Abstract
B9L/BCL9‐2, a novel β‐catenin‐interacting protein, plays an important role in colorectal carcinogenesis by translocating β‐catenin to the nucleus and enhancing β‐catenin–T‐cell factor‐mediated transcription. To elucidate the role of B9L in breast cancers, we studied B9L expression in ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) of the breast immunohistochemically and compared it to the immunohistochemical expression of known proteins involved in breast carcinogenesis. In breast tissues, B9L immunoreactivity was present exclusively in the nuclei of normal and neoplastic ductal cells. In DCIS, immunohistochemical B9L expression was significantly associated with the tumor nuclear grade, comedo necrosis and the expression of ErbB2/HER‐2, c‐myc and p53. In IDC, B9L expression was correlated with ErbB2/HER‐2 expression and tumor nuclear grade only. In both DCIS and IDC, immunohistochemical B9L expression was not related to the expression of cytoplasmic β‐catenin. We demonstrated that nuclear B9L expression was closely associated with the high nuclear grade cancer phenotype and the expression of ErbB2/HER‐2 in breast cancers. (Cancer Sci 2007; 98: 484–490)
The Wnt/Wingless signaling transduction pathway is conserved across all animal taxa and plays important roles in development and tumorigenesis.( 1 , 2 , 3 , 4 ) In the Wnt pathway, the central player is β‐catenin, which is a transcription cofactor with T‐cell factor (TCF)/lymphoid enhancer factor (LEF) and a structural adaptor protein linking cadherins to the actin cytoskeleton in cell‐to‐cell adhesion.( 3 , 5 ) Cytoplasmic or signaling β‐catenin is unstable and rapidly targeted to proteasome‐mediated destruction. Wnt signaling inhibits this degradation, resulting in the accumulation of β‐catenin in the nucleus and its association with the TCF/LEF family, which leads to the activation of Wnt target genes.
In human cancers, the deregulation of Wnt signaling is often linked to colorectal cancer. About 80% of all colorectal cancers show inactivation of the adenomatous polyposis coli (APC) tumor suppressor.( 6 ) Furthermore, some of the colorectal cancers that have wild‐type APC have activating mutations in β‐catenin and Axin, which are also found in cancers of many other organs.( 6 ) Colorectal cancer cells with mutant APC or β‐catenin have high levels of TCF‐mediated transcription, which is thought to be the basis for tumorigenesis.( 6 ) It has been reported that various factors regulate β‐catenin–TCF‐mediated transcription, such as the Groucho family members, inhibitor of β‐catenin and TCF‐4 (ICAT), p300, and the BCL9 homolog Lgs.( 7 , 8 , 9 , 10 , 11 , 12 ) In particular, p300 activates β‐catenin–TCF‐mediated transcription and is critical for β‐catenin‐mediated neoplastic transformation.( 9 , 10 ) We previously identified a BCL9‐related β‐catenin‐binding protein, named B9L, which translocates β‐catenin to the nucleus and enhances β‐catenin–TCF‐mediated transcription.( 13 , 14 ) Brembeck et al. also identified this protein as BCL9‐2.( 14 ) B9L is overexpressed in approximately 43% of human colorectal tumors relative to its expression in the corresponding non‐neoplastic tissues.( 13 ) Furthermore, we found that immunoreactivity to the B9L protein is correlated with progressive grade of colorectal neoplasia, as is the expression of B9L mRNA.( 15 )
Recently, the incidence of ductal carcinoma in situ (DCIS) has increased, probably due to the development of early detection techniques, such as mammography for screening.( 16 ) In breast carcinogenesis, a substantial proportion (14–53%) of DCIS may become invasive given sufficient time.( 17 , 18 , 19 , 20 ) The subtypes of DCIS are classified by nuclear grade, architecture and necrosis, principally according to the 1995 Philadelphia Consensus on DCIS.( 21 ) A high nuclear grade was a significant prognostic factor for the development of ipsilateral breast recurrences in numerous retrospective and prospective studies.( 22 ) A substantial proportion of cases of DCIS overexpress the receptor tyrosine kinase ErbB2/HER‐2, particularly the comedocarcinoma subtype.( 23 ) Overexpression of ErbB2/HER‐2 has been reported as an adverse prognostic factor in invasive carcinoma and is therefore considered to be a marker of a more aggressive biology.( 23 )
Although the importance of the Wnt pathway in the progression of DCIS is unclear, previous studies have proposed a clear association among histological nuclear grade, comedo necrosis, ErbB2/HER‐2 expression, p53 expression and estrogen receptor (ER) negativity in DCIS.( 23 , 24 , 25 ) Moreover, the coexpression of p53 and β‐catenin was found to be associated significantly with worse overall survival.( 26 ) To elucidate the role of B9L in tumorigenesis other than in the colorectum, we investigated B9L expression in DCIS and invasive ductal carcinoma (IDC) of the breast using immunohistochemical methods and compared it to the immunohistochemical expression of known proteins related to breast carcinogenesis.
Materials and Methods
Patient characteristics and tumor tissues. Tumor tissues were obtained from 102 patients with DCIS who underwent surgery for breast cancer at Gunma University Hospital, Dokkyo University Hospital, or Maebashi Red Cross Hospital from 1991 to 2005 and 40 patients with IDC who underwent surgery for breast cancer at Gunma University Hospital from 2003 to 2004.
The clinicopathological features of the patients studied are shown in Table 1. The patients with DCIS ranged from 29 to 79 years in age with a mean age of 54.2 years. The patients with IDC ranged from 31 to 75 years with a mean age of 51.7 years. In the patients with IDC, the pT stage was pT1 in 21 (52.5%) tumors and pT2 in 19 (47.5%) tumors.
