Abstract
Malignant fibrous histiocytoma (MFH) is one of the most common soft tissue sarcomas. MFH has been proposed to be a lesion accompanied with inflammatory responses. During chronic inflammation, reactive nitrogen and oxygen species generated from inflammatory cells are considered to participate in carcinogenesis by causing DNA damage. 8‐nitroguanine is a mutagenic nitrative DNA lesion formed during chronic inflammation. We examined whether nitrative DNA damage is related to the prognosis of MFH patients. We performed immunohistochemical analyses to examine the distribution of DNA damage and the expression of inflammation‐related molecules including inducible nitric oxide synthase (iNOS), nuclear factor‐κB (NF‐κB), and cyclooxygenase‐2 (COX‐2) in clinical specimens from 25 patients with MFH. We also analyzed the correlation of DNA damage or the expression of these genes with the prognosis of MFH patients. Immunohistochemical staining revealed that the formation of 8‐nitroguanine and 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG), an oxidative DNA lesion, occurred to a much greater extent in MFH tissue specimens from deceased patients than in live patients. iNOS, NF‐κB and COX‐2 were colocalized with 8‐nitroguanine in MFH tissues. It is noteworthy that the statistical analysis using the Kaplan‐Meier method demonstrated strong 8‐nitroguanine staining to be associated with a poor prognosis. In conclusion, 8‐nitroguanine appears to participate in not only the initiation and promotion of MFH, but also in the progression of MFH, and could therefore be used as a promising biomarker to evaluate the prognosis of cancer patients. (Cancer Sci 2007; 98: 163–168)
Abbreviations:
- MFH
malignant fibrous histiocytoma
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- 8‐oxodG
8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- NF‐κB
nuclear factor‐κB
- COX‐2
cyclooxygenase‐2
Malignant fibrous histiocytoma has been regarded as one of the most common soft tissue sarcomas occurring in adult patients,( 1 ) and it occurs most frequently in the extremities, trunk and retroperitoneum.( 2 ) MFH is classified into four subtypes including storiform‐pleomorphic (60–70%), myxoid (10–20%), giant cell (approximately 10%) and inflammatory (relatively rare).( 3 ) MFH has been proposed to be a lesion accompanied with inflammatory responses. The expression of cytokines in a certain type of MFH (inflammatory MFH) may account for the local inflammatory infiltrate and the aggressive nature observed in these malignant cells.( 4 ) In the early phases of experimentally induced rat sarcoma, an inflammatory reaction characterized by an infiltration of lymphocytes, monocytes and macrophages was observed.( 5 ) It has been hypothesized that many malignancies arise from areas of inflammation.( 6 , 7 ) Moreover, chronic inflammation has also been proposed to contribute to the development of various cancers.( 6 , 7 ) These findings led us to an idea that the inflammatory responses thus play a role in the pathogenesis of MFH.
During chronic inflammation, RNS and ROS are generated from inflammatory and epithelial cells, and therefore are considered to play a key role in both carcinogenesis and tumor progression.( 8 , 9 ) ROS can induce the formation of 8‐oxod), an indicator of oxidative DNA damage.( 10 , 11 , 12 ) Nitric oxide can mediate the formation of 8‐nitroguanine, a marker of nitrative DNA damage,( 13 ) which has been proposed to account for inflammation‐mediated carcinogenesis as a potentially mutagenic lesion.( 9 , 14 ) In MFH patients, the tumor tissues have been reported to have mutations in proto‐oncogenes and tumor suppressor genes.( 15 , 16 , 17 ) We have previously performed clinical studies to examine the formation of 8‐nitroguanine and 8‐oxodG in patients with various inflammatory diseases.( 18 , 19 ) We have discovered that these DNA lesions were specifically formed at sites of carcinogenesis under various inflammatory conditions.( 20 , 21 , 22 , 23 , 24 ) 8‐nitroguanine was apparently formed in gastric gland epithelial cells of patients with H. pylori infection( 20 ) and in hepatocytes of patients with chronic hepatitis C.( 21 ) Furthermore, we recently performed a molecular epidemiological study which suggested that the formation of 8‐nitroguanine is possibly associated with a poor prognosis in cancer patients.( 22 ) In MFH patients, we demonstrated the prognostic significance of the expression of bone morphogenetic protein‐2.( 25 ) However, the role of nitrative DNA damage in carcinogenesis in soft tissues remains to be clarified.
In this study, we obtained surgical specimens from MFH patients, and performed a double immunofluorescent staining procedure to examine the formation of 8‐nitroguanine and 8‐oxodG. Moreover, we examined the expression of iNOS, NF‐κB and COX‐2 in sarcomas of MFH patients by immunohistochemistry. NF‐κB is a key player in inflammation which regulates the expression of various genes involved in controlling inflammatory response such as proinflammatory mediators, iNOS and COX‐2.( 26 , 27 ) To investigate the prognostic factors of cancer patients, we analyzed the relation of 8‐nitroguanine formation and the expression of inflammation‐related molecules with the survival rate of MFH patients.
Materials and Methods
Subjects. MFH patients undergoing surgical resections from 1990 to 2004 at the Department of Orthopaedic Surgery, Mie University Graduate School of Medicine, Japan, participated in this study. This study was approved by the Ethics Committee of Mie University Graduate School of Medicine. The gross appearance of surgical specimens obtained from 25 patients was classified as Stage IIa (1 patient), Stage IIb (2 patients), Stage III (17 patients) and Stage IV (5 patients) at the original diagnosis (Table 1). The International Union Against Cancer TNM classification and the staging system by the American Joint Committee on Cancer (AJCC) were used for tumor assessment.
