Abstract
Granulocyte colony‐stimulating factor (G‐CSF)‐supported, post‐remission chemotherapy (Cx) for adult acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LBL) was evaluated. One hundred and forty‐three eligible patients (median age, 41 years) including 126 ALL and 17 LBL receiving induction Cx (vincristine, cyclophosphamide, prednisolone [PSL], doxorubicin, L‐asparaginase, intrathecal‐methotrexate [IT‐MTX]) were analyzed. For patients achieving complete response (CR), two courses of post‐remission Cx (course A of daunorubicin, cytosine arabinoside, vindesine, PSL plus IT‐MTX; course B of mitoxantrone, etoposide, vincristine, PSL plus IT‐MTX) with the use of G‐CSF were repeated alternately; thereafter, maintenance Cx including MTX and 6‐mercaptopurine was given for 2 years. One hundred and nineteen (83%) patients achieved CR, while 14 (10%) died during induction. Among the 119 patients achieving CR, five died in remission, 76 relapsed, and the remaining 38 were alive without disease. The median survival time of the 143 eligible patients was 26 months (95% confidence interval, 19–34). At a median follow‐up time of 9 years, the 5‐year survival rate was 32% and the 5‐year progression‐free survival (PFS) rate was 26%. The 5‐year survival rate of 36 patients who underwent autologous (n = 20) or allogeneic stem cell transplantation (SCT; n = 16) in the first CR group was 58%. Compared with the authors’ previous trials, survival and PFS were markedly improved. In conclusion, G‐CSF‐supported, intensive post‐remission Cx and subsequent SCT are worthy of further investigation for the treatment of adult ALL and LBL. (Cancer Sci 2007; 98: 1350–1357)
Acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) show overlapping clinical features including a mediastinal mass, leukemic manifestation, and a high prevalence of central nervous system (CNS) involvement.( 1 ) ALL is categorized as a precursor B‐ or T‐cell malignancy, and T‐ALL and T‐LBL show similar immunophenotypes.( 2 ) Based on these common characteristics, patients with ALL and those with LBL have been treated with similar chemotherapy regimens.( 1 , 3 , 4 , 5 , 6 , 7 ) Generally, the prognoses of adult patients are unfavorable compared with pediatric patients.( 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 )
In 1981, the Lymphoma Study Group of the Japan Clinical Oncology Group (JCOG‐LSG) initiated a phase III trial for non‐Hodgkin lymphoma including LBL, using first‐generation chemotherapy, VEPA (vincristine [VCR], cyclophosphamide [CPA], prednisolone [PSL] and doxorubicin [DXR]) or VEPA‐M (VEPA plus methotrexate [MTX]); however, the outcome for patients with LBL was poor.( 14 ) With the aim of improving the results against LBL, JCOG‐LSG conducted a phase II study, JCOG8702, an alternating non‐cross‐resistant combination chemotherapy consisting of VEPA, L‐asparaginase (L‐asp) and intermediate‐dose MTX without maintenance therapy with 6‐mercaptopurine (6‐MP) and MTX for ALL and LBL.( 15 ) Although the complete response (CR) rate (78%, 36/46) was within the range of satisfaction, the long‐term survival rate (15%) was inferior to those of published programs incorporating maintenance therapy.( 9 , 10 , 11 , 12 ) The authors concluded that simplified JCOG8702 therapy without maintenance therapy does not deserve further investigation.
The primary goal of the present phase II study (JCOG9004) was to evaluate post‐remission therapy for patients achieving CR with induction therapy. The following three kinds of strategies were undertaken: (i) intensive post‐remission therapy with the use of granulocyte colony‐stimulating factor (G‐CSF); (ii) long‐term maintenance therapy including 6‐MP and MTX; and (iii) allogeneic and autologous stem cell transplantation (SCT). In addition, the authors evaluated the dose‐intensified induction regimen, in which the doses of CPA were escalated, and the numbers of administering VCR and DXR were increased as compared to the induction regimen in the previous study, JCOG8702.( 15 )
Patients and Methods
Patients. One hundred and forty‐seven patients aged 15–69 years were registered. ALL and LBL were diagnosed according to the French–American–British (FAB) Classification,( 16 ) and Working Formulation,( 17 ) respectively, at each institution. Patients with LBL at any clinical stage were eligible. Patients having the following disorders were ineligible: preceding hematologic disorders including myelodysplastic syndrome, performance status (PS) 4 (World Health Organization [WHO]),( 18 ) liver dysfunction (liver cirrhosis or hepatic transaminase ≥ 4 × upper normal limits), renal dysfunction (serum creatinine ≥ 2.0 mg/dL), hypoxemia (arterial blood gas < 70 mmHg), diabetes mellitus necessitating insulin treatment, severe infection, active tuberculosis, cardiac failure, cardiac disorders such as ischemic heart disease and cardiomyopathy, active concurrent cancer, severe psychiatric disorders, pregnancy, or other severe complications. Patients showing liver and/or renal dysfunction considered to be caused by neoplastic cell infiltration were eligible. Patients who had received anticancer agents at the time of entry were ineligible, but those receiving radiation or glucocorticoids were eligible. Patients with central nervous system (CNS) involvement were not excluded but received additional CNS therapy.
Immunophenotyping was performed at each institution, and the data collected were used in the central pathology review. Flow cytometric analysis with a panel of monoclonal antibodies was conducted as an immunofluorescent assay on suspended cells. At least 11 monoclonal antibodies against major antigens (CD1, 2, 3, 5, 7, 10, 13, 19, 20 and 33, human leukocyte antigen‐D‐related [HLA‐DR]), and assays for terminal deoxynucleotidyl transferase (TdT), surface membrane immunoglobulin (S‐Ig) and cytoplasmic immunoglobulin (C‐Ig) were recommended. Samples were considered positive if more than 20% of cells showed specific labeling above that of controls. B‐lineage was defined as CD19+ or CD20+; and T‐lineage as CD2+, CD3+, CD5+ or CD7+. Cases expressing S‐Ig were categorized as mature B‐cell phenotype. Patients with myeloperoxidase‐negative blasts expressing only myeloid antigens were reclassified as acute myeloid leukemia, M0,( 19 ) and were ineligible. For the biopsied lymph node or tumor samples, immunohistochemical analysis was conducted. Even if the biopsied specimen showed histologic findings consistent with LBL, patients having 25% tumor cells or more in their bone marrow (BM) were diagnosed as having ALL.( 2 ) It was recommended that a pretreatment peripheral blood (PB), BM or biopsied specimen be subjected to cytogenetic analysis. Biopsy specimens of patients diagnosed with LBL at each institution were submitted to the central pathology for review.