Table 1.
Clinical and pathological characteristics of the patients
| Characteristic | Cases | |
|---|---|---|
| n | % | |
| Ductal carcinoma in situ | ||
| Patients (n) | 102 | |
| Age (years) | ||
| Mean | 54.16 | |
| Range | 29–79 | |
| Nuclear grade | ||
| Low | 24 | 23.5 |
| Intermediate | 42 | 41.2 |
| High | 36 | 35.3 |
| Comedo | ||
| Absent | 59 | 57.8 |
| Present | 43 | 42.2 |
| Predominant architecture | ||
| Cribriform | 31 | 30.4 |
| Solid | 13 | 12.7 |
| Micropapillary | 24 | 23.5 |
| Others | 11 | 10.8 |
| Pure comedo | 23 | 22.6 |
| Invasive ductal carcinoma | ||
| Patients (n) | 40 | |
| Age (years) | ||
| Mean | 51.65 | |
| Range | 31–75 | |
| Nuclear grade | ||
| Low | 5 | 12.5 |
| Intermediate | 16 | 40.0 |
| High | 19 | 47.5 |
| Predominant architecture | ||
| Papillotubular | 19 | 47.5 |
| Scirrhous | 11 | 27.5 |
| Solid‐tubular | 10 | 25.0 |
| pT stage | ||
| 1 | 21 | 52.5 |
| 2 | 19 | 47.5 |
| pN stage | ||
| 0 | 29 | 72.5 |
| 1 | 11 | 27.5 |
| Stage | ||
| I | 16 | 40.0 |
| IIa | 17 | 42.5 |
| IIb | 7 | 17.5 |
In the histological classification of nuclear grade, of the 102 DCIS, 24 tumors (23.5%) were classified as low grade, 42 tumors (41.2%) as intermediate, and 36 tumors (35.3%) as high grade. Comedo‐type necrosis was observed in 43 (42.2%) DCIS. In IDC, the distribution of nuclear grade was similar to that in DCIS (Table 1). Surgically resected specimens were fixed with 10% formalin and embedded in paraffin for routine pathological examination. We prepared and used 4 µm‐thick paraffin sections from a paraffin block containing histology representative of the tumor.
Histological analysis of DCIS. We evaluated the nuclear grade of all tumors histologically and further subdivided them into three grades defined using the criteria of Lagios: low, intermediate and high.( 27 ) The DCIS were also analyzed for the presence of comedo‐type necrosis.
Immunohistochemical procedure. The paraffin sections were dewaxed with xylene, and endogenous peroxidase activity was quenched with absolute methanol containing 0.3% H2O2 for 30 min at room temperature. The sections were rehydrated through a graded ethanol series and treated with antigen retrieval, as shown in Table 2. To block non‐specific binding of the primary antibody, the slides were treated with 10% normal rabbit or mouse serum in phosphate‐buffered saline for 30 min at room temperature. Then, the sections were reacted with anti‐B9L polyclonal antibody or other primary antibodies overnight at 4°C. The antibody to B9L was obtained from rabbits immunized with recombinant glutathione S‐transferase (GST)–B9L (amino acids 245–564), as described previously.( 13 ) Details of the primary antibodies are shown in Table 2. After thoroughly washing the sections with Tris‐buffered saline containing 0.1% Triton X‐100, they were incubated with horseradish peroxidase‐labeled polymer secondary antibody from DAKO Envision (Dako, Glostrup, Denmark) or with the secondary biotinylated antimouse IgG for 30 min at room temperature, and then incubated with avidin–biotin–peroxidase complex solution (Vectastain; Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. Finally, the slides were visualized with 0.02% 3,3′‐diaminobenzidine tetrahydrochloride solution containing 0.005% H2O2 and counterstained lightly with hematoxylin.
Table 2.
Details of the primary antibodies used in the present study
| Antigen | Clone | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|
| B9L | Polyclonal | 1:1600 | A | Laboratory of Molecular and Genetic Information, Institute for Molecular and Cellular Biosciences, Tokyo, Japan |
| β‐catenin | Monoclonal, 14 | 1:2000 | A | Transduction Laboratories, Newington, NH, USA |
| ErbB2/HER‐2 | Polyclonal | 1:50 | A | DAKO Cytomation, Glostrup, Denmark |
| ER | Monoclonal, 1D5 | 1:100 | A | DAKO Cytomation, Glostrup, Denmark |
| p53 | Monoclonal, DO7 | 1:50 | B | Novocastra Laboratories, Newcastle upon Tyne, UK |
| c‐myc | Monoclonal, 9E10.3 | 1:200 | A | NeoMarkers, Fremont, CA, USA |
| Cyclin D1 | Monoclonal, DCS‐6 | 1:50 | C | Novocastra Laboratories, Newcastle upon Tyne, UK |
A, boiled in 0.01 mol/L sodium phosphate‐citrate buffer, pH 6.0 at 98°C for 20 min; B, boiled in 20% ZnSO4 solution at 98°C for 20 min; C, 0.001 mol/L ethylenediaminetetraacetate solution, pH 8.0 at 98°C for 40 min. ER, estrogen receptor.
In order to check specific staining for B9L, anti‐B9L polyclonal antibody was absorbed with recombinant GST–B9L protein and used for the immunohistochemistry instead of the primary antibody.