Table 1.
Clinicopathological data of the malignant fibrous histiocytoma patients
| No. | Age | Sex | AJCC stage | Location | Size(cm) | Recurrence | Metastasis | Histological type | Follow‐up duration (month) | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 47 | F | IV | Buttock | 6 × 4 × 3 | + | Lung, skin, thigh, pleura | Storiform‐pleomorphic | 45 | DOD |
| 2 | 50 | F | III | Buttock | 6 × 5 × 5 | + | Lung | Storiform‐pleomorphic | 68 | NED |
| 3 | 73 | F | III | Chest wall | 12 × 10 × 6 | – | Intrathoracic | Storiform | 33 | DOD |
| 4 | 76 | F | III | Back | 8 × 4 × 2 | + | Axillary lymph node | Storiform | 24 | DOD |
| 5 | 64 | F | III | Thigh | 12 × 4 × 4 | + | – | Storiform | 102 | NED |
| 6 | 54 | M | III | Back | 10 × 8 × 6 | – | – | Storiform | 15 | AWD |
| 7 | 75 | M | IV | Buttock | 18 × 11 × 5 | + | Lung, inguina | Storiform | 8 | DOD |
| 8 | 73 | M | IV | Thigh | 20 × 15 × 13 | + | Lung | Storiform | 9 | DOD |
| 9 | 81 | F | III | Thigh | 19 × 13 × 10 | – | – | Storiform | 38 | CDF |
| 10 | 69 | F | III | Thigh | 16 × 10 × 8 | + | – | Storiform | 64 | NED |
| 11 | 75 | M | III | Thigh | 5 × 5 × 4 | + | Liver | Storiform | 42 | NED |
| 12 | 66 | M | III | Thigh | 10 × 10 × 8 | + | Lung | Pleomorphic | 12 | DOD |
| 13 | 49 | M | IV | Thigh | 15 × 10 × 10 | + | Lung | Pleomorphic | 15 | DOD |
| 14 | 67 | M | III | Upper arm | 16 × 10 × 10 | – | Lung | Pleomorphic | 12 | DOD |
| 15 | 57 | F | III | Thigh | 22 × 16 × 10 | – | – | Pleomorphic | 87 | CDF |
| 16 | 69 | M | III | Upper arm | 13 × 8 × 7 | – | – | Pleomorphic | 33 | CDF |
| 17 | 46 | F | IIb | Knee | 6 × 5 × 2 | – | – | Pleomorphic | 52 | CDF |
| 18 | 42 | M | III | Lower leg | 9 × 8 × 6 | – | – | Myxoid | 40 | CDF |
| 19 | 70 | F | IIb | Thigh | 11 × 11 × 4 | – | Lung | Myxoid | 156 | NED |
| 20 | 77 | M | IIa | Upper arm | 10 × 9 × 4 | + | Axillary lymph node | Myxoid | 78 | DOD |
| 21 | 69 | F | III | Thigh | 12 × 9 × 6 | – | Intrapelvic | Myxoid | 29 | DOD |
| 22 | 27 | M | IV | Thigh | 20 × 20 × 5 | – | Lung | Myxoid | 5 | DOD |
| 23 | 85 | F | III | Back | 15 × 13 × 8 | + | Lung | Myxoid | 4 | DOD |
| 24 | 54 | F | III | Thigh | 11 × 10 × 4 | – | – | Myxoid | 41 | CDF |
| 25 | 53 | M | III | Retroperitoneum | 18 × 13 × 11 | – | – | Myxoid | 43 | CDF |
AJCC, American Joint Committee on Cancer; DOD, dead of disease; CDF, continuous disease free; NED, no evidence of disease; AWD, alive with disease.
Specimen collection and storage. MFH cases were diagnosed by physicians based on the clinicopathological findings. Surgical specimens were immediately fixed in 20% neutralized formalin buffer solution at room temperature, and then, paraffin blocks were made.
Immunohistological staining. Immunohistochemical staining was performed using the LSAB (labeled streptavidin‐biotin) method; 3‐µm sections were cut using a microtome (MICROM GmbH, Type HM400R, Walldorf, Germany) and incubated overnight at 55°C. These sections were deparaffinized in xylene and rehydrated in descending gradations of ethanol. To enhance the immunostaining, the sections were placed in citrate buffer (pH 6) and autoclaved at 120°C for 10 min for antigen unmasking. After gradually quenching at room temperature, they were washed by PBS and immersed in APK buffer solution (Ventana Medical Systems, Inc., Tucson, AZ, USA). The sections were then placed on a NexES IHC automated immunostainer (Ventana Medical Systems, Inc.). We produced highly sensitive and specific anti‐8‐nitroguanine rabbit polyclonal antibody as described previously,( 28 ) and used as primary antibody at a concentration of 2 µg/mL. Other primary antibodies were also employed and their working dilutions were as follows: anti‐8‐oxodG mouse monoclonal antibody (1:100, Japan Institute for the Control of Aging, Fukuroi, Japan), anti‐NF‐κB p65 rabbit polyclonal antibody (1:1000, Abcam, Cambridge, UK), anti‐iNOS rabbit polyclonal antibody (1:300, Calbiochem‐Novabiochem, Darmstadt, Germany) and anti‐COX‐2 rabbit polyclonal antibody (1:300, Oxford Biomedical Research, Oxford, MI, USA). In a NexES IHC i‐VIEW DAB KIT, Bluing Reagent and hematoxylin (Ventana Medical Systems) were employed. As secondary antibodies, anti‐mouse IgG, anti‐mouse IgM and anti‐rabbit IgG antibodies (Ventana Medical Systems) were used. Then, we performed quantitative analysis for immunoreactivities of DNA lesions and inflammation‐related molecules by measuring the staining rates using Lumina Vision software (version 1.11, Mitani Shoji Corporation, Japan).
