Abstract
Macrophage scavenger receptor (MSR)‐positive inflammatory cells and tumor‐associated macrophages (TAMs) have been reported to regulate the growth of various cancers. In this study, the infiltration of MSR‐positive cells and TAMs was analyzed to predict the outcome of repeat biopsy in men diagnosed as having no malignancy at the first prostate biopsy. Repeat biopsy of the prostate was carried out in 92 patients who were diagnosed as having no malignancy at the first biopsy. Of these, 30 patients (32.6%) were positive for prostate cancer at the repeat biopsy. Tumor‐associated macrophages and MSR‐positive cells were immunohistochemically stained with mAbs CD68 and CD204, respectively. Six ocular measuring fields were chosen randomly under a microscope at ×400 power in the initial negative biopsy specimens, and the mean TAM and MSR counts for each case were determined. No difference in TAM count was found between the cases with or without prostate cancer. By contrast, the MSR count in patients with cancer was significantly lower than that in patients without cancer at the repeat biopsy (P < 0.001). Logistic regression analysis indicated that the MSR count at first biopsy is a significantly better predictive factor for positive repeat biopsy than PSA velocity, interval between first and repeat biopsies, or TAM count. Decreased infiltration of MSR‐positive cells in negative first biopsy specimens was correlated with positive findings in the repeat biopsy. The MSR count might be a good indicator for avoiding unnecessary repeat biopsies. (Cancer Sci 2010)
Prostate cancer (PCa) is one of the most common cancers in males in developed countries.( 1 ) Prostate‐specific antigen (PSA) is currently the most useful serum tumor marker for detecting Pca.( 2 ) However, because of the low specificity of PSA, many patients undergo unnecessary needle biopsies of the prostate.( 3 ) Most patients who undergo prostate biopsies and have negative pathological findings are followed up with periodic PSA measurements. A repeat biopsy might be indicated by the presence of high‐grade prostatic intraepithelial neoplasia (HGPIN) on the initial biopsy, a low percentage of free PSA, an increasing PSA velocity, elevated PSA density, or changes on digital rectal examination.( 4 , 5 , 6 , 7 , 8 ) Several additional biomarkers and molecular markers prompt us to carry out a repeat biopsy, including prostate cancer gene 3 mRNA in the urine,( 9 , 10 ) hypermethylation of the glutathione S‐transferase pi 1 gene promoter in post‐biopsy urine specimens,( 11 ) and telomerase activity in prostate massage samples.( 12 )
Recurrent or chronic inflammation has been implicated in the development of many human cancers, including gastric, liver, colon, and urinary bladder cancers.( 13 ) It has been reported that some inflammatory cells contribute positively to carcinogenesis or cancer progression in the prostate.( 14 , 15 , 16 , 17 ) Of the many kinds of inflammatory cell, decreased infiltration of macrophage scavenger receptor 1 (MSR1)‐positive cells has been reported to play important roles in the progression of PCa.( 18 ) Germ‐line mutations in the MSR1 gene leading to defective expression are involved in prostate carcinogenesis.( 19 , 20 , 21 ) Therefore, we examined the association between tumor‐associated macrophage (TAM) infiltration or the expression of MSR1 and the rate of detection of PCa at a repeat biopsy of the prostate in patients in whom the first biopsy was negative.
Materials and Methods
Patients. Between 1997 and 2000, 373 patients underwent first prostate biopsy because of elevated serum PSA, positive digital rectal examination (DRE), or positive findings in transrectal ultrasonography. Of these, 222 patients (59.5%) with negative biopsy were followed up periodically (usually at 6‐month intervals) with a serum PSA check or DRE. From this group, 92 patients (42.8%) ranging in age from 51 to 82 years (median, 69 years) underwent repeat biopsies of the prostate because of the increasing serum PSA level, appearance of abnormal nodules in the prostate by DRE, or their anxiety. Of these 92 patients, 30 (32.6%) were positive for cancer at the repeat biopsy. Written informed consent was obtained from all patients.