Evaluation of immunohistochemical staining. To evaluate the B9L immunoreactivity, we applied a modified scoring system that was used for β‐catenin immunohistochemistry, as described previously.( 26 ) The intensity of B9L immunoreactivity was evaluated in comparison with that of adjacent normal duct epithelium as an internal positive control and scored as follows: 0 if negative; 1+ if equivalent to that of normal ductal cells; and 2+ if stronger than that of normal ductal cells (Fig. 1). The staining intensity of β‐catenin was graded on a scale from 0 to 2+ (0, negative to weak; 1+, moderate; 2+, strong). To evaluate the ErbB2/HER‐2 immunoreactivity, the standard scoring system was modified as follows: 0, negative; 1+, score 1; 2+, score 2–3. The ER immunoreactivity was evaluated in a manner similar to that for ErbB2/HER‐2 and was graded from 0 to 2+ (0, negative; 1+, score 1–3; 2 +, score 4–8).( 28 ) To evaluate p53 expression, the percentage of tumor cells positive for nuclear staining was scored as follows: 0, <10%; 1+, 10–50%; 2+, >50%. To evaluate c‐myc and cyclin D1 expression, the percentage of tumor cells positive for nuclear staining was scored as follows: 0, negative; 1+, <10%; 2+, >10%.
Figure 1.
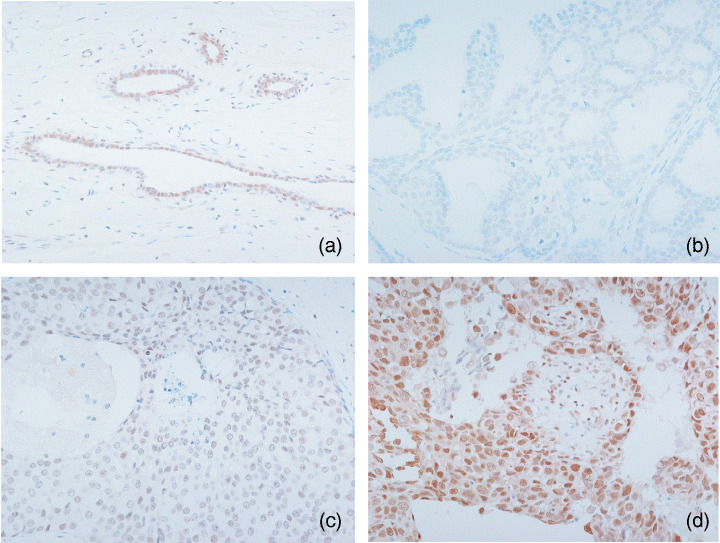
Immunohistochemical results for B9L in ductal carcinoma in situ (DCIS). (a) B9L immunoreactivity of normal breast tissues (×200). (b) Low nuclear grade DCIS (score 0, ×200). (c) Intermediate nuclear grade DCIS (score 1+, ×200). (d) High nuclear grade DCIS (score 2+, ×200).
Statistical analysis. Statistical analysis was carried out using SPSS 11.0 software (SPSS Japan, Tokyo, Japan). The Kruskal–Wallis test and Spearman's correlation of rank coefficients were used to examine the correlation of B9L with other protein and pathological factors. Statistical significance was set at P < 0.05.
Results
Immunohistochemical results for DCIS
B9L and β‐catenin. In normal breast tissues, B9L immunoreactivity was present in the nuclei of acinar and ductal cells, but not myoepithelial cells (Fig. 1). Small infiltrating lymphocytes were immunohistochemically positive for B9L. B9L immunoreactivity was present exclusively in the nuclei, and the nuclear immunoreactivity was relatively constant, but varied from case to case. B9L was immunohistochemically positive for 88 (86.3%) of 102 DCIS, and 37 (36.3%) and 51 (50%) tumors with scores of 1+ and 2+, respectively (Table 3).
Table 3.
Correlation of various histopathological features with expression of B9L in ductal carcinoma in situ
| Feature | n | B9L | P‐value † | ||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | |||
| No. cases | 14 | 37 | 51 | ||
| Nuclear grade | |||||
| Low | 24 | 4 | 10 | 10 | 0.010 |
| Intermediate | 42 | 8 | 16 | 18 | |
| High | 36 | 0 | 11 | 25 | |
| Comedo | |||||
| Absence | 59 | 8 | 28 | 23 | 0.035 |
| Presence | 43 | 6 | 9 | 28 | |
| Predominant architecture | |||||
| Cribriform | 31 | 2 | 17 | 12 | 0.418 |
| Solid | 13 | 2 | 6 | 5 | |
| Micropapillary | 24 | 3 | 7 | 14 | |
| Others | 11 | 4 | 2 | 5 | |
| Pure comedo | 23 | 3 | 5 | 15 | |
| β‐catenin | |||||
| 0 | 18 | 6 | 5 | 7 | 0.058 |
| 1 | 55 | 4 | 25 | 26 | |
| 2 | 29 | 4 | 7 | 18 | |
| ErbB2/HER‐2 | |||||
| 0 | 32 | 9 | 11 | 12 | 0.027 |
| 1 | 35 | 4 | 14 | 17 | |
| 2 | 35 | 1 | 12 | 22 | |
| ER | |||||
| 0 | 28 | 2 | 7 | 19 | 0.088 |
| 1 | 13 | 3 | 4 | 6 | |
| 2 | 61 | 9 | 26 | 26 | |
| p53 | |||||
| 0 | 37 | 13 | 12 | 12 | <0.001 |
| 1 | 24 | 1 | 12 | 11 | |
| 2 | 41 | 0 | 13 | 28 | |
| c‐myc | |||||
| 0 | 61 | 12 | 25 | 24 | 0.008 |
| 1 | 30 | 2 | 6 | 22 | |
| 2 | 11 | 0 | 6 | 5 | |
ER, estrogen receptor. †Bold type indicates a significant correlation.