To examine the colocalization of 8‐nitroguanine and the expression of inflammation‐related molecules, we performed double immunofluorescence technique. Deparaffinized sections were washed in PBS and then immersed in PBS including 0.1% Triton‐X for 30 min. Thereafter, they were again washed in PBS. After autoclaving in citrate buffer (pH 6) at 120°C for 10 min, the sections were quenched at room temperature. Next, the sections were blocked with 1% skim milk for 30 min and treated with rabbit polyclonal anti‐8‐nitroguanine antibody and mouse monoclonal antibody (anti‐8‐oxodG, anti‐NF‐κB p65, anti‐iNOS [1:300; Sigma, St. Louis, MO, USA] or anti‐COX‐2 [1:50, Cayman Chemical, Ann Arbor, MI, USA] antibody) overnight at room temperature. After washing in PBS, they were incubated for 3 h with Alexa 594‐labeled goat antibody against rabbit IgG and Alexa 488‐labeled goat antibody against mouse IgG (Molecular Probes, Eugene, OR, USA, 1:400 each). The stained sections were examined under both an inverted microscope and a fluorescence microscope (BX50F‐3, Olympus Optical Co. Ltd., Tokyo, Japan).
Histopathological study. A histopathological study was performed by hematoxylin and eosin staining in paraffin sections by the standard method. The deparaffinized sections were washed in running tap water for 5 min, and then the slides were immersed in Mayer's Hematoxylin (Merck Sharp & Dohme, Whitehouse Station, NJ, USA) for 2–3 min. After washing in running tap water for 5 min, the slides were immersed in 70% alcohol for 2 min, in eosin alcohol for 1–2 min, and in 90% alcohol for a period and then were dehydrated.
Statistical analysis. The patient samples were categorized into six subgroups according to the staining rates (<7.5%, 7.5–15.0%, 15.0–22.5%, 22.5–30.0%, 30.0–37.5% and >37.5%), evaluated as described above, and then statistical differences of the immunoreactivities between deceased and living patients were analyzed by the χ2‐test. Spearman's and Pearson's rank correlation coefficients served to analyze the correlations for qualitative and quantitative data, respectively. Survival between the two subgroups, high‐grade (staining rates; 15% or more) and low‐grade (less than 15%) groups, was compared using the life‐table method of Kaplan‐Meier, and then statistically analyzed by the generalized Wilcoxon test. P‐values of less than 0.05 were considered to be statistically significant.
Results
Clinical data of MFH patients. The 25 MFH patients undergoing surgical resections comprised 12 male and 13 female patients, ranging in age from 27 to 85 years (mean ± SD, 62.7 ± 14.1 years). Survival data were available for all patients. The follow‐up duration ranged from 4 to 156 months (median, 46 months). These specimens were verified by histopathological studies and the histological subtypes were categorized into storiform‐pleomorphic (2 patients), storiform (9 patients), pleomorphic (6 patients) and myxoid (8 patients) by two independent researchers according to Enzinger and Weiss's Soft Tissue Tumors criteria.( 1 ) One of the two patients demonstrating a storiform‐pleomorphic subtype, 4/9 patients with a storiform subtype, 3/6 with a pleomorphic subtype and 4/8 with a myxoid subtype died. There was no significant difference in the prognosis among histological subtypes. All tumors measured more than 6 cm diameter in size. The tumors were located in the thigh (12 patients), limb (4 patients), buttock (3 patients), back (3 patients), chest wall (1 patient), knee (1 patient) and retroperitoneum (1 patient). Recurrences were found in 12 cases (48%) and distant metastases were found in 15 cases (60%). Among these 15 cases, 10 had lung metastasis. The prognosis was classified as follows: DOD, death of disease (12 patients); NED, no evidence of disease (5 patients); CDF, continuous disease free (7 patients); AWD, alive with disease (1 patient). The clinical data of these patients are summarized in Table 1.
Histopathological findings and 8‐nitroguaine formation in tumor tissues of MFH patients. Figure 1 shows the histopathological findings and 8‐Nitroguanine formation in MFH patients. The patients with storiform, pleomorphic and myxoid types showed such characteristic histological features as described previously.( 1 , 2 ) 8‐nitroguanine formation was observed in the nuclei of the tumor cells and inflammatory cells within the MFH tissue specimens. The immunoreactivity of 8‐nitroguanine was observed to a significantly greater extent in the patients who died due to any histological subtype (storiform, pleomorphic and myxoid) than in the living patients (Fig. 1 and Table 2).
Figure 1.