Registration involved a telephone call or facsimile from participating physicians to the JCOG Statistical or Data Center (1991–1997: Statistical Center; 1998–: Data Center).( 20 ) When a patient achieving first CR met the following eligibility criteria for SCT, the physician was requested to register the patient. For allogeneic SCT, patients should meet the following criteria: (i) age of 15–39 years; (ii) HLA‐matched sibling donor; (iii) continuous CR after the first cycle of Consolidation B; (iv) PS of 0 or 1; (v) arterial blood gas ≥ 80 mmHg and percentage diffusing capacity of carbon monoxide ≥ 60%; (vi) cardiac ejection fraction ≥ 50%; (vii) creatinine clearance ≥ 60 mL/min; (viii) total bilirubin ≤ 2.0 mg/dL; (ix) negative hepatitis B virus surface antigen; and (x) informed consent. For autologous SCT, patients should meet the following criteria: (i) age of 15–49 years; and (ii) no matched sibling donor, in addition to the above‐described criteria (iii) to (x) for allogeneic SCT.
The physician was responsible for submitting periodic data reports on toxicity, response and survival. The protocol was approved by the Protocol Review Committee of the JCOG and by the institutional review board at each institution. Before treatment, written or oral informed consent was obtained from each patient or his/her family member.
Treatment.
Chemotherapy. The total treatment plan is shown in Fig. 1, and drug doses and administration schedules are listed in Table 1. The treatment regimens consisted of one course of Induction, two alternating courses of Consolidation A and Consolidation B, and four alternating courses of Maintenance C and Maintenance D. During and after administration of L‐asp in Induction therapy, it was recommended that fresh frozen plasma or anti‐thrombin be given to patients showing marked decrease in fibrinogen and anti‐thrombin‐III. In Maintenance D containing 400 mg/m2 MTX IV, plasma concentrations of MTX were measured 24, 48, and 72 h after the administration of MTX. When high concentrations of MTX were detected, additional leucovorin was administered.
Figure 1.
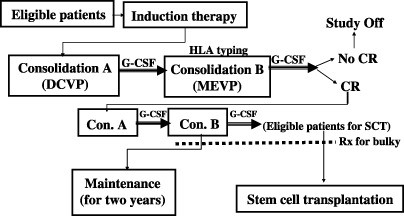
Total treatment scheme of the present, JCOG9004, protocol. CR, complete response; DCVP7, daunorubicin, cytosine arabinoside, vindesine, prednisolone; G‐CSF, granulocyte colony‐stimulating factor; SCT, stem cell transplantation.
Table 1.
Chemotherapy courses and drug doses in the present study, JCOG9004
| Agent | Route | Dose (≥50 years old) | Schedule (≥50 years old) |
|---|---|---|---|
| Induction: VEPA‐L | |||
| Vincristine | IV | 1.4 mg/m2 | day 1, 8, 15, 22, 29 |
| Cyclophosphamide | IV | 600 mg/m2 (400 mg/m2) | day 1, 15, 29 |
| Prednisolone | PO | 40 mg/m2 | day 1–28, then tapered |
| Doxorubicin | IV | 40 mg/m2 (30 mg/m2) | day 1, 8, 22 |
| L‐asparaginase | IV | 6000 U/m2 (4000 U/m2) | day 25–34 (25–31) |
| Methotrexate | IT | 10 mg/m2 | day 15, 29 |
| Prednisolone | IT | 20 mg/patient | day 15, 29 |
| Consolidation A: DCVP | |||
| Daunorubicin | IV | 40 mg/m2 (30 mg/m2) | day 1–3 |
| Cytosine arabinoside | CI | 80 mg/m2 | day 1–6 (1–5) |
| Vindesine | IV | 2.4 mg/m2 (2 mg/m2) | day 1 |
| Prednisolone | PO | 40 mg/m2 | day 1–6, then tapered |
| Methotrexate | IT | 10 mg/m2 | day 4 |
| Prednisolone | IT | 20 mg/patient | day 4 |
| Filgrastim | SC | 125 µg/patient | day 9– |
| Consolidation B: MEVP | |||
| Mitoxantrone | IV | 6 mg/m2 (4 mg/m2) | day 1–3 |
| Etoposide | IV | 100 mg/m2 (80 mg/m2) | day 1–5 |
| Vincristine | IV | 1.4 mg/m2 | day 1 |
| Prednisolone | PO | 40 mg/m2 | day 1–5, then tapered |
| Methotrexate | IT | 10 mg/m2 | day 4 |
| Prednisolone | IT | 20 mg/patient | day 4 |
| Filgrastim | SC | 125 µg/patient | day 8– |
| Maintenance C: FEPA‐MP | |||
| Vindesine | IV | 2.4 mg/m2 | day 1, 8 |
| Cyclophosphamide | IV | 600 mg/m2 | day 1 |
| Prednisolone | PO | 40 mg/m2 | day 1–8, then tapered |
| Doxorubicine | IV | 40 mg/m2 | day 1 |
| Methotrexate | PO | 20 mg/m2 | day 29, 36, 43, 50, 57 |
| 6‐Mercaptopurine | PO | 60 mg/m2 | day 29–56 |
| Maintenance D: M‐VEPA‐MP | |||
| Methotrexate | IV | 400 mg/m2 | day 1* |
| Leucovorin | Any | 15 mg/patient | day 2, 3** |
| Vincristine | IV | 1.4 mg/m2 | day 8, 15 |
| Etoposide | IV | 140 mg/m2 | day 8, 15 |
| Prednisolone | PO | 40 mg/m2 | day 8–15, then tapered |
| Nimustine | IV | 60 mg/m2 | day 8 |
| Methotrexate | PO | 20 mg/m2 | day 36, 43, 50, 57 |
| 6‐Mercaptopurine | PO | 60 mg/m2 | day 36–56 |
100 mg/m2 IV bolus followed by 300 mg/m2 drip infusion for 4 h.