The immunohistochemical expression of β‐catenin was observed only on the membrane or in the cytoplasm, but not in the nucleus (Fig. 2b). We simply set the total level of β‐catenin expression as the sum of membrane and cytoplasmic staining. β‐Catenin expression was observed in 84 (82.3%) of 102 DCIS, and 55 (53.9%) and 29 (28.4%) tumors with scores of 1+ and 2+, respectively.
Figure 2.
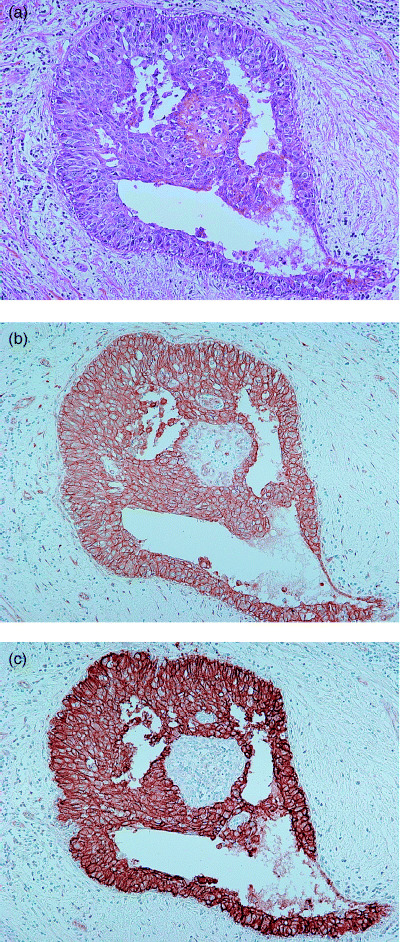
Immunohistochemical results for β‐catenin and ErbB2/HER‐2 in high nuclear grade ductal carcinoma in situ. (a) Hematoxylin–eosin staining (×100). (b) Membranous staining of β‐catenin (score 2+, ×100). (c) Expression of ErbB2/HER‐2 (score 2+, ×100).
ER, ErbB2/HER‐2 and p53. The expression of ER was observed in 74 (72.5%) of 102 DCIS and 13 (12.7%) and 61 (59.8%) tumors with scores of 1+ and 2+, respectively (Table 3). The expression of ErbB2/HER‐2 was observed on the membrane (Fig. 2c). ErbB2/HER‐2 was expressed in 70 (68.6%) of 102 DCIS and 35 (34.3%) and 35 (34.3%) tumors with scores of 1+ and 2+, respectively.
The expression of p53 was observed in the nucleus and 65 (63.7%) tumors were positive for p53. Twenty‐four (23.5%) and 41 (40.2%) tumors were detected with scores of 1+ and 2+, respectively.
C‐myc, cyclin D1. c‐myc was expressed in 41 (40.2%) of 102 DCIS and 30 (29.4%) and 11 (10.8%) tumors with scores of 1+ and 2+, respectively (Table 3). Cyclin D1 was expressed in 50 (74.6%) of only 67 DCIS and 25 (37.3%) and 25 (37.3%) tumors with scores of 1+ and 2+, respectively (data not shown).
Immunohistochemical results for IDC. In IDC, B9L immunoreactivity was present exclusively in the nuclei (Fig. 3). The nuclear immunoreactivity was relatively constant. β‐catenin was expressed only on the membrane or in the cytoplasm, but not in the nucleus, as in DCIS. B9L was evaluated immunohistochemically as positive in 34 (85%) tumors with an intensity of 1+ and 2+ in 23 (57.5%) and 11 (27.5%) tumors, respectively (Table 4). β‐catenin immunoreactivity was observed in 28 (70%) of 40 tumors with scores 1+ and 2+ in 17 (42.5%) and 11 (27.5%) tumors, respectively. ER immunoreactivity was seen in 28 (70%) of 40 tumors with scores of 1+ and 2+ in 2 (5%) and 26 (65%) tumors, respectively. The expression of ErbB2/HER‐2 and p53 in IDC was similar in DCIS. ErbB2/HER‐2 immunoreactivity was seen in 28 (70%) of 40 tumors with scores of 1+ and 2+ in 16 (40%) and 12 (30%) tumors, respectively. For p53 expression, 14 (35%) tumors were positive, with scores of 1+ and 2+ in 3 (7.5%) and 11 (27.5%) tumors, respectively. c‐myc was expressed in 33 (82.5%) of 40 tumors with scores of 1+ and 2+ in 14 (35%) and 19 (47.5%) tumors, respectively. Cyclin D1 was expressed in 32 (80%) of 40 tumors with scores of 1+ and 2+ in 11 (27.5%) and 21 (52.5%) tumors, respectively.
Figure 3.