Immunohistochemistry. Biopsy specimens were fixed in 10% neutral buffered formalin and routinely processed for paraffin embedding. Serial 5‐μm‐thick sections were cut, and one section was stained with H&E and reviewed by a pathologist (K.A., one of the authors) to obtain the pathological diagnosis. Tumor‐associated macrophages and MSR‐positive inflammatory cells were labeled immunohistochemically using mAbs CD68 (1:100 dilution; Dako, Glostrup, Denmark) and CD204 (1:100 dilution; Trans Genic, Kobe, Japan), respectively, and were visualized using an LSAB kit (Dako). For systematic counting, six ocular measuring fields, each with a real area of 0.06175 mm2, were chosen randomly under a microscope at ×400 in the first negative biopsy specimens. For each case, the mean numbers of the TAMs and MSR‐positive cells were determined as the TAM and MSR count of each case.
Statistical analysis. Statistical analysis was carried out using StatView (SAS Institute, Cary, NC, USA). Differences in the PSA value, TAM count, and MSR count between the cancer and normal groups were evaluated using the Mann–Whitney U‐test. The correlation between TAMs, MSR infiltration by immunohistochemistry, and categorical variables were evaluated using the chi‐square test and Fisher’s exact probability test. Statistical significance was considered at P < 0.05. Logistic regression analysis was carried out to determine significant useful predictors for positive repeat biopsy.
Results
Immunohistochemical findings. Representative immunohistochemical findings are shown in Figure 1. Macrophage scavenger receptor‐positive cells were observed among the connective tissue. Prostatic epithelial cells, interstitial cells, or basal cells were not stained with antibody CD204.
Figure 1.
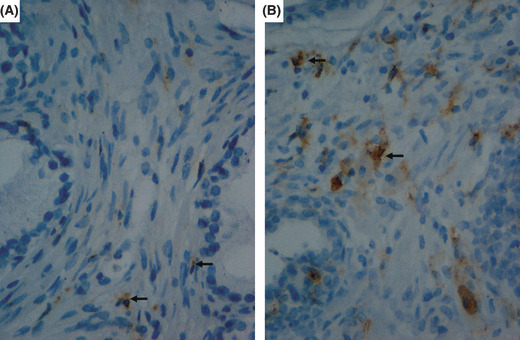
Representative immunostaining for macro‐phage scavenger receptor (MSR) cells from men who underwent repeat biopsy of the prostate. Representative cases with low (A) and high (B) MSR counts are shown. Arrowheads indicate MSR‐positive cells.
Prostate‐specific antigen and pathological diagnosis. The PSA value at the first biopsy ranged from 1.7 to 43.12 ng/mL (median, 8.80). The median interval from the first biopsy to the second was 21.6 months (range, 4–84 months). These data are summarized in Table 1. The serum PSA levels at the first biopsy (mean ± SD) did not differ statistically between the patients with final pathological diagnoses of PCa and benign prostate, as shown in Figure 2(A) (11.95 ± 8.39 versus 9.19 ± 4.60; P = 0.075). In contrast, the PSA level at the repeat biopsy was significantly higher in patients with PCa than in patients with benign prostate (19.65 ± 14.19 versus 11.40 ± 8.19; P = 0.004; Fig. 2B). No statistical difference in the PSA velocity between PCa and benign prostate was found, as shown in Table 2.
Table 1.
Patients’ characteristics
| Range | Median | |
|---|---|---|
| Age at the first biopsy (years old) | 51 to 82 | 69 |
| PSA value at the first biopsy (ng/mL) | 1.70 to 43.12 | 8.80 |
| PSA value at the repeat biopsy (ng/mL) | 1.90 to 52.68 | 10.00 |
| Time from the first to the repeat biopsy (months) | 4 to 84 | 21.6 |
| PSA velocity (ng/mL/year) | −3.77 to 37.50 | 1.6 |
PSA, prostate‐specific antigen.
Figure 2.