Six times starting 24 h after administration of methotrexate every 6 h. Any, IV, IM, or PO; CI, continuous infusion; IM, intramuscularly; IT, intrathecally; IV, intravenously; PO, per os (orally); SC, subcutaneously.
The total duration of treatment was 30 months. For patients who did not achieve CR with induction therapy, one course of each of Consolidation A and Consolidation B was given. For those who did not achieve CR until then, protocol treatment was terminated. Testicular irradiation or CNS irradiation was not administered prophylactically, but irradiation to a mass lesion initially presenting as over 5 cm in diameter was recommended after consolidation therapy.
Trimethoprim/sulfamethoxazole was recommended for the prophylaxis of Pneumocystis jirovecii pneumonia. G‐CSF (filgrastim) was not recommended in induction therapy unless a life‐threatening infection developed, but was prophylactically used after chemotherapy in Consolidation A and B.
SCT. For patients aged 15–39 years with an HLA‐matched related donor who achieved CR and met the additional criteria for SCT, allogeneic SCT was recommended after each course of Consolidation A and B. For patients aged 15–49 years without an HLA‐matched related donor who achieved CR, non‐purged autologous SCT was recommended. For conditioning before SCT, high‐dose busulfan (1 mg/kg × 4/day orally for 4 days on day –7 to –4) and high‐dose CPA (60 mg/kg/day × 2 days on day –3 and –2) were given to recipients of allogeneic or autologous SCT.( 21 ) For prophylaxis of acute graft‐versus‐host disease (GVHD), cyclosporin A and short‐term MTX were given;( 22 ) however, the doses of MTX were reduced to 10 mg/m2 on day 1, and 7 mg/m2 on days 3 and 5.( 23 )
Modification of doses and schedules. For patients aged 50 years or older, the following dose reductions and schedule modifications were performed: CPA, DXR and L‐asp in Induction, DNR, VDS and Ara‐C in Consolidation A, and MIT and ETP in Consolidation B (Table 1). The total dose was kept at 280 mg/m2 for DXR, at 240 mg/m2 for DNR, and at 36 mg/m2 for MIT in patients aged 15–49 years; and at 250 mg/m2 for DXR, at 180 mg/m2 for DNR, and at 24 mg/m2 for MIT in patients aged 50 years or older.
In all patients, treatment was postponed if the AST (GOT) level was over 200 U or if the total bilirubin was over 2.0 mg/dL. 400 mg/m2 MTX in Maintenance D was omitted when blood urea nitrogen was more than 20 mg/dL, serum creatinine was more than 1.2 mg/dL, creatinine clearance was less than 60 mL/min, serum AST (GOT) was over two times higher than the upper normal limit, or total bilirubin was over the normal upper limit. DXR, DNR or MIT was omitted when cardiotoxicity such as tachycardia, arrhythmia, ejection fraction less than 40% and cardiomyopathy developed. In patients seropositive for hepatitis‐B virus (HBV) surface antigen or hepatitis‐C virus (HCV) antibody, PSL was omitted to avoid the activation of HBV or HCV. In patients who developed an infection of grade 3 or greater, treatment was postponed, and the doses of CPA, DXR, DNR, MIT, Ara‐C, ETP were reduced to 75% in subsequent courses. In patients developing constipation of grade 3 or greater caused by Vinca alkaloids, their administrations were skipped. In patients who developed grade 4 paralytic ileus, all treatments were discontinued. In patients suffering from VCR‐induced neurotoxicity, VCR could be changed to VDS. In those who developed CPA‐induced hemorrhagic cystitis, CPA was omitted in subsequent courses. Hyperglycemia caused by PSL or chemotherapy was treated with insulin, but in patients for whom this was uncontrollable, PSL was reduced in doses or discontinued.
Criteria for response. Patients with ALL were judged to have achieved CR when the results of a BM examination became normal (blasts < 5%) along with the normalization of all abnormal laboratory findings such as absolute neutrophil count (ANC) > 1000/µL and platelets > 100 000/µL, and all extramedullary diseases had resolved. Patients with LBL were judged to have achieved CR when the tumor or swollen lymph nodes disappeared for more than 4 weeks, according to the WHO response criteria.( 18 )
Assessment of toxicity. Toxicity was assessed according to the WHO toxicity criteria.( 18 )
Statistical analysis. All analyses were performed using SAS statistical software (SAS for Windows, Version 6.12, SAS Institute Inc., Cary, NC, USA). CR rate was evaluated in 143 eligible patients. Overall survival (OS) and progression‐free survival (PFS) were evaluated in 143 eligible patients and 119 patients who achieved CR, respectively. Fisher's exact test was used to compare the relative frequencies of therapy‐related death (TRD) by the induction therapy among age groups. The chi‐squared test was used to assess the association between CR rate and each patient characteristic. Survival was calculated from the date of registration to the last follow‐up date or death. Duration of PFS was defined from the date of registration to the date of relapse or progression, death or date of last follow up. Survival curves were obtained using the Kaplan–Meier method. The difference between survival curves according to each characteristic was analyzed using the log‐rank test. The LIFETEST procedure of SAS software was used for the Kaplan–Meier method and the log‐rank test.( 24 ) Cox proportional hazards regression analysis was performed to assess the association between each patient characteristic and survival using the PHREG procedure.( 25 )
Results
Patient characteristics. Between February 1991 and November 1994, 147 newly diagnosed patients with ALL or LBL aged 15–69 years were registered from 31 institutions, and 143 (97%) were eligible (Table 2). Three enrolled patients under the institutional diagnosis of LBL were judged ineligible by the central pathology review, because the diagnosis was diffuse large B‐cell lymphoma, diffuse mixed B‐cell lymphoma, and Burkitt lymphoma, respectively. The remaining patient was ineligible due to a subsequent report from the institution with the diagnosis of Burkitt lymphoma. In six patients, the clinical diagnosis was changed to ALL because the neoplastic cells in BM were 25% or more.( 2 ) The 143 eligible patients ranged in age from 16 to 69 years, with a median age of 41 years. There were 87 men (61%) and 56 women (39%). The FAB subtype could be determined in 116 patients (92%): 36 (29%) were L1, 75 (60%) were L2, and five (4%) were L3.