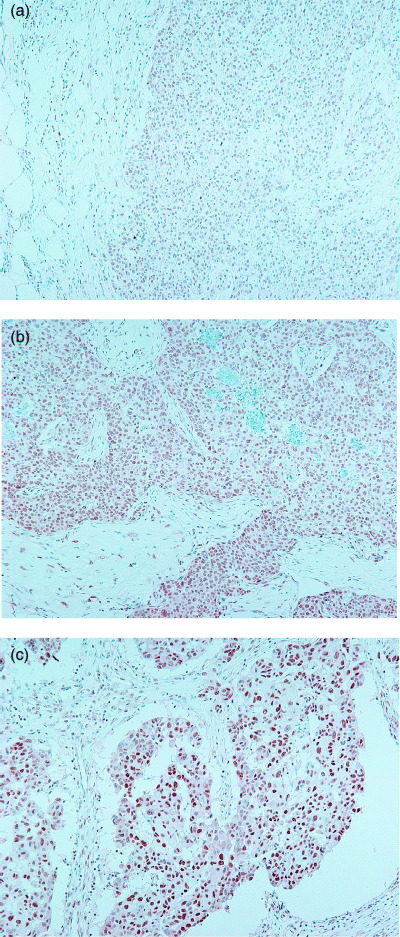
Immunohistochemical results for B9L in invasive ductal carcinoma. (a) Solid‐tubular carcinoma, low nuclear grade (score 0, ×100). (b) Solid‐tubular carcinoma, intermediate nuclear grade (score 1+, ×100). (c) Papillotubular carcinoma, high nuclear grade (score 2+, ×100).
Table 4.
Correlation of various histopathological features with expression of B9L in invasive ductal carcinoma
| Feature | n | B9L | P‐value † | ||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | |||
| No. cases | 6 | 23 | 11 | ||
| Nuclear grade | |||||
| Low | 5 | 3 | 2 | 0 | <0.001 |
| Intermediate | 16 | 3 | 11 | 2 | |
| High | 19 | 0 | 10 | 9 | |
| β‐catenin | |||||
| 0 | 12 | 3 | 7 | 2 | 0.193 |
| 1 | 17 | 0 | 11 | 6 | |
| 2 | 11 | 3 | 5 | 3 | |
| ErbB2/HER‐2 | |||||
| 0 | 12 | 2 | 7 | 3 | 0.035 |
| 1 | 16 | 4 | 10 | 2 | |
| 2 | 12 | 0 | 6 | 6 | |
| ER | |||||
| 0 | 12 | 2 | 7 | 3 | 0.127 |
| 1 | 2 | 0 | 0 | 2 | |
| 2 | 26 | 4 | 16 | 6 | |
| p53 | |||||
| 0 | 26 | 4 | 14 | 8 | 0.681 |
| 1 | 3 | 0 | 2 | 1 | |
| 2 | 11 | 2 | 7 | 2 | |
| c‐myc | |||||
| 0 | 7 | 0 | 6 | 1 | 0.177 |
| 1 | 14 | 2 | 5 | 7 | |
| 2 | 19 | 4 | 12 | 3 | |
| Cyclin D1 | |||||
| 0 | 8 | 1 | 5 | 2 | 0.999 |
| 1 | 11 | 4 | 2 | 5 | |
| 2 | 21 | 1 | 16 | 4 | |
ER, estrogen receptor. †Bold type indicates a significant correlation.
Statistical analysis of histopathological factors and B9L expression in DCIS and IDC. In DCIS, the immunohistochemical B9L expression was significantly associated with the nuclear grade, comedo necrosis, and the expression of ErbB2/HER‐2, c‐myc and p53 (Table 3). B9L expression tended to be correlated with β‐catenin and ER. We subdivided the pattern of β‐catenin expression into membrane only and membrane/cytoplasm. The membrane only pattern of β‐catenin was significantly correlated with B9L expression, whereas the membrane/cytoplasm pattern was not (Table 5). According to Spearman's correlation of rank coefficients, B9L expression had the third‐highest correlation with the nuclear grade of DCIS (γ = 0.27) (Table 6).
Table 5.
Correlation of β‐catenin expression pattern with B9L expression in ductal carcinoma in situ
| Expression pattern | n | B9L | P‐value† | ||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | |||
| No. cases | 14 | 37 | 51 | ||
| Membrane only | |||||
| 0 | 11 | 6 | 3 | 2 | 0.004 |
| 1 | 42 | 4 | 18 | 20 | |
| 2 | 17 | 1 | 5 | 11 | |
| Membrane/cytoplasm | |||||
| 0 | 7 | 0 | 2 | 5 | 0.628 |
| 1 | 13 | 0 | 7 | 6 | |
| 2 | 12 | 3 | 2 | 7 | |
† Bold type indicates a significant correlation.
Table 6.
Spearman's correlation of rank coefficients of various histopathological features and expression of proteins in ductal carcinoma in situ
| Protein | Nuclear grade | Comedo | B9L | ErbB2/HER‐2 | p53 |
|---|---|---|---|---|---|
| Nuclear grade | 1 | ||||
| Comedo | 0.63† | 1 | |||
| B9L | 0.27† | 0.26† | 1 | ||
| ErbB2/HER‐2 | 0.52† | 0.37† | 0.33† | 1 | |
| p53 | 0.19 | 0.09 | 0.42† | 0.38 † | 1 |
Correlation is significant at the 0.01 level (two‐tailed).
In IDC, B9L expression was correlated with ErbB2/HER‐2 expression (P = 0.035) and nuclear grade (P < 0.001). In addition, the expression of ER showed an inverse relationship with p53 expression and nuclear grade. The expression of c‐myc and cyclin D1, which are targets of Wnt signaling, was not correlated with the expression of either B9L or β‐catenin (Table 4).