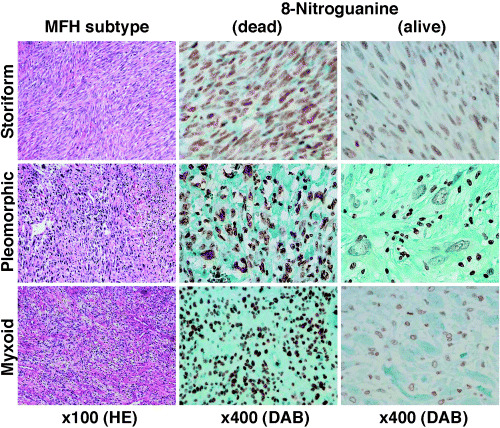
Histopathological findings and 8‐nitroguanine formation in malignant fibrous histiocytoma (MFH) patients. 8‐nitroguanine formation is observed in the nuclei of tumor cells and inflammatory cells within MFH tissue specimens. The immunoreactivity of 8‐nitroguanine is found to be significantly greater in the patients who died than in the living subjects regardless of any histologic subtype.
Table 2.
Difference in DNA damage and the expression of imflammation‐related molecules between deceased and living malignant fibrous histiocytoma patients
| Staining rate (%) | 8‐Nitroguanine | 8‐oxodG | iNOS | NF‐κB (nucleus) | COX‐2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients(%) | ||||||||||
| Alive (13) | Dead (12) | Alive (13) | Dead (12) | Alive (13) | Dead (12) | Alive (13) | Dead (12) | Alive (13) | Dead (12) | |
| 0.0–7.5 | 5 | 0 | 11 | 1 | 5 | 1 | 7 | 1 | 8 | 0 |
| 7.5–15.0 | 8 | 1 | 2 | 0 | 4 | 0 | 4 | 1 | 4 | 1 |
| 15.0–22.5 | 0 | 7 | 0 | 6 | 3 | 3 | 2 | 3 | 0 | 0 |
| 22.5–30.0 | 0 | 2 | 0 | 5 | 1 | 7 | 0 | 4 | 1 | 2 |
| 30.0–37.5 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 7 |
| >37.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| P‐value | P = 0.0003 | P = 0.0001 | P = 0.0163 | P = 0.0092 | P = 0.0007 | |||||
DNA damage and expression of inflammation‐related molecules in tumor tissues of MFH patients. Figure 2 shows DNA damage and the expression of inflammation‐related molecules in tumor tissues and adjacent non‐tumor tissues of MFH patients. The formation of 8‐nitroguanine and 8‐oxodG was clearly observed in the nucleus of tumor cells. iNOS and COX‐2 proteins were localized in the cytoplasm of tumor cells. The expression of NF‐κB was more evidently detected in the nucleus compared with the cytoplasm of tumor cells. On the other hand, DNA damage and the expression of these inflammation‐related genes were not observed in adjacent non‐tumor tissues (Fig. 2). No immunoreactivity was observed in tumor tissues when the primary antibody was omitted (data not shown). Figure 3 shows the colocalization of 8‐nitroguanine (red) with 8‐oxodG, NF‐κB, iNOS and COX‐2 (green), as evaluated by the double immunofluorescence technique. 8‐Nitroguanine and 8‐oxodG were colocalized in the same tumor cells. The expression of iNOS and COX‐2 was observed in the cytoplasm in most 8‐nitroguanine‐positive tumor cells. NF‐κB expression was detected mainly in the nucleus and colocalized with 8‐nitroguanine. In addition, the immunoreactivities of 8‐nitroguanine, 8‐oxodG, NF‐κB and COX‐2 were also observed in giant cells and inflammatory cells in MFH tissues.
Figure 2.
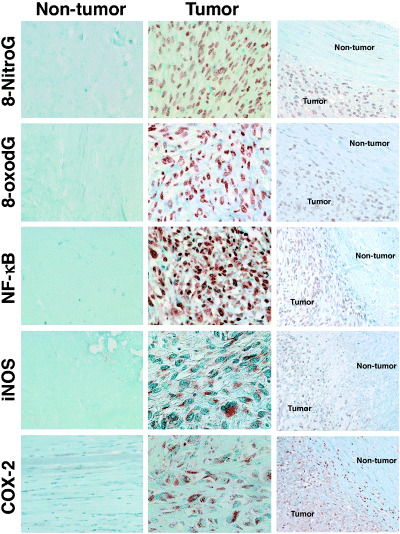
DNA damage and the expression of nuclear factor‐κB (NF‐κB), inducible nitric oxide synthase (iNOS) and cyclooxygenase‐2 (COX‐2) in malignant fibrous histiocytoma tissues. The formation of 8‐nitroguanine and 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG) is observed in the nucleus of tumor cells. iNOS and COX‐2 proteins are expressed in the cytoplasm in most tumor cells. NF‐κB is more clearly detected in the nucleus than in the cytoplasm of tumor cells. Little or no immunoreactivity of these DNA lesions and proteins was observed in adjacent non‐tumor tissues. Magnification: ×400 (left and center columns) and ×200 (right column).
Figure 3.