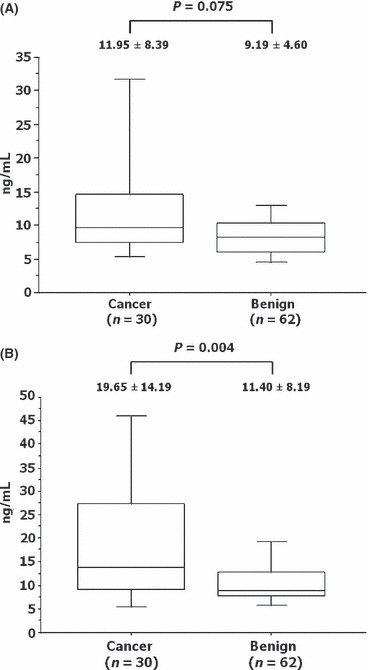
Association between the final pathological results and the serum prostate‐specific antigen level at the first (A) and repeat (B) biopsies of the prostate. Boxed areas represent the 25th and 75th percentiles, and error values; error bars represent the 10th and 90th percentiles.
Table 2.
Association of clinicopathological parameters with biopsy results
| Benign prostate | Prostatic cancer | P‐value | |
|---|---|---|---|
| Age at the repeat biopsy (years) | 68.38 ± 6.27 | 68.98 ± 7.09 | 0.356 |
| PSA at the first biopsy (ng/mL) | 9.19 ± 4.60 | 11.95 ± 8.39 | 0.075 |
| PSA at the repeat biopsy (ng/mL) | 11.40 ± 8.19 | 19.65 ± 14.19 | 0.004 |
| PSA velocity (ng/mL/year) | 1.49 ± 4.45 | 2.72 ± 4.74 | 0.083 |
| TAM count | 15.70 ± 4.24 | 16.13 ± 5.85 | 0.364 |
| MSR count | 38.70 ± 9.95 | 27.13 ± 5.28 | <0.001 |
MSR, macrophage scavenger receptor; PSA, prostate‐specific antigen; TAM, tumor‐associated macrophage.
Tumor‐associated macrophage count and pathological diag‐nosis. The TAM count at the first biopsy did not differ between the patients who were diagnosed with PCa and those with benign prostate (16.13 ± 5.85 versus 15.70 ± 4.24; P = 0.364), as shown in Figure 3.
Figure 3.
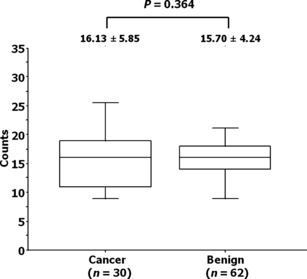
Association between the tumor‐associated macrophage count and pathological results in patients who underwent repeat biopsies of the prostate. Boxed areas represent the 25th and 75th percentiles, and error values; error bars represent the 10th and 90th percentiles.
Macrophage scavenger receptor count and pathological diagnosis. The MSR count at the first biopsy was significantly lower in those patients who were diagnosed with PCa than in those with benign prostate (27.13 ± 5.28 versus 38.70 ± 9.95; P < 0.001) as shown in Figure 4.
Figure 4.
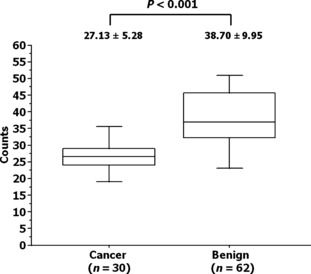
Association between the macrophage scavenger receptor count and pathological results in patients who underwent repeat biopsies of the prostate. Boxed areas represent the 25th and 75th percentiles, and error values; error bars represent the 10th and 90th percentiles.
Sensitivity and specificity of PSA level, and TAM and MSR counts at first biopsy. The PSA at the first biopsy and the TAM and MSR counts were divided into high and low groups compared to the mean values of 8.80 ng/mL, 16, and 32, respectively. Based on these groupings, sensitivity, specificity, positive predictive value, and negative predictive value were calculated as markers for detecting PCa in the repeat biopsy (Table 3). In Table 3, categories positively associated with cancer are placed upper for PSA, TAM, and MSR. In terms of sensitivity and specificity, the MSR count was superior to the TAM count or PSA level at the first biopsy.
Table 3.
