Abstract
Head and neck squamous cell carcinoma has still a poor prognosis. Since angiogenesis is crucial for tumor growth, a better understanding of the potential clinical relevance as well as the interactions between the numerous proangiogenic growth factors is essential to develop improved therapeutic strategies in these tumors. Expression levels of eight growth factors known to induce angiogenesis (HGF, bFGF, VEGF‐A, VEGF‐D, PDGF‐AB, PDGF‐BB, G‐CSF, and GM‐CSF) were quantitatively measured by ELISA in homogenates of 41 head and neck squamous cell carcinomas. In addition, microvessel density and protein localization of growth factors were assessed by immunohistochemistry. Statistical analysis was performed to assess interrelationships between growth factors analyzed and to correlate protein levels with patient outcome. In 90% of the tissues at least 4/8 growth factors analyzed were detectable. Highest amounts and most frequent expression were found for HGF, bFGF and VEGF‐A while PDGF‐AB and PDGF‐BB were present in two‐thirds and G‐CSF and GM‐CSF in approximately half of the cases. Although there was no significant relation to microvessel density, we identified significant associations for bFGF with HGF and G‐CSF as well as of PDGF‐AB with those of VEGF‐A and PDGF‐BB. For the first time we demonstrate that expression levels of HGF as well as that of bFGF and G‐CSF in head and neck squamous tumors are negative prognostic factors for patient survival. Our data indicate a network of interrelated and prognostically relevant growth factors in these tumors that have to be taken into consideration when planning an antiangiogenic and antitumor therapy. (Cancer Sci 2009; 100: 1210–1218)
Abbreviations:
- bFGF
basic fibroblast growth factor
- CUP
carcinoma of unknown primary
- G‐CSF
granulocyte colony‐stimulating factor
- GM‐CSF
granulocyte macrophage colony‐stimulating factor
- HGF
hepatocyte growth factor
- HNSCC
head and neck squamous cell carcinoma
- PDGF
platelet derived growth factor
- VEGF
vascular endothelial growth factor
- MVD
microvessel density
Head and neck squamous cell carcinoma (HNSCC) is an aggressive epithelial malignancy that was ranked as the eighth leading cause of cancer death worldwide in 2000.( 1 ) Although sharing the paramount risk factors of tobacco smoking and drinking of alcohol it is a heterogeneous group of tumors arising from different anatomical sites of the upper aero‐digestive tract with differences in etiology and clinical outcome.( 2 ) Head and neck cancer and its treatment have severe impact on the quality of life of patients, causing disfigurement and affecting speech and the basic survival functions of breathing and swallowing.( 3 ) Any form of recurrence is strongly associated with high mortality, and the total 5‐year survival rate today is only 50%.( 4 ) Novel approaches such as combined modality therapy, and advances in surgical and radiotherapeutic techniques have resulted in an improvement in terms of function and quality of life.( 5 ) Nevertheless, standard treatment has only marginally increased the survival rates of patients with this disease in the last decades.( 6 ) Therefore, there is urgent need for novel therapeutic approaches.
Tumor growth and metastasis are angiogenesis‐dependent. To expand beyond the size of a few mm3, solid tumors such as HNSCC have to gain access to the host vascular system and generate their own blood supply.( 7 ) Tumor and stroma cells secrete growth factors such as vascular endothelial growth factor A (VEGF‐A) and basic fibroblast growth factor (bFGF) that target surrounding endothelial cells, which in turn are stimulated to form microvessels towards the angiogenic stimulus.( 8 ) This induction of a tumor vasculature, termed the ‘angiogenic switch’ is a rate‐limiting step in tumor progression. When enough tumor cells become angiogenic, the tumor can expand progressively and shed metastatic cells.( 9 , 10 )
bFGF is known to have potent angiogenic activity and has been identified in a wide variety of malignancies including HNSCC.( 11 ) While it is 20‐fold more potent than its acidic analog,( 12 ) it significantly increases vessel density only when VEGF‐A and bFGF are coexpressed.( 13 ) Further, a stable vascular network is only induced if bFGF is secreted in combination with platelet derived growth factor BB (PDGF‐BB)( 14 ) indicating that a network of closely interacting angiogenic growth factors is required to elicit an effective angiogenic response.