Table 2.
Patient characteristics
| Total (n) | Acute lymphoblastic leukemia (n) | Lymphoblastic lymphoma (n) | |
|---|---|---|---|
| Enrolled | 147 | 120 † | 27 † |
| Eligible | 143 | 126 † | 17 † |
| Evaluable | 143 | 126 † | 17 † |
| ‡ Median age (years; range) | 41 (16–69) | 43 (16–69) | 32 (18–58) |
| ‡ Sex (M/F) | 87/56 | 74/52 | 13/4 |
Four enrolled patients under the institutional diagnosis of lymphoblastic lymphoma were judged ineligible by the central pathology review and a subsequent report from the institution. In six patients, the clinical diagnosis was changed to acute lymphoblastic leukemia because the neoplastic cells in BM were 25% or more.
‡ The data of the 143 eligible patients. F, female; M, male.
An immunophenotypic analysis was successfully performed in 125 (99%) of 126 ALL, in 16 (94%) of 17 LBL cases, and in 141 (98%) of 143 total eligible cases (Table 3). Among 126 ALL cases, 90 (72%) were categorized as B‐lineage cases, whereas 28 (22%) were T‐cell lineage. Cases of mixed type (T/B positive) or unclassified type (lack of lineage marker) were categorized as ‘others’. Among LBL cases, 13 were T‐cell lineage, two B‐cell lineage and one categorized as ‘other’. Cytogenetic analysis was performed in 111 (78%) of 143 cases. Philadelphia chromosome was detected in 23 (20%) cases. Other abnormal results were also reported in 23 cases (data not shown), normal karyotype in 47 and indeterminate results in 18.
Table 3.
Other patient characteristics
| Total (n; %) | Acute lymphoblastic leukemia (n; %) | Lymphoblastic lymphoma (n; %) | |
|---|---|---|---|
| Immunophenotype | |||
| T‐lineage | 41 (29) | 28 (22) | 13 (76) |
| B‐lineage | 92 (64) | 90 (72) | 2 (12) |
| Others | 8 (6) | 7 (6) | 1 (6) |
| NA | 2 (1) | 1 (1) | 1 (6) |
| FAB subtype | |||
| L1 | 36 (29) | ||
| L2 | 75 (60) | ||
| L3 | 5 (4) | ||
| NA | 10 (8) | ||
| Philadelphia chromosome | |||
| Detected | 23 (16) | 23 (18) | 0 |
| Not detected | 88 (62) | 80 (64) | 8 (47) |
| NA | 32 (22) | 23 (18) | 9 (53) |
FAB, French–American–British Classification; NA, not available.
Therapeutic results. The therapeutic results are summarized in Table 4. 119 (83%; 95% confidence interval [CI], 78–90%) of the 143 eligible patients achieved CR. Their median age was 40 years, ranging from 15 to 68 years. Sixty patients (50%) were 40 years or younger. 106 patients with ALL and 13 with LBL were included. Among the patients who achieved CR, 60 proceeded to maintenance therapy. At the time of last follow up, 38 patients remained alive without progression. The overall survival curve of all 143 eligible patients is shown in Fig. 2. The median survival for all 143 eligible patients was 26 months (95% CI, 19–34 months). The estimated probability of overall survival at 5 years was 32% (95% CI; 25–40%) and 31% (95% CI, 24–39%) at 7 years. The estimated probability of PFS at 5 years was 26% (95% CI, 18–33%), as shown in Fig. 3. The overall survival curve of 119 patients who achieved CR is shown in Fig. 4. The estimated probability of survival at 5 years was 38% (95% CI; 29–47%). The PFS curve of 119 patients who achieved CR is shown in Fig. 5. The estimated probability of PFS at 5 years was 31% (95% CI; 22–39%).
Table 4.
Therapeutic results
| Eligible | 143 |
| CR | 119 (83%; 95% CI, 78–90) |
| Therapy‐related death | 17 |
| Induction phase | 14 |
| Consolidation phase | 3 |
| Relapsed | 76 |
| Died in CR † , † | 5 |
| Alive in CR | 38 |
| Median survival time ‡ | 26 months (95% CI, 19–34) |
| 7 years‐OS ‡ | 31% (95% CI, 24–39) |
| 7 years‐PFS ‡ | 26% (95% CI, 19–33) |
Three patients suffered from TRD during consolidation/maintenance therapy.
†Two patients died in CR, one hemorrhagic cystitis after SCT, and etiology was not reported in the other.
Median follow‐up time; 9 years. CI, confidence interval; CR, complete response, OS, overall survival; PFS, progression‐free survival.
Figure 2.
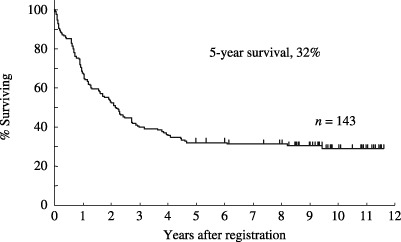
Overall survival of 143 eligible patients with acute lymphoblastic leukemia (n = 126) and lymphoblastic lymphoma (n = 17). The median survival for all 143 eligible patients was 26 months (95% CI, 19–34 months). The estimated probability of overall survival at 5 years was 32% (95% CI; 25–40%) and 31% (95% CI, 24–39%) at 7 years.
Figure 3.