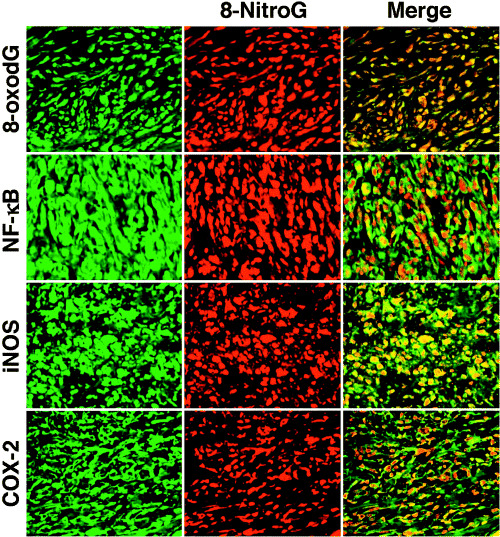
Colocalization of 8‐nitroguanine with 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine (8‐oxodG), nuclear factor‐κB (NF‐κB), inducible nitric oxide synthase (iNOS) and cyclooxygenase‐2 (COX‐2). 8‐Nitroguanine and 8‐oxodG are colocalized in the same tumor cells. iNOS and COX‐2 are expressed in the cytoplasm in most 8‐nitroguanine‐positive tumor cells. NF‐κB is colocalized with 8‐nitroguanine in the nucleus. Magnification: ×400.
Influences of DNA damage and inflammation‐related molecules on survival of MFH patients. We statistically analyzed the difference of DNA damage and the expression of inflammation‐related molecules between deceased and living patients as shown in Table 2. Immunoreactivity of 8‐nitroguanine was significantly stronger in the tumor tissue of the deceased patients than in those of the living patients (P = 0.0003). Similarly, the immunoreactivities of 8‐oxodG (P = 0.0001), iNOS (P = 0.0163), NF‐κB (P = 0.0092) and COX‐2 (P = 0.0007) were significantly stronger in the patients who died (Table 2).
We also performed generalized Wilcoxon test using the Kaplan‐Meier method to evaluate the effects of DNA damage and inflammation‐related molecules on prognosis of MFH patients as shown in Figure 4. MFH patients with high‐grade levels (staining rate; ≥15%) of 8‐nitroguanine had a significantly shorter survival (P = 0.0002) than those with low‐grade levels (<15%) (Fig. 4). In addition, the patients with high‐grade levels of 8‐oxodG (P = 0.0002), iNOS (P = 0.0278), COX‐2 (P = 0.0007) and nuclear NF‐κB (P = 0.0080) exhibited a significantly poorer prognosis in comparison to those with low‐grade levels (Fig. 4). These results suggest that the formation of DNA lesions and the expression of these molecules are significantly associated with poor survival. Moreover, MFH patients with metastasis also had a significantly shorter survival (P = 0.0005) than those without metastasis (Fig. 4).
Figure 4.
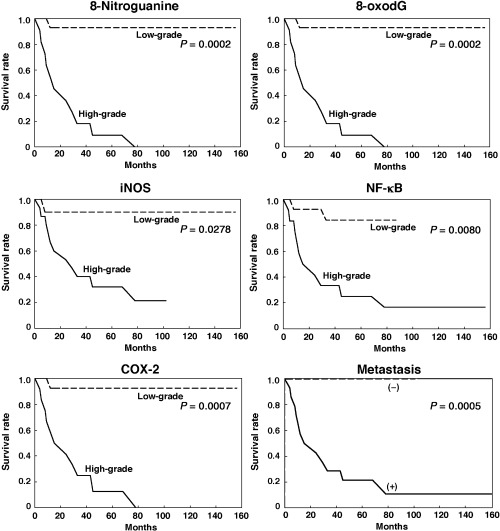
Effects of DNA damage and inflammation‐related molecules on survival of malignant fibrous histiocytoma patients (Kaplan‐Meier method). Solid line, patients with high‐grade levels of DNA lesion (8‐nitroguanine or 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine [8‐oxodG]) or inflammation‐related molecules (nuclear factor‐κB [NF‐κB], inducible nitric oxide synthase [iNOS] or cyclooxygenase‐2 [COX‐2]). Broken line, patients with low‐grade levels. In the case of metastases, solid and broken lines represent patients with (+) and without (–) metastases, respectively.
Correlation of the 8‐nitroguanine formation with metastasis, recurrence and the expression of inflammation‐related molecules. The prognosis for MFH patients (deceased or alive) showed a much more remarkable correlation with metastasis (R = 0.852, P = 0.0000) than with recurrence (R = 0.4423, P = 0.0268). 8‐nitroguanine formation was strongly correlated with metastasis (R = 0.7040, P = 0.0001). 8‐Nitroguanine formation was correlated with the formation of 8‐oxodG (R = 0.8207, P = 0.0000) and expression of iNOS (R = 0.5846, P = 0.0022), NF‐κB (R = 0.6276, P = 0.0008) and COX‐2 (R = 0.7392, P = 0.0000). NF‐κB expression was correlated with the expression of iNOS (R = 0.8200, P = 0.0000) and COX‐2 (R = 0.7254, P = 0.0000) in MFH tissues.
Discussion
Chronic inflammation has been proposed to contribute to the onset and development of various cancers.( 6 , 7 ) ROS and RNS are considered to play an important role in the pathogenesis of inflammation‐related cancer due to the fact that they cause DNA damage.( 8 , 9 ) We have previously demonstrated that 8‐nitroguanine is formed at the sites of carcinogenesis in humans and experimental animals with bacterial, viral and parasite infection.( 18 , 19 , 20 , 21 , 29 , 30 ) Based on the findings of our clinical studies, we proposed the hypothesis that 8‐nitroguanine formation contributes to tumor initiation and promotion.