We also analyzed the association of each protein and histopathological factor in DCIS and IDC. In DCIS, the analyses showed a mutual relationship among the expression of B9L and ErbB2/HER‐2, nuclear grade and the presence of comedo necrosis (Fig. 4a). The expression of β‐catenin was significantly correlated with nuclear grade and comedo necrosis, and tended to be correlated with B9L expression. The expression of ER was inversely related to ErbB2/HER‐2 expression, nuclear grade and comedo necrosis. In contrast, in IDC, β‐catenin expression was completely independent of the other parameters investigated in this study (Fig. 4b).
Figure 4.
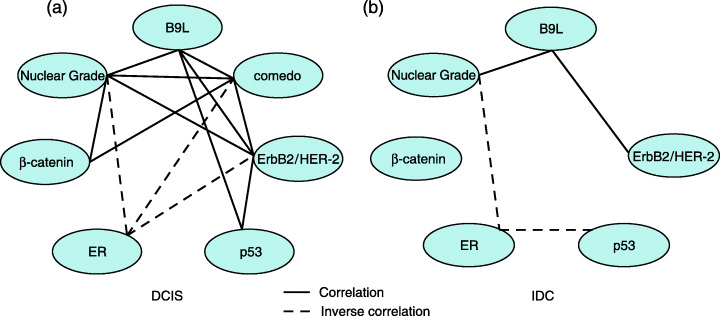
Correlation or inverse correlation between cell biological factors and histopathological features in (a) ductal carcinoma in situ and (b) invasive ductal carcinoma. Solid lines show the correlations between cell biological factors and histopathological features. Dotted lines show inverse correlations.
Discussion
In human breast cancers, the hyperactivation of Wnt signaling caused by mutations in genes such as β‐catenin, APC and Axin is infrequent.( 29 ) However, strong immunohistochemical evidence indicates that β‐catenin is overexpressed in over 50% of breast cancers, which suggests the activation of this signal pathway.( 30 , 31 ) Moreover, in mammary gland development, the Wnt signal is strongly implicated in the initial development of the mammary rudiments, and in the ductal branching and alveolar morphogenesis that occurs during pregnancy.( 29 ) Therefore, the activation of the Wnt signal pathway is inevitable in mammary gland development and breast carcinogenesis. We hypothesized that the overexpression of B9L contributes to the elevated β‐catenin levels in early breast cancers. We therefore selected DCIS tumors, rather than IDC tumors, to investigate the expression of B9L in breast carcinogenesis. We found that B9L is detected in the nucleus exclusively, whereas β‐catenin is detected in the cell membrane and cytoplasm, but not the nucleus, as reported previously.( 26 , 32 , 33 , 34 , 35 , 36 ) Therefore, the upregulation of B9L might activate Wnt signaling even when β‐catenin is not prominently accumulated in the nucleus.
Immunohistochemical B9L expression was significantly associated with the nuclear grade, comedo necrosis and the expression of ErbB2/HER‐2, c‐myc and p53 in DCIS. B9L expression tended to be correlated with β‐catenin and ER. Increased B9L expression may be related to c‐myc gene activation in DCIS. Nevertheless, increased B9L expression did not seem to cause cyclin D1 activation, although 75% of DCIS expressed cyclin D1. In addition, it has been shown that the Wnt and estrogen signaling pathways cross‐talk in vivo through the functional interaction between ERα and β‐catenin.( 37 ) The tendency toward a correlation between B9L expression and ER expression may concur with the cross‐talk in human DCIS.
In IDC, B9L expression was significantly correlated with nuclear grade and ErbB2/HER‐2 expression only. Although 80% of IDC expressed both c‐myc and cyclin D1, no correlation was detected with B9L expression, suggesting that increased B9L expression is not the major cause of c‐myc and cyclin D1 gene activation in IDC. This finding is consistent with previous studies showing that c‐myc overexpression does not accompany β‐catenin accumulation in invasive breast cancer.( 35 ) It was also reported that β‐catenin does not penetrate the nuclei, despite the fact that both c‐myc and cycling D1 are markedly elevated in invasive breast cancers.( 38 , 39 ) Although immunohistochemical B9L expression was significantly associated with p53 in DCIS, B9L expression was not correlated with p53 in IDC. Furthermore, cross‐talk between the Wnt and estrogen signaling pathways( 37 ) was not observed in our immunohistochemical study of IDC. The reason for the difference in DCIS and IDC is uncertain, but the small sample size of IDC studied might have been responsible. All DCIS may not always progress to IDC.( 17 , 18 , 19 , 20 ) Therefore, the immunoexpression of ER, B9L, β‐catenin, p53 and c‐myc might differ among pure DCIS, the DCIS component in IDC, and the invasive component in IDC.
Based on the pathological and genetic features, lobular carcinoma is remarkably similar to low‐grade ductal carcinoma.( 40 , 41 , 42 ) Consistent with this, we detected B9L expression in acinar cells (Fig. 1a). Although it was reported that the β‐catenin expression pattern in lobular carcinoma differs from that in ductal carcinoma,( 43 , 44 , 45 , 46 ) it is possible that B9L is expressed in lobular carcinoma even when β‐catenin is not accumulated in the nucleus, like expression in ductal carcinoma.
The association of B9L expression, nuclear grade status and ErbB2/HER‐2 expression is a common phenomenon in breast carcinogenesis in both DCIS and IDC. These three factors are common in cancer cells with highly atypical phenotypes, which may be a common cell lineage in the progression from DCIS to IDC.( 47 ) Therefore, B9L is one of the factors that may play an important role in this cell lineage. B9L might be a biomarker for detecting highly malignant DCIS as it progresses to IDC and could be used to follow the patients after surgery. Further studies are necessary to confirm that the upregulation of B9L contributes to breast carcinogenesis by activating Wnt signaling. In addition, the mechanisms of B9L overexpression and degradation and of its interaction with other molecules remain uncharacterized. Moreover, the mechanism of nuclear translocation of B9L remains to be investigated. Further elucidation of B9L functions may provide insight into the mechanisms of tumorigenesis and assist in the development of antitumor reagents and diagnostic methods in new clinical strategies for breast cancer.