Sensitivity, specificity, PPV and NPV stratified by PSA, TAM count and MSR count at the first biopsy
| Parameter | Benign | Cancer | Sensitivity/Specificity | PPV*/NPV |
|---|---|---|---|---|
| High PSA (≥8.8) | 27 | 21 | 21/30 | 21/48 |
| Low PSA (<8.8) | 35 | 9 | 35/62 | 35/44 |
| High TAM count (≥16) | 25 | 14 | 14/30 | 14/39 |
| Low TAM count (<16) | 37 | 16 | 37/62 | 37/53 |
| Low MSR count (<32) | 18 | 25 | 44/62 | 44/49 |
| High MSR count (≥32) | 44 | 5 | 25/30 | 25/43 |
PSA, prostate‐specific antigen; TAM, tumor‐associated macrophage; MSR, macrophage scavenger receptor; *PPV, positive predictive value; NPV, negative predictive value.
Logistic regression analysis. Among the clinicopathologic parameters analyzed, MSR count was the only useful predictor for positive repeat biopsy (Table 4).
Table 4.
Logistic regression
| Parameters | Regression coefficient | P value |
|---|---|---|
| Age | 0.039 | 0.5583 |
| PSA at the first biopsy | 0.0433 | 0.5182 |
| PSA at the repeat biopsy | 0.0531 | 0.1983 |
| PSA velocity | −0.0639 | 0.2838 |
| Periods between the first and the repeat biopsy | 0.3048 | 0.1514 |
| TAM count | 0.0321 | 0.6269 |
| MSR count | −0.1972 | 0.0004 |
PSA, prostate‐specific antigen; TAM, tumor‐associated macrophage; MSR, macrophage scavenger receptor.
Discussion
With widespread PSA screening, PCa is often detected at an early stage.( 2 ) However, because of the low positive predictive value of PSA, up to 75% of men with gray zone PSA (4–10 ng/mL) cannot escape an unnecessary biopsy.( 3 ) Based on periodic checks, patients with an increasing serum PSA undergo repeat prostate biopsy. Even in those cases, the detection rate of PCa remains between 10% and 35%.( 8 , 21 ) This means that many people undergo unnecessary biopsies. One of the most serious problems is that indications for repeat biopsy and the timing of the procedure are not clearly defined.( 4 ) Therefore, it is very important to discover a good indicator for a repeat biopsy of the prostate.
In our institute, repeat biopsies were carried out based on the doctors’ preference and policy according to PSA increase, but not based on definite criteria. Therefore, the interval from the first biopsy to a repeat biopsy was not constant. As shown in Table 2, the PSA levels at the first biopsy of patients with cancer at repeat biopsy were not significantly higher than those of patients without cancer, whereas the PSA levels at the repeat biopsy were higher in patients with cancer than those in patients without cancer. Moreover, PSA velocity tended to be greater in patients with cancer than in patients without cancer. In general, repeat biopsies were likely to be carried out based on the PSA velocity, increased PSA density, low free/total PSA ratio, or the presence of HGPIN.( 22 , 23 , 24 )
Chronic inflammation of the prostate has been reported to be one of the risk factors for prostate carcinogenesis.( 14 , 15 , 16 , 17 ) Several specific inflammatory cells have been implicated in carcinogenesis or the progression of PCa. The infiltration of TAMs is more prominent in cancer tissue than in normal prostate tissue, and an association between increased infiltration of TAMs and progression of PCa has also been reported.( 25 , 26 ) Therefore, TAMs might work as cancer‐stimulating factors and are usually seen at the tumor–normal tissue interface.( 27 , 28 )
Among several species of macrophages, MSR‐positive inflammatory cells have been also considered to be M2‐polarized and to be involved in the progression of glioma and ovarian epithelial tumor.( 29 , 30 ) Germ‐line mutations of the MSR1 gene are involved in alterations in host defense immunity as associated with an increased risk of PCa. It has been postulated that defective expression of MSR1, which is associated with macrophage function, could lead to serious inflammatory damage resulting in carcinogenesis.( 19 , 20 , 21 ) Yang et al. ( 18 ) reported that reduced expression of MSR1 is associated with progression of PCa. Our previous data also showed that decreased infiltration of MSR‐positive cells in the prostate biopsy specimens was associated with poor prognosis of the PCa.( 31 ) Moreover, the expression of MSR is inhibited by transforming growth factor‐β1 in human monocyte/macrophage cell line THP‐1.( 32 ) In humans, an increased level of transforming growth factor‐β1 is associated with PCa progression and metastasis.( 33 ) These findings all give us a good reason to accept the results that decreased MSR‐positive staining was well correlated with the presence of PCa in the repeat biopsy. Macrophage scavenger receptor‐positive cells in the prostate might play protective roles in tumor progression, contrary to other types of malignancies, in spite of no apparent reason.
