalloproteinase‐1 (MT1‐MMP) and matrix metalloproteinase‐2 (MMP‐2) in human melanoma cells in vitro and in vivo . Int J Cancer 2000; 87: 12–19. [DOI] [PubMed] [Google Scholar]
- 31. Eric EG, Thanh H‐X, Alain M et al. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie 2005; 87: 361–8. [DOI] [PubMed] [Google Scholar]