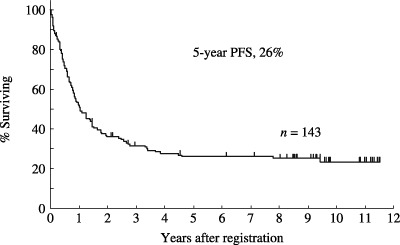
Progression‐free survival (PFS) of 143 eligible patients with acute lymphoblastic leukemia (n = 126) and lymphoblastic lymphoma (n = 17). The estimated probability of PFS at 5 years was 26% (95% CI, 18–33%).
Figure 4.
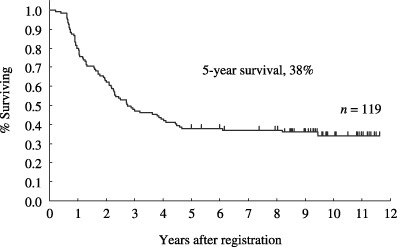
Overall survival of 119 patients with acute lymphoblastic leukemia (n = 106) and lymphoblastic lymphoma (n = 13) who achieved complete response. The estimated probability of overall survival at 5 years was 38% (95% CI, 29–47%).
Figure 5.
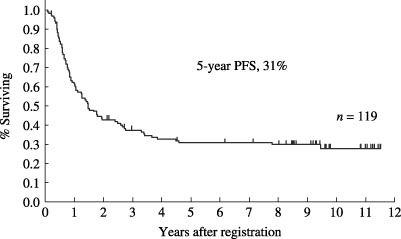
Progression‐free survival (PFS) of 119 patients with acute lymphoblastic leukemia (n = 106) and lymphoblastic lymphoma (n = 13) who achieved complete response. The estimated probability of PFS at 5 years was 31% (95% CI, 22–39%).
Twenty patients who achieved CR were registered and underwent SCT according to the protocol, using BM as the stem cell source, and high‐dose busulfan and CPA as the conditioning regimen. However, several advances in this area have enabled treating physicians to choose the total body irradiation (TBI)‐based conditioning regimen, peripheral blood stem cell as the stem cell source, and an unrelated donor. As a result, 16 patients who achieved CR according to this protocol underwent SCT outside the protocol. To assess the role of SCT, all 36 patients who underwent SCT in the first CR were evaluated as shown in Table 5. Their median age was 25 years and their age ranged from 15 to 48 years. 28 patients (78%) were male, and 28 (78%) with ALL and eight (22%) with LBL were included. Autologous and allogeneic stem cells were used in 20 patients (BM in 11 patients and PB in 9 patients) and 16 patients (related donor BM in 13 patients and unrelated donor BM in 3 patients), respectively. The overall survival of the 36 patients at median follow up of 5 years is shown in Fig. 6. Median survival was not reached and the estimated probability of survival at 5 years was 58% (95% CI, 42–74%). The overall survival according to the stem cell source is shown in Fig. 7. The estimated survival probabilities of autologous and allogeneic SCT were 39% (95% CI, 18–61%) and 75% (95% CI, 54–96%) at 5 years, respectively.
Table 5.
Patients undergoing stem cell transplantation during first complete response
| Patient characteristic | n | |
| Tota | 36 | |
| Registered | 20 | |
| Report only | 16 | |
| Median age (years; range) | 25 (15–48) | |
| Sex (male/female) | 28/8 | |
| Disease | Acute lymphoblastic leukemia | 28 |
| Chromosome | Philadelphia | 1 |
| Stem cell | Autologous (bone marrow/peripheral blood) | 11/9 |
| Allogeneic (related/unrelated) | 13/3 | |
| Conditioning | ||
| Busulfan/Cy‐based | 25 | |
| Total body irradiation‐based | 10 | |
| Other | 1 | |
| Results | ||
| Died <100 days after stem cell transplantation | 1 | |
| Death after relapse | 16 | |
| Other cause of death | 1 | |
| 5‐year overall survival † | 58% (95% CI, 42–74%) | |
| Alive at last follow up | 20 | |
Median follow‐up time, 5 years. CI, confidence interval; Cy, cyclophosphamide.
Figure 6.

Overall survival of 36 patients who underwent stem cell transplantation in first remission. The estimated probability of survival at 5 years was 58% (95% CI, 42–74%).
Figure 7.
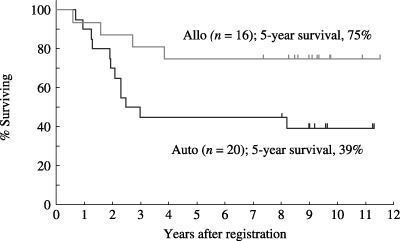
Overall survival of 16 patients who underwent allogeneic (Allo) stem cell transplantation (SCT) and 20 patients who underwent autologous (Auto) SCT. The estimated survival probabilities of autologous and allogeneic SCT were 39% (95% CI, 18–61%) and 75% (95% CI, 54–96%) at 5 years, respectively.
Subgroup analysis. CR rates were compared between subsets according to the following eight variables; age (≤40 vs >40 years), sex (male vs female), clinical diagnosis (LBL vs ALL), WHO PS (0, 1 vs 2–4), leukocytes at initial presentation (<10 000/µL vs≥10 000/µL), immunophenotype of neoplastic cells (T vs non‐T), C‐reactive protein (CRP; normal vs elevated), Philadelphia chromosome anomaly (not detected vs detected); however, significant factors influencing CR‐rates (P < 0.05) were not identified.
Concerning overall survival, small P‐values using the log‐rank test were obtained in the following subsets: age of ≤40 years versus older, sex of male versus female, PS of 0 or 1 versus 2–4, Philadelphia chromosome anomaly of not detected versus detected (Table 6). Cox proportional hazards regression analysis also showed that older age, female sex, and the detection of Philadelphia chromosome anomaly were associated with shortened survival.
Table 6.