In this study, to investigate whether 8‐nitroguanine can be a prognostic factor of inflammation‐related cancer, we examined the formation of these DNA lesions in patients with MFH. In all subtypes of MFH, the infiltration of inflammatory cells was observed in tumor tissue specimens of MFH patients and our findings were consistent with those of previous studies.( 2 ) Both 8‐nitroguanine and 8‐oxodG formed in sarcoma tissues to a much greater extent in deceased patients than in living subjects. Although among 13 living patients, 5 and 11 cases showed low staining rate (0–7.5%) of 8‐nitroguanine and 8‐oxoguanine, respectively, their immunoreactivity was higher than that of non‐tumor tissues where no immunoreactivity was observed. This result suggests that nitrative and oxidative DNA damage participates in not only initiation but also progression of MFH. Moreover, we demonstrated for the first time that intensive 8‐nitroguanine formation in MFH patients is associated with a poor prognosis. Relevantly, we recently performed a molecular epidemiological study, which suggested that 8‐nitroguanine formation is possibly associated with a poor prognosis in cholangiocarcinoma patients.( 22 ) These findings raise the hypothesis that 8‐nitroguanine formation participates in tumor progression in addition to tumor initiation and promotion. This hypothesis is supported by our recent study showing that subcutaneous implantation of a plastic plate induced soft tissue sarcoma in experimental animals via 8‐nitroguanine formation.( 31 ) Moreover, 8‐nitroguanine may be used as a better biomarker to predict the potential risk of tumor progression than histological features, because no significant difference was observed in the prognosis between each subtype of MFH.
We also examined the expression of inflammation‐related genes in MFH patients in relation to 8‐nitroguanine formation and the prognosis of these patients. NF‐κB is considered to be a key player in inflammation since it regulates the expression of various genes involved in controlling inflammatory response, including iNOS and COX‐2 expression.( 26 ) In addition, NF‐κB functions as a tumor promoter in inflammation‐associated cancer.( 32 ) In this study, 8‐nitroguanine was colocalized with iNOS and NF‐κB in tumor cells of MFH patients. NF‐κB expression in the nucleus of tumor cells was closely correlated with iNOS expression in MFH patients. These results lead to the hypothesis that 8‐nitroguanine formation is dependent on iNOS expression mediated by NF‐κB activation. This is the first study to propose the molecular mechanism of inflammation‐related carcinogenesis in soft tissues. The inhibition of NF‐κB by the expression of its inhibitory molecule IκBα decreased the viability of human sarcoma cells.( 33 ) iNOS expression was observed in soft tissue sarcoma cells and the treatment with an iNOS inhibitor reduced the growth of the sarcomas in an animal model.( 34 ) These studies support the involvement of NF‐κB‐mediated iNOS expression in the pathogenesis of MFH. Furthermore, we clearly demonstrated COX‐2 expression in MFH, which was colocalized with 8‐nitroguanine formation and was significantly associated with a poor prognosis in MFH patients. A selective COX‐2 inhibitor has also been reported to inhibit cell growth in a human MFH cell line.( 35 ) In addition, a clinical analysis demonstrated that COX‐2 expression was noted in a large part of MFHs, but not in benign fibrohistiocytic tumors, while COX‐2‐positive MFH showed a poorer survival than negative ones.( 36 ) COX‐2 expression is associated with tumor growth and neovascularity in a variety of malignancies.( 36 , 37 , 38 ) Based on our results, it is therefore reasonable to consider that in MFH patients, both NF‐κB expression and its nuclear translocation occur, thereby leading to iNOS expression and 8‐nitorguanine formation. COX‐2 expression appears to be induced by a similar mechanism, thus participating in additional inflammatory responses, and also resulting in tumor progression. NO can stimulate tumor growth and metastasis by promoting the migratory, invasive, and angiogenic abilities of tumor cells, which may also be triggered by the activation of COX‐2.( 39 ) MFH has an aggressive behavior and a highly metastatic potential to other organs which can thus result in the eventual death of such patients with soft‐tissue sarcomas.( 1 ) Most metastases occur in the lung( 2 ) and consistently, in this study, 10 cases had lung metastasis among the 15 patients with distant metastases. Interestingly, 8‐nitroguanine formation was correlated with distant metastases in MFH patients, thus suggesting that the formation of this DNA lesion participates in tumor progression and metastasis. These findings support the hypothesis that DNA damage mediated by inflammatory responses contributes to tumor progression in soft tissues.