No reliable or definitive predictor of repeat biopsy outcome is currently available. Other than PSA‐related markers, only a few factors have been discussed. Among them, prostate cancer gene 3 mRNA in the urine has been reported to be very useful for patients with elevated serum PSA levels and negative biopsy findings.( 9 , 10 ) The microvessel density and presence of HGPIN in biopsy specimens have also been reported as useful markers for considering repeat biopsy.( 34 ) In addition, telomerase activity in prostate massage samples and hypermethylation of the glutathione S‐transferase gene promoter in the urine after prostate biopsy might be predictive markers, although they have not been evaluated as indicators for a repeat biopsy.( 11 , 12 )
Our data needs to be evaluated in a larger sample or pursuing validation study. However, this kind of approach is essential for the discovery of new predicting markers to avoid unnecessary prostate biopsy.
Acknowledgments
This study was supported by Grants‐in‐Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology (No. 21592044).
References
- 1. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- 2. Partin AW, Oesterling JE. The clinical usefulness of prostate‐specific antigen: update 1994. J Urol 1994; 152: 1358–68. [DOI] [PubMed] [Google Scholar]
- 3. Kirby SR, Christmas TJ, Brawer MK. Prostate Cancer. London: Mosby, 2001. [Google Scholar]
- 4. Djavan B, Zlotta A, Remzi M et al. Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1,051 men. J Urol 2000; 163: 1144–8. [PubMed] [Google Scholar]
- 5. O’Dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology 2000; 55: 553–9. [DOI] [PubMed] [Google Scholar]
- 6. Fowler JE Jr, Bigler SA, Miles D, Yalkut DA. Predictors of first repeat biopsy cancer detection with suspected local stage prostate cancer. J Urol 2000; 163: 813–8. [PubMed] [Google Scholar]
- 7. Benecchi L, Pieri AM, Melissari M, Potenzoni M, Pastizzaro CD. A novel nomogram to predict the probability of prostate cancer on repeat biopsy. J Urol 2008; 180: 146–9. [DOI] [PubMed] [Google Scholar]
- 8. Shariat SF, Karakiewicz PI, Roehrborn CG, Kattan MW. An updated catalog of prostate cancer predictive tools. Cancer 2008; 113: 3075–99. [DOI] [PubMed] [Google Scholar]
- 9. Marks LS, Fradet Y, Deras IL et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007; 69: 532–5. [DOI] [PubMed] [Google Scholar]
- 10. Haese A, De La Taille A, Van Poppel H et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol 2008; 54: 1081–8. [DOI] [PubMed] [Google Scholar]
- 11. Gonzalgo ML, Pavlovich CP, Lee SM et al. Prostate cancer detection by GSTP1 methylation analysis of postbiopsy urine specimens. Clin Cancer Res 2003; 9: 2673–7. [PubMed] [Google Scholar]
- 12. Vicentini C, Gravina GL, Angelucci A et al. Detection of telomerase activity in prostate massage samples improves differentiating prostate cancer from benign prostatic hyperplasia. J Cancer Res Clin Oncol 2004; 130: 217–21. [DOI] [PubMed] [Google Scholar]
- 13. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palapattu GS, Sutcliffe S, Bastian PJ et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis 2005; 26: 1170–81. [DOI] [PubMed] [Google Scholar]
- 15. Wagenlehner FM, Elkahwaji JE, Algaba F et al. The role of inflammation and infection in the pathogenesis of prostate carcinoma. BJU Int 2007; 100: 733–7. [DOI] [PubMed] [Google Scholar]
- 16. De Marzo AM, Platz EA, Sutcliffe S et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer 2007; 7: 256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun J, Turner A, Xu J, Gronberg H, Isaacs W. Genetic variability in inflammation pathways and prostate cancer risk. Urol Oncol 2007; 25: 250–9. [DOI] [PubMed] [Google Scholar]