Univariate analysis of factors affecting survival
| Characteristic | Category | Distribution (n) | P‐value † | Risk ratio ‡ (95% CI) |
|---|---|---|---|---|
| Age (years) | ≤40/>40 | 70/73 | 0.016 | 1.624 (1.019–2.419) |
| Sex | Male/female | 87/56 | 0.008 | 1.692 (1.140–2.517) |
| Diagnosis | ALL/LBL | 126/17 | 0.196 | 0.639 (0.322–1.268) |
| Performance status | 0,1/2–4 | 110/32 | 0.052 | 1.564 (0.992–2.464) |
| Leukocytes | <1 × 104/µL/≥1 × 104/µL | 71/66 | 0.123 | 1.370 (0.917–2.045) |
| Immunophenotype | Non‐T/T | 102/41 | 0.124 | 0.699 (0.441–1.106) |
| C‐reactive protein | Normal/elevated | 56/67 | 0.152 | 1.372 (0.888–2.119) |
| Philadelphia chromosome | Not detected/detected | 88/23 | 0.013 | 1.875 (1.134–3.098) |
P‐value by log rank test.
‡ Risk ratio by Cox proportional hazards regression analysis. ALL, acute lymphoblastic leukemia; CI, confidence interval; LBL, lymphoblastic lymphoma.
Toxicity. The number of patients who resulted in therapy‐related death (TRD) was 17; their median age was 60 years, ranging from 21 to 69 years. Fourteen patients died in the induction phase, and three in the consolidation phase. The causes of death were infection in 11 patients, intracranial event in five, and hemorrhage in one. The relative frequencies of TRD were significantly different between age groups: 2% (2/97) and 26% (12/46) in patients <50 years and those at ≥50 years (P < 0.0001), and 4% (5/125) and 50% (9/18) in patients <60 years and those at ≥60 years (P < 0.0001).
The numbers of patients who developed non‐hematologic toxicities of grade 3 or greater, except for alopecia and nausea/vomiting, are shown in Table 7. The major non‐hematologic toxicities of grade 3 or greater encountered in patients who received induction therapy were ALT (GPT) elevation (29%), infection (14%) and peripheral neuropathy (4%). Among the 120 patients treated with consolidation therapy, 19 (16%) developed ALT (GPT) elevation and 12 (10%) infection of grade 3 or greater.
Table 7.
Numbers of patients who developed non‐hematologic toxicities of grade 3 or greater
| Treatment regimen | Induction (n; %) | Consolidation A/B † (n; %) |
|---|---|---|
| Total treated patients | 147 | 120 |
| Total evaluated courses | 146 | 120 |
| Toxicity | ||
| Infection | 20 (14) | 12 (10) |
| Diarrhea | 3 (2) | 1 (1) |
| Constipation | 3 (2) | 1 (1) |
| ALT (GPT) elevation | 43 (29) | 19 (16) |
| Peripheral neuropathy | 6 (4) | 0 |
| Pulmonary | 5 (3) | 2 (2) |
| Arrhythmia | 2 (1) | 0 |
Toxicities in the first cycle of Consolidation A/B were evaluated.
Discussion
In the present study, JCOG9004, 83% of all eligible patients achieved CR. As compared to the previous study. JCOG8702, the CR rate was slightly improved from 78%.( 15 ) These results using induction therapy are equivalent to those from other multi‐institutional trials.( 3 , 5 , 6 , 9 , 10 , 11 , 12 , 13 ) The major problem in induction therapy was TRD in the older age group. Considering the high incidence of TRD in older age groups (26%[12/46] in patients at ≥50 years and 50%[9/18] in those at ≥60 years) despite the dose reduction in patients at ≥50 years (Table 1), further modifications of dose and schedule in induction therapy may be necessary for older patients. The CR rate (76%, 13/17) of patients with LBL was similar to those in other reports.( 3 , 5 , 6 , 7 )
In the present study, JCOG9004, mitoxantrone, etoposide and cytosine arabinoside were incorporated to intensify the consolidation phase with the support of G‐CSF. In the maintenance therapy, long‐term administration of MTX and 6‐MP was combined with pulse chemotherapy containing an intermediate dose of MTX. The toxicity of the two consolidation therapies (A and B) was tolerable in most patients, although three (3%) of the 120 patients developed TRD. It is likely that these strategies in post‐remission chemotherapy improved the overall therapeutic results, compared with the authors’ previous study, JCOG 8702,( 15 ) although there remains the possibility that other factors including the increased dose intensity of the induction regimen, incorporation of stem cell transplantation, and changes in supportive care and patient selection between the two protocols may have been an influence. The median survival time was improved to 26 months from 14 months in JCOG 8702, and the estimated probability of survival at 7 years was improved to 31% from 15%.
In prognostic factor analysis, female sex was unexpectedly an unfavorable factor. Similar results were reported in a randomized study of filgrastim during induction and consolidation for adult ALL conducted by Cancer and Leukemia Group B.( 12 ) The reasons for the unfavorable prognostic impact of female sex are unclear; however, it is possibly because patients receiving SCT in first CR were male dominant in the present series. Other prognostic factors picked up in this study showed consistent results with other reports,( 10 , 11 , 12 , 13 , 26 , 27 , 28 ) although there were some discrepancies. The proportion of patients with Philadelphia chromosome anomaly in ALL (23/103, 22%) was not different from other reports, and they showed an unfavorable prognosis similar to other studies.( 11 , 12 , 13 , 26 , 27 , 28 ) C‐reactive protein did not have an impact in the present study, in contrast to the authors’ previous trial, JCOG 8702.( 15 )
Patients with LBL showed a tendency toward a more favorable prognosis than those with ALL, although there was no significant difference, as shown in Table 6. These results suggest that a favorable outcome in patients with LBL can be achieved with an ALL‐type treatment regimen including SCT, similar to other reports.( 6 , 7 , 29 )
Because there is considerable heterogeneity in the stem cell source, conditioning regimen, etc., in the 36 patients who underwent SCT in first CR in this series, it is impossible to draw meaningful conclusions; however, the results of overall survival suggest that SCT, particularly allogeneic SCT, in first CR is worthy of further investigation.
Although some advantages have been obtained by various strategies in the treatment of adult ALL and LBL, the present therapeutic outcome is still unsatisfactory. One of the plausible reasons for the unfavorable therapeutic outcome is age. In the present study, the median age of the 143 eligible patients was 41 years, and that of the 126 eligible patients with ALL was 43 years. When this was compared with the median ages of 25–40 years in the reported results,( 9 , 10 , 11 , 12 , 13 , 15 , 26 , 27 , 28 ) the median age in the present series is the oldest. Considering that age is a strong prognostic factor in most studies for adult ALL and LBL,( 30 ) the therapeutic results in this study could be worse.