ROS and RNS are considered to be involved in the pathogenesis of inflammation‐related carcinogenesis through DNA damage.( 8 , 9 ) These reactive species can induce 8‐oxodG formation thus leading to G → T transversions.( 40 , 41 ) On the other hand, RNS generated during inflammation can cause 8‐nitroguanine formation. 8‐Nitroguanine formed in DNA is chemically unstable, and thus can be spontaneously released, thereby resulting in the formation of an apurinic site.( 13 ) The apurinic site can form a pair with adenine during DNA synthesis, thus resulting in G → T transversions( 42 ) Translesion DNA synthesis past an apurinic site mediated by DNA polymerase ζ may contribute to point mutations.( 43 ) Therefore, 8‐nitroguanine is a potentially mutagenic DNA lesion as well as 8‐oxodG. Gene mutations in tumor tissue specimens from MFH patients have been reported. In MFH patients, the tumor tissues contained G → T transversions in the H‐ras proto‐oncogene( 15 ) and an exon of the p53 tumor suppressor gene.( 16 ) These clinical studies support our findings, which show the formation of mutagenic DNA lesions to be mediated by the inflammatory responses that participate in the carcinogenesis of soft tissues. ROS are generated from multiple sources, including carcinogenic chemicals and their metabolites, and electron transport chain in mitochondria in addition to inflammation. On the other hand, NO is generated specifically during inflammation in inflammatory and epithelial cells. Therefore, 8‐nitroguanine is formed mainly under inflammatory conditions and would play a substantial role in inflammation‐related carcinogenesis including MFH, although 8‐oxodG can be generated during inflammation.
We have previously reported that various infectious and inflammatory diseases induce the formation of 8‐oxodG and 8‐nitroguanine and these DNA lesions would participate in the initiation and/or promotion of carcinogenesis.( 18 , 19 ) In this study, we demonstrated for the first time that the formation of 8‐nitroguanine and the expression of the molecules involved in DNA damage are significantly associated with a poor prognosis for cancer patients. The progression of a large majority of human and experimental tumors therefore seems to be stimulated by NO which results from the activation of iNOS.( 39 ) These findings raise a new hypothesis that 8‐nitroguanine contributes to tumor progression in addition to initiation and/or promotion. Therefore, the formation of DNA lesions mediated by inflammatory responses could contribute to multiple steps of carcinogenesis, thus leading to tumor invasion and metastasis. In conclusion, 8‐nitroguanine is therefore considered to be a potential biomarker to evaluate the potential risk and prognosis of patients with inflammation‐related cancer.
Acknowledgments
This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. We would like to thank Mr Brian Quinn for reviewing the English usage in our manuscript.
References
- 1. Weiss SW, Goldbum JR. Enzinger and Weiss's Soft Tissue Tumors, 4th edn. St. Louis: Mosby, 2002. [Google Scholar]
- 2. Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer 1978; 41: 2250 – 66. [DOI] [PubMed] [Google Scholar]
- 3. Randall RL, Albritton KH, Ferney BJ, Layfield L. Malignant fibrous histiocytoma of soft tissue: an abandoned diagnosis. Am J Orthop 2004; 33: 602 – 8. [PubMed] [Google Scholar]
- 4. Melhem MF, Meisler AI, Saito R, Finley GG, Hockman HR, Koski RA. Cytokines in inflammatory malignant fibrous histiocytoma presenting with leukemoid reaction. Blood 1993; 82: 2038 – 44. [PubMed] [Google Scholar]
- 5. Richter KK, Parham DM, Scheele J, Hinze R, Rath FW. Presarcomatous lesions of experimentally induced sarcomas in rats: morphologic, histochemical, and immunohistochemical features. In Vivo 1999; 13: 349 – 55. [PubMed] [Google Scholar]
- 6. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357: 539 – 45. [DOI] [PubMed] [Google Scholar]
- 7. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860 – 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer 2003; 3: 276 – 85. [DOI] [PubMed] [Google Scholar]
- 9. Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation‐induced carcinogenesis. Arch Biochem Biophys 2003; 417: 3 – 11. [DOI] [PubMed] [Google Scholar]
- 10. Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine‐specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res 2001; 488: 65 – 76. [DOI] [PubMed] [Google Scholar]
- 11. Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 1996; 313: 17 – 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tazawa H, Okada F, Kobayashi T et al. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation‐associated carcinogenesis and tumor progression. Am J Pathol 2003; 163: 2221 – 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H. Formation of 8‐nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis 1995; 16: 2045 – 50. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki N, Yasui M, Geacintov NE, Shafirovich V, Shibutani S. Miscoding events during DNA synthesis past the nitration‐damaged base 8‐nitroguanine. Biochemistry 2005; 44: 9238 – 45. [DOI] [PubMed] [Google Scholar]
- 15. Yoo J, Robinson RA, Lee JY. H‐ras and K‐ras gene mutations in primary human soft tissue sarcoma: concomitant mutations of the ras genes. Mod Pathol 1999; 12: 775 – 80. [PubMed] [Google Scholar]