Acknowledgments
We thank T. Nakano, K. Muroya and S. Aoki for valuable suggestions and assistance; H. Ito for supplying samples; and T. Hikino for excellent technical assistance. This work was supported in part by the Harunasou Foundation Cancer Research Subsidizing Fund, the Kanetsu Chuo Hospital Research Fund, the Katoh Surgery and Medical Hospital Fund, the Maebashi North Hospital in Maebashi, the Keiaido Hospital in Midori, Gunma, and the Research Fund of the Uchida Clinic in Inamachi, Saitama.
References
- 1. Polakis P. Wnt signaling and cancer. Genes Dev 2000; 14: 1837–51. [PubMed] [Google Scholar]
- 2. Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 2000; 103: 311–20. [DOI] [PubMed] [Google Scholar]
- 3. Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 1997; 11: 3286–305. [DOI] [PubMed] [Google Scholar]
- 4. Akiyama T. Wnt/beta‐catenin signaling. Cytokine Growth Factor Rev 2000; 11: 273–82. [DOI] [PubMed] [Google Scholar]
- 5. Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol 2002; 4: E101–8. [DOI] [PubMed] [Google Scholar]
- 6. Thompson B, Townsley F, Rosin‐Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol 2002; 4: 367–73. [DOI] [PubMed] [Google Scholar]
- 7. Sekiya T, Nakamura T, Kazuki Y et al. Overexpression of Icat induces G2 arrest and cell death in tumor cell mutants for adenomatous polyposis coli, β‐catenin, or Axin. Cancer Res 2002; 62: 3322–6. [PubMed] [Google Scholar]
- 8. Tago K, Nakamura T, Nishita M et al. Inhibition of Wnt signaling by ICAT, a novel β‐catenin‐interacting protein. Genes Dev 2000; 14: 1741–9. [PMC free article] [PubMed] [Google Scholar]
- 9. Hecht A, Vleminckx K, Stemmler MP, Van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of β‐catenin in vertebrates. EMBO J 2000; 19: 1839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Y, Kolligs FT, Hottiger MO, Mosavin R, Fearon ER, Nabel GJ. Regulation of β‐catenin transformation by the p300 transcriptional coactivator. Proc Natl Acad Sci USA 2000; 97: 12 613–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kramps T, Peter O, Brunner E et al. Wnt/wingless signaling requires BCL9/legless‐mediated recruitment of pygopus to the nuclear β‐catenin–TCF complex. Cell 2002; 109: 47–60. [DOI] [PubMed] [Google Scholar]
- 12. Willis TG, Zalcberg IR, Coignet LJ et al. Molecular cloning of translocation t(1;14) (q21;q32) defines a novel gene (BCL9) at chromosome 1q21. Blood 1998; 91: 1873–81. [PubMed] [Google Scholar]
- 13. Adachi S, Jigami T, Yasui T et al. Role of a BCL9‐related β‐catenin‐binding protein, B9L, in tumorigenesis induced by aberrant activation of Wnt signaling. Cancer Res 2004; 64: 8496–501. [DOI] [PubMed] [Google Scholar]
- 14. Brembeck FH, Schwarz‐Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9‐2 in the switch between β‐catenin's adhesive and transcriptional functions. Genes Dev 2004; 18: 2225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sakamoto I, Ohwada S, Toya H et al. Up‐regulation of a BCL9‐related β‐catenin‐binding protein, B9L, in different stages of sporadic colorectal adenoma. Cancer Sci 2007; 98: 83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moriya T, Kasami M, Akiyama F et al. A proposal for the histopathological diagnosis of ductal carcinoma in situ of the breast. Breast Cancer 2000; 7: 321–5. [DOI] [PubMed] [Google Scholar]
- 17. Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses’ Health Study. Cancer 2005; 103: 1778–84. [DOI] [PubMed] [Google Scholar]
- 18. Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow‐up after biopsy only. Cancer 1982; 49: 751–8. [DOI] [PubMed] [Google Scholar]
- 19. Eusebi V, Feudale E, Foschini MP et al. Long‐term follow‐up of in situ carcinoma of the breast. Semin Diagn Pathol 1994; 11: 223–35. [PubMed] [Google Scholar]
- 20. Rosen P, Snyder RE, Foote FW, Wallace T. Detection of occult carcinoma in the apparently benign breast biopsy through specimen radiography. Cancer 1970; 26: 944–52. [DOI] [PubMed] [Google Scholar]
- 21. Consensus conference on the classification of ductal carcinoma in situ . Hum Pathol 1997; 28: 1221–5. [DOI] [PubMed] [Google Scholar]
- 22. Roka S, Rudas M, Taucher S et al. High nuclear grade and negative estrogen receptor are significant risk factors for recurrence in DCIS. Eur J Surg Oncol 2004; 30: 243–7. [DOI] [PubMed] [Google Scholar]
- 23. Claus EB, Chu P, Howe CL et al. Pathobiologic findings in DCIS of the breast: morphologic features, angiogenesis, HER‐2/neu and hormone receptors. Exp Mol Pathol 2001; 70: 303–16. [DOI] [PubMed] [Google Scholar]
- 24. Baqai T, Shousha S. Oestrogen receptor negativity as a marker for high‐grade ductal carcinoma in situ of the breast. Histopathology 2003; 42: 440–7. [DOI] [PubMed] [Google Scholar]