To further improve the therapeutic results, application of a dose‐intensive pediatric regimen,( 31 ) incorporation of effective new agents such as nelarabine for T‐ALL and T‐LBL,( 32 ) imatinib and new tyrosine kinase inhibitors for Philadelphia chromosome‐positive ALL,( 33 , 34 , 35 , 36 ) may be needed, in addition to SCT.
In conclusion, intensive post‐remission chemotherapy was performed safely with the prophylactic use of G‐CSF, and resulted in the marked improvement of therapeutic outcomes in adult patients with ALL and LBL, as compared with the authors’ previous study, JCOG8702. Intensive post‐remission therapy followed by SCT is considered as a promising strategy for the treatment of adult patients with ALL and LBL.
List of participants
C. Mikuni and K. Aikawa (Sapporo National Hospital); M. Kasai and Y. Kiyama (Sapporo Hokuyu Hospital); I. Miura and A. Miura (Akita University); T. Sai (Iwaki Kyoritsu General Hospital); Y. Sasaki and K. Itoh (National Cancer Center Hospital East); M. Shimoyama, K. Tobinai, K. Minato, T. Takenaka, K. Takeyama and A. Kohno (National Cancer Center Hospital); U. Sawada (Nihon University); I. Aoki, K. Kawano (Kyorin University); T. Ibuka (Tokyo Metropolitan Komagome General Hospital); T. Miwa and A. Togawa (International Medical Center of Japan); H. Yamada and S. Iwase (The Jikei University School of Medicine, Aoto Hospital); K. Deura and S. Seki (Saku Central Hospital); M. Ogura, Y. Kagami and H. Suzuki (Aichi Cancer Center); H. Nagai (Nagoya National Hospital); T. Hotta and T. Kinoshita (Nagoya University); M. Hirano and M. Okamoto (Fujita Health University); S. Shirakawa, T. Kobayashi, M. Masuya and M. Yamaguchi (Mie University); S. Konda and Y. Masaki (Kanazawa Medical University); T. Suzuki (Shiga Medical Center for Adults); S. Fukuhara and H. Ohno (Kyoto University); T. Abe and M. Taniwaki (Kyoto Prefectural University of Medicine); Y. Ohno (Tenri Hospital); S. Irino and M. Nagai (Kagawa Medical School); H. Toki and K. Okabe (National Shikoku Cancer Center); M. Kozuru and N. Uike (National Kyusyu Cancer Center); S. Okamoto (National Kyusyu Medical Center); K. Fujita and Y. Izumi (Kokura Memorial Hospital); Y. Shimamoto and H. Fukushima (Saga Medical School); K. Yamaguchi and K. Takatsuki (Kumamoto University); M. Matsumoto, S. Hanada, K. Uozumi and A. Utsunomiya (Kagoshima University); K. Araki and I. Ohshiro (University of the Ryukyus).
Acknowledgments
We are greatly appreciative of all members of JCOG‐LSG for their excellent cooperation in this long‐term follow‐up study. We thank the many treating physicians, nurses, laboratory technicians and patients. We wish to thank Ms. Yuko Watanabe and Dr Taro Shibata (JCOG Data Center) for data management and statistical analyses. This study was supported by Grants‐in‐Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan (2S‐1, 5S‐1, 8S‐1, 11S‐1, 14S‐1, 17S‐1, 1‐1, 4‐5, 7‐29, 9‐10) (1990–present), for the Second‐Term Ten‐Year Strategy for Cancer Control from the Ministry of Health and Welfare (1994–present), and for Basic Research from the Science and Technology Agency (1991–1993).
References
- 1. Slater DE, Mertelsmann R, Koziner B et al . Lymphoblastic lymphoma in adults. J Clin Oncol 1986; 4: 57–67. [DOI] [PubMed] [Google Scholar]
- 2. Brunning RD, Borowitz M, Matutes E et al . Precursor T lymphoblastic leukaemia/lymphoblastic lymphoma (precursor, T‐cell acute lymphoblastic leukaemia). In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2001; 115–17. [Google Scholar]
- 3. Coleman CN, Picozzi VJ Jr, Cox RS et al . Treatment of lymphoblastic lymphoma in adults. J Clin Oncol 1986; 4: 1628–37. [DOI] [PubMed] [Google Scholar]
- 4. Gaynor J, Chapman D, Little C et al . A cause‐specific hazard rate analysis of prognostic factors among 199 adults with acute lymphoblastic leukemia: the Memorial Hospital Experience since 1969. J Clin Oncol 1988; 6: 1014–30. [DOI] [PubMed] [Google Scholar]
- 5. Willemze R, Zijlmans JM, Den Ottolander GJ et al . High‐dose ara‐C for remission induction and consolidation of previously untreated adults with ALL or lymphoblastic lymphoma. Ann Hematol 1995; 70: 71–4. [DOI] [PubMed] [Google Scholar]
- 6. Hoelzer D, Gokbuget N, Digel W et al . Outcome of adult patients with T‐lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood 2002; 99: 4379–85. [DOI] [PubMed] [Google Scholar]
- 7. Thomas DA, O’Brien S, Cortes J et al . Outcome with the hyper‐CVAD regimens in lymphoblastic lymphoma. Blood 2004; 104: 1624–30. [DOI] [PubMed] [Google Scholar]
- 8. Rivera GK, Pinkel D, Simone JD et al . Treatment of acute lymphoblastic leukemia: 30 years’ experience at St. Jude Children's Research Hospital. N Engl J Med 1993; 329: 1289–95. [DOI] [PubMed] [Google Scholar]
- 9. Hoelzer D, Thiel E, Loeffler H et al . Intensified therapy in acute lymphoblastic and acute undifferentiated leukemia in adults. Blood 1984; 64: 38–47. [PubMed] [Google Scholar]
- 10. Hoelzer D, Thiel E, Loeffler H et al . Prognostic factors in a multicenter study of acute lymphoblastic leukemia in adults. Blood 1988; 71: 123–31. [PubMed] [Google Scholar]