- 16. Jiao YF, Nakamura S, Sugai T et al. p53 gene mutation and MDM2 overexpression in a case of primary malignant fibrous histiocytoma of the jejunum. APMIS 2002; 110: 165 – 71. [DOI] [PubMed] [Google Scholar]
- 17. Sakamoto A, Oda Y, Itakura E et al. H‐, K‐, and N‐ras gene mutation in atypical fibroxanthoma and malignant fibrous histiocytoma. Hum Pathol 2001; 32: 1225 – 31. [DOI] [PubMed] [Google Scholar]
- 18. Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation‐related carcinogenesis. Biol Chem 2006; 387: 365 – 72. [DOI] [PubMed] [Google Scholar]
- 19. Kawanishi S, Hiraku Y. Oxidative and nitrative DNA damage as biomarker for carcinogenesis with special reference to inflammation. Antioxid Redox Signal 2006; 8: 1047 – 58. [DOI] [PubMed] [Google Scholar]
- 20. Ma N, Adachi Y, Hiraku Y et al. Accumulation of 8‐nitroguanine in human gastric epithelium induced by Helicobacter pylori infection. Biochem Biophys Res Commun 2004; 319: 506 – 10. [DOI] [PubMed] [Google Scholar]
- 21. Horiike S, Kawanishi S, Kaito M et al. Accumulation of 8‐nitroguanine in the liver of patients with chronic hepatitis C. J Hepatol 2005; 43: 403 – 10. [DOI] [PubMed] [Google Scholar]
- 22. Pinlaor S, Sripa B, Ma N et al. Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J Gastroenterol 2005; 11: 4644 – 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaiyarit P, Ma N, Hiraku Y et al. Nitrative and oxidative DNA damage in oral lichen planus in relation to human oral carcinogenesis. Cancer Sci 2005; 96: 553 – 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma N, Tagawa T, Hiraku Y, Murata M, Ding X, Kawanishi S. 8‐Nitroguanine formation in oral leukoplakia, a premalignant lesion. Nitric Oxide 2006; 14: 137 – 43. [DOI] [PubMed] [Google Scholar]
- 25. Asano N, Yamakazi T, Seto M, Matsumine A, Yoshikawa H, Uchida A. The expression and prognostic significance of bone morphogenetic protein‐2 in patients with malignant fibrous histiocytoma. J Bone Joint Surg Br 2004; 86: 607 – 12. [PubMed] [Google Scholar]
- 26. Surh YJ, Chun KS, Cha HH et al. Molecular mechanisms underlying chemopreventive activities of anti‐inflammatory phytochemicals: down‐regulation of COX‐2 and iNOS through suppression of NF‐κB activation. Mutat Res 2001; 480–481: 243 – 68. [DOI] [PubMed] [Google Scholar]
- 27. Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature 2004; 431: 405 – 6. [DOI] [PubMed] [Google Scholar]
- 28. Pinlaor S, Hiraku Y, Ma N et al. Mechanism of NO‐mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation‐mediated carcinogenesis. Nitric Oxide 2004; 11: 175 – 83. [DOI] [PubMed] [Google Scholar]
- 29. Pinlaor S, Ma N, Hiraku Y et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8‐nitroguanine and 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis 2004; 25: 1535 – 42. [DOI] [PubMed] [Google Scholar]
- 30. Ding X, Hiraku Y, Ma N et al. Inducible nitric oxide synthase‐dependent DNA damage in mouse model of inflammatory bowel disease. Cancer Sci 2005; 96: 157 – 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tazawa H, Tatemichi M, Sawa T et al. Oxidative and nitrative stress caused by subcutaneous implantation of a foreign body accelerate sarcoma development in Trp53+/– mice. Carcinogenesis in press. [DOI] [PubMed]
- 32. Pikarsky E, Porat RM, Stein I et al. NF‐κB functions as a tumour promoter in inflammation‐associated cancer. Nature 2004; 431: 461 – 6. [DOI] [PubMed] [Google Scholar]
- 33. Feig BW, Lu X, Hunt KK et al. Inhibition of the transcription factor nuclear factor‐κB by adenoviral‐mediated expression of IκBαM results in tumor cell death. Surgery 1999; 126: 399 – 405. [PubMed] [Google Scholar]
- 34. De Wilt JH, Manusama ER, Van Etten B et al. Nitric oxide synthase inhibition results in synergistic anti‐tumour activity with melphalan and tumour necrosis factor alpha‐based isolated limb perfusions. Br J Cancer 2000; 83: 1176 – 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamashita H, Osaki M, Honjo S, Yoshida H, Teshima R, Ito H. A selective cyclooxygenase‐2 inhibitor, NS‐398, inhibits cell growth by cell cycle arrest in a human malignant fibrous histiocytoma cell line. Anticancer Res 2003; 23: 4671 – 6. [PubMed] [Google Scholar]
- 36. Yamashita H, Osaki M, Ardyanto TD, Yoshida H, Ito H. Cyclooxygenase‐2 in human malignant fibrous histiocytoma: correlations with intratumoral microvessel density, expression of vascular endothelial growth factor and thymidine phosphorylase. Int J Mol Med 2004; 14: 565 – 70. [PubMed] [Google Scholar]
- 37. Iniguez MA, Rodriguez A, Volpert OV, Fresno M, Redondo JM. Cyclooxygenase‐2: a therapeutic target in angiogenesis. Trends Mol Med 2003; 9: 73 – 8. [DOI] [PubMed] [Google Scholar]
- 38. Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991; 325: 1593 – 6. [DOI] [PubMed] [Google Scholar]
- 39. Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol 2001; 2: 149 – 56. [DOI] [PubMed] [Google Scholar]
- 40. Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation‐damaged base 8‐oxodG. Nature 1991; 349: 431 – 4. [DOI] [PubMed] [Google Scholar]
- 41. Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8‐oxoguanine in DNA. Nature 2000; 403: 859 – 66. [DOI] [PubMed] [Google Scholar]
- 42. Loeb LA, Preston BD. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet 1986; 20: 201 – 30. [DOI] [PubMed] [Google Scholar]
- 43. Wu X, Takenaka K, Sonoda E et al. Critical roles for polymerase ζ in cellular tolerance to nitric oxide‐induced DNA damage. Cancer Res 2006; 66: 748 – 54. [DOI] [PubMed] [Google Scholar]