- 25. Rajan PB, Scott DJ, Perry RH, Griffith CD. p53 protein expression in ductal carcinoma in situ (DCIS) of the breast. Breast Cancer Res Treat 1997; 42: 283–90. [DOI] [PubMed] [Google Scholar]
- 26. Chung GG, Zerkowski MP, Ocal IT et al. β‐Catenin and p53 analyses of a breast carcinoma tissue microarray. Cancer 2004; 100: 2084–92. [DOI] [PubMed] [Google Scholar]
- 27. Lagios MD. Duct carcinoma in situ. Pathology and treatment. Surg Clin North Am 1990; 70: 853–71. [DOI] [PubMed] [Google Scholar]
- 28. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 29. Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia 2004; 9: 119–31. [DOI] [PubMed] [Google Scholar]
- 30. Lin SY, Xia W, Wang JC et al. Beta‐catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA 2000; 97: 4262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of β‐catenin by inhibiting its interaction with APC. Nat Cell Biol 2001; 3: 793–801. [DOI] [PubMed] [Google Scholar]
- 32. Dolled‐Filhart M, McCabe A, Giltnane J, Cregger M, Camp RL, Rimm DL. Quantitative in situ analysis of β‐catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res 2006; 66: 5487–94. [DOI] [PubMed] [Google Scholar]
- 33. Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen‐Dale AL. E‐cadherin and α‐, β‐, and γ‐catenin protein expression in relation to metastasis in human breast carcinoma. J Pathol 1998; 185: 262–6. [DOI] [PubMed] [Google Scholar]
- 34. Bankfalvi A, Terpe HJ, Breukelmann D et al. Immunophenotypic and prognostic analysis of E‐cadherin and β‐catenin expression during breast carcinogenesis and tumour progression: a comparative study with CD44. Histopathology 1999; 34: 25–34. [DOI] [PubMed] [Google Scholar]
- 35. Wong SC, Lo SF, Lee KC, Yam JW, Chan JK, Hsiao WL. Expression of frizzled‐related protein and Wnt‐signalling molecules in invasive human breast tumours. J Pathol 2002; 196: 145–53. [DOI] [PubMed] [Google Scholar]
- 36. Gillett CE, Miles DW, Ryder K et al. Retention of the expression of E‐cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast. J Pathol 2001; 193: 433–41. [DOI] [PubMed] [Google Scholar]
- 37. Kouzmenko AP, Takeyama K, Ito S et al. Wnt/β‐catenin and estrogen signaling converge in vivo . J Biol Chem 2004; 279: 40 255–8. [DOI] [PubMed] [Google Scholar]
- 38. Jonsson M, Borg A, Nilbert M, Andersson T. Involvement of adenomatous polyposis coli (APC)/β‐catenin signalling in human breast cancer. Eur J Cancer 2000; 36: 242–8. [DOI] [PubMed] [Google Scholar]
- 39. Bukholm IK, Nesland JM, Borresen‐Dale AL. Re‐expression of E‐cadherin, α‐catenin and β‐catenin, but not of γ‐catenin, in metastatic tissue from breast cancer patients. J Pathol 2000; 190: 15–19. [DOI] [PubMed] [Google Scholar]
- 40. Simpson PT, Gale T, Fulford LG, Reis‐Filho JS, Lakhani SR. The diagnosis and management of pre‐invasive breast disease: pathology of atypical lobular hyperplasia and lobular carcinoma in situ . Breast Cancer Res 2003; 5: 258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reis‐Filho JS, Lakhani SR. The diagnosis and management of pre‐invasive breast disease: genetic alterations in pre‐invasive lesions. Breast Cancer Res 2003; 5: 313–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu YJ, Osin P, Lakhani SR, Di Palma S, Gusterson BA, Shipley JM. Comparative genomic hybridization analysis of lobular carcinoma in situ and atypical lobular hyperplasia and potential roles for gains and losses of genetic material in breast neoplasia. Cancer Res 1998; 58: 4721–7. [PubMed] [Google Scholar]
- 43. Mastracci TL, Tjan S, Bane AL, O'Malley FP, Andrulis IL. E‐cadherin alterations in atypical lobular hyperplasia and lobular carcinoma in situ of the breast. Mod Pathol 2005; 18: 741–51. [DOI] [PubMed] [Google Scholar]
- 44. Turashvili G, Bouchal J, Burkadze G, Kolar Z. Differentiation of tumours of ductal and lobular origin. I. Proteomics of invasive ductal and lobular breast carcinomas. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2005; 149: 57–62. [DOI] [PubMed] [Google Scholar]
- 45. Karayiannakis AJ, Nakopoulou L, Gakiopoulou H, Keramopoulos A, Davaris PS, Pignatelli M. Expression patterns of β‐catenin in in situ and invasive breast cancer. Eur J Surg Oncol 2001; 27: 31–6. [DOI] [PubMed] [Google Scholar]
- 46. Rieger‐Christ KM, Pezza JA, Dugan JM, Braasch JW, Hughes KS, Summerhayes IC. Disparate E‐cadherin mutations in LCIS and associated invasive breast carcinomas. Mol Pathol 2001; 54: 91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simpson PT, Reis‐Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol 2005; 205: 248–54. [DOI] [PubMed] [Google Scholar]