- 11. Larson RA, Dodge RK, Burns CP et al . A five‐drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and Leukemia Group B study 8811. Blood 1995; 85: 2025–37. [PubMed] [Google Scholar]
- 12. Larson RA, Dodge RK, Linker CA et al . A randomized controlled trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood 1998; 92: 1556–64. [PubMed] [Google Scholar]
- 13. Thomas X, Boiron JM, Huguet F et al . Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA‐94 trial. J Clin Oncol 2004; 22: 4075–86. [DOI] [PubMed] [Google Scholar]
- 14. Shimoyama M, Ota K, Kikuchi M et al . Major prognostic factors of adult patients with advanced T‐cell lymphoma/leukemia. J Clin Oncol 1988; 6: 1088–97. [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi T, Tobinai K, Shimoyama M et al . Long‐term follow‐up results of adult patients with acute lymphocytic leukemia or lymphoblastic lymphoma treated with short‐term, alternating non‐cross‐resistant chemotherapy: Japan Clinical Oncology Group Study 8702. Jpn J Clin Oncol 1999; 29: 340–8. [DOI] [PubMed] [Google Scholar]
- 16. Bennett JM, Catovsky D, Daniel MT et al . Proposals for the classification of acute leukemias. Br J Haematol 1976; 33: 451–8. [DOI] [PubMed] [Google Scholar]
- 17. The Non‐Hodgkin's Lymphoma Pathologic Classification Project: National Cancer Institute sponsored study of classifications of non‐Hodgkin's lymphoma . Summary and description of a Working Formulation for clinical usage. Cancer 1982; 49: 2112–35. [DOI] [PubMed] [Google Scholar]
- 18. WHO Handbook for Reporting Results of Cancer Treatment. WHO offset publication no. 48, Geneva: World Health Organization, 1979. [Google Scholar]
- 19. Bennett JM, Catovsky D, Daniel MT et al . Proposal for the recognition of minimally differentiated acute myeloid leukaemia (AML‐M0). Br J Haematol 1991; 78: 325–9. [DOI] [PubMed] [Google Scholar]
- 20. Shimoyama M, Fukuda H, Saijo N et al . Japan Clinical Oncology Group (JCOG). Jpn J Clin Oncol 1998; 28: 158–62. [DOI] [PubMed] [Google Scholar]
- 21. Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood 1987; 70: 1382–8. [PubMed] [Google Scholar]
- 22. Storb R, Deeg HJ, Whitehead J et al . Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986; 314: 729–35. [DOI] [PubMed] [Google Scholar]
- 23. Morishima Y, Morishita Y, Tanimoto M et al . Low incidence of acute graft‐versus‐host disease by the administration of methotrexate and cyclosporine in Japanese leukemia patients after bone marrow transplantation from human leukocyte antigen compatible siblings; possible role of genetic homogeneity. Blood 1989; 74: 2252–6. [PubMed] [Google Scholar]
- 24. SAS Institute Inc . SAS/STAT User's Guide, Version 6, 4th edn, Vol. 1. & 2. Cary NC: SAS Institute Inc., 1989. [Google Scholar]
- 25. SAS. Institute Inc . SAS Technical Report P‐229, SAS/STAT Software: Changes and Enhancements, Release 6.07. Cary NC: SAS Institute Inc., 1992. [Google Scholar]
- 26. Takeuchi J, Kyo T, Naito K et al . Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG‐ALL93 study. Leukemia 2002; 16: 1259–66. [DOI] [PubMed] [Google Scholar]
- 27. Kantarjian H, O’Brien S, Smith TL et al . Results of treatment with Hyper‐CVAD, a dose‐intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000; 18: 547–61. [DOI] [PubMed] [Google Scholar]
- 28. Kantarjian H, Thomas D, O’Brien S et al . Long‐term follow‐up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper‐CVAD), a dose‐intensive regimen, in adult acute lymphocytic leukemia. Cancer 2004; 101: 2788–801. [DOI] [PubMed] [Google Scholar]
- 29. Levine JE, Harris RE, Loberiza FR Jr. et al . A comparison of allogeneic and autologous bone marrow transplantation for lymphoblastic lymphoma. Blood 2003; 101: 2476–82. [DOI] [PubMed] [Google Scholar]
- 30. Larson RA. Acute lymphoblastic leukemia: older patients and newer drugs. Hematology Am Soc Hematol Educ Program 2005; 0: 131–6. [DOI] [PubMed] [Google Scholar]
- 31. DeAngelo DJ. The treatment of adolescents and young adults with acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program 2005; 0: 123–30. [DOI] [PubMed] [Google Scholar]
- 32. Berg SL, Blaney SM, Devidas M et al . Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T‐cell malignancies: a report from the Children's Oncology Group. J Clin Oncol 2005; 23: 3376–82. [DOI] [PubMed] [Google Scholar]
- 33. Yanada M, Takeuchi J, Sugiura I et al . High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR‐ABL‐positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol 2006; 24: 460–6. [DOI] [PubMed] [Google Scholar]
- 34. Wassmann B, Pfeifer H, Goekbuget N et al . Alternating versus concurrent schedules of imatinib and chemotherapy as front‐line therapy for Philadelphia‐positive acute lymphoblastic leukemia (Ph+ ALL). Blood 2006; 108: 1469–77. [DOI] [PubMed] [Google Scholar]
- 35. Talpaz M, Shah NP, Kantarjian H et al . Dasatinib in imatinib‐resistant Philadelphia chromosome–positive leukemias. N Engl J Med 2006; 354: 2531–41. [DOI] [PubMed] [Google Scholar]
- 36. Kantarjian H, Giles F, Wunderle L et al . Nilotinib in imatinib‐resistant CML and Philadelphia chromosome‐positive ALL. N Engl J Med 2006; 354: 2542–51. [DOI] [PubMed] [Google Scholar]