- 18. Yang G, Addai J, Tian WH, Frolov A, Wheeler TM, Thompson TC. Reduced infiltration of class A scavenger receptor positive antigen‐presenting cells is associated with prostate cancer progression. Cancer Res 2004; 64: 2076–82. [DOI] [PubMed] [Google Scholar]
- 19. Xu J, Zheng SL, Komiya A et al. Germline mutations and sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Nat Genet 2002; 32: 321–5. [DOI] [PubMed] [Google Scholar]
- 20. Xu J, Zheng SL, Komiya A et al. Common sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Am J Hum Genet 2003; 72: 208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raja J, Ramachandran N, Munneke G, Patel U. Current status of transrectal ultrasound‐guided prostate biopsy in the diagnosis of prostate cancer. Clin Radiol 2006; 61: 142–53. [DOI] [PubMed] [Google Scholar]
- 22. Mian BM, Naya Y, Okihara K, Vakar‐Lopez F, Troncoso P, Babaian RJ. Predictors of cancer in repeat extended multisite prostate biopsy in men with previous negative extended multisite biopsy. Urology 2002; 60: 836–40. [DOI] [PubMed] [Google Scholar]
- 23. Lopez‐Corona E, Ohori M, Scardino PT, Reuter VE, Gonen M, Kattan MW. A nomogram for predicting a positive repeat prostate biopsy in patients with a previous negative biopsy session. J Urol 2003; 170: 1184–8. [DOI] [PubMed] [Google Scholar]
- 24. Puppo P. Repeated negative prostate biopsies with persistently elevated or rising PSA: a modern urologic dilemma. Eur Urol 2007; 52: 639–41. [DOI] [PubMed] [Google Scholar]
- 25. Joseph IB, Isaacs JT. Macrophage role in the anti‐prostate cancer response to one class of antiangiogenic agents. J Natl Cancer Inst 1998; 90: 1648–53. [DOI] [PubMed] [Google Scholar]
- 26. Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC. Reduced infiltration of tumor‐associated macrophages in human prostate cancer: association with cancer progression. Cancer Res 2000; 60: 5857–61. [PubMed] [Google Scholar]
- 27. Pak CC, Fidler IJ. Molecular mechanisms for activated macrophage recognition of tumor cells. Semin Cancer Biol 1991; 2: 189–95. [PubMed] [Google Scholar]
- 28. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour‐associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti‐cancer therapy. Eur J Cancer 2006; 42: 717–27. [DOI] [PubMed] [Google Scholar]
- 29. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti‐inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008; 216: 15–24. [DOI] [PubMed] [Google Scholar]
- 30. Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony‐stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol Int 2009; 59: 300–5. [DOI] [PubMed] [Google Scholar]
- 31. Takayama H, Nonomura N, Nishimura K et al. Decreased immunostaining for macrophage scavenger receptor is associated with poor prognosis of prostate cancer. BJU Int 2009; 103: 470–4. [DOI] [PubMed] [Google Scholar]
- 32. Nishimura N, Harada‐Shiba M, Tajima S et al. Acquisition of secretion of transforming growth factor‐beta 1 leads to autonomous suppression of scavenger receptor activity in a monocyte‐macrophage cell line, THP‐1. J Biol Chem 1998; 273: 1562–7. [DOI] [PubMed] [Google Scholar]
- 33. Wikstrom P, Stattin P, Franck‐Lissbrant I, Damber JE, Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 1998; 37: 19–29. [DOI] [PubMed] [Google Scholar]
- 34. Sinha AA, Quast BJ, Reddy PK et al. Microvessel density as a molecular marker for identifying high‐grade prostatic intraepithelial neoplasia precursors to prostate cancer. Exp Mol Pathol 2004; 77: 153–9. [DOI] [PubMed] [Google Scholar]