However, knowledge about the complex interactions between growth factors is still incomplete and their functional activity is not restricted to angiogenesis alone. For instance, VEGF‐A, a key regulator of angiogenesis, has been identified as a multifunctional factor involved not only in angiogenesis but also in tumor progression, immunosuppression and immune tolerance.( 15 ) In a previous study examining the secretion of several angiogenic proteins in culture supernatants of HNSCC cells, we suggested a major role of VEGF and the platelet derived growth factor AB (PDGF‐AB) and that the additional secretion of granulocyte colony‐stimulating factor (G‐CSF) or granulocyte macrophage colony‐stimulating factor (GM‐CSF) might contribute to a poorer prognosis.( 11 ) However, since these data were obtained from cultivated tumor cells, further evaluation in tumor tissues containing tumor and stromal cells and thus an additional source of angiogenic proteins besides the tumor cells, is warranted in order to confirm the clinical importance of these growth factors.
A more detailed understanding of the regulatory pathways and the interactions between growth factors in HNSCC is essential to improve the clinical prognosis of this tumor type. To investigate the clinical relevance and their possible interactions, we quantified a set of eight growth factors in HNSCC tumors derived from HNSCC‐relevant tumor localizations. In the past, for all of these growth factors induction of angiogenesis has been demonstrated.( 16 ) It is noteworthy that with hepatocyte growth factor (HGF) we included a molecule which is in contrast to the other angiogenic growth factors analyzed in our study predominantly expressed by stromal cells.( 17 ) Expression levels were correlated with conventional clinicopathological parameters including overall survival, demonstrating HGF, bFGF, and G‐CSF to be unfavorable prognostic factors for patient survival. Finally, based on significant interrelations seen between expression levels of certain growth factors, proteins analyzed could be attributed to two distinct subgroups with the key players VEGF‐A, PDGF‐AB, and ‐BB belonging to the first group and HGF, bFGF, G‐CSF, as well as GM‐CSF building the second group.
Materials and Methods
Samples. Tissue specimens of 41 HNSCC tumors were obtained intraoperatively and snapfrozen immediately after surgery in liquid nitrogen and stored at –80°C until processing. Informed consent was obtained from each patient according to the research proposals approved by the Institutional Review Board at the Medical Faculty Heidelberg. The specimens were derived in equal numbers from the four major localizations of HNSCC: oropharynx (n = 10), hypopharynx (n = 10), larynx (n = 10) and carcinoma of unknown primary (CUP; n = 11). Histological evaluation was performed by the local pathologist. Clinical data of the respective patients concerning tumor localization, tumor stage, TNM classification, tumor grade, therapy and survival are summarized in Table 1. Treatment in all except two patients consisted of surgery and radiotherapy. In addition, 13 of 41 patients received chemotherapy.
Table 1.
Clinical data of study samples
| Tissue code | Localization | Stage | pTNM | Grading | Op | RTx | CTx | Disease‐free interval (weeks) | Overall survival (weeks) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CUP | IV | Tx N2b Mx | G2 | + | + | – | 164 | 164 |
| 2 | CUP | IV | Tx N3 Mx | G4 | + | + | – | 95 | 95 |
| 3 | CUP | IV | Tx N2 c Mx | G3 | + | + | – | 172 | 172 † |
| 4 | CUP | IV | Tx N2b Mx | G3 | + | + | – | 160 | 160 † |
| 5 | CUP | IV | Tx N2b Mx | G3 | + | + | – | 17 | 17 |
| 6 | CUP | IV | Tx N2a Mx | G3 | + | – | – | 3664 | 3664 † |
| 7 | CUP | III | Tx N1 Mx | G3 | + | + | – | 176 | 176 † |
| 8 | CUP | IV | Tx N2 c Mx | G4 | + | + | + | 96 | 224 |
| 9 | CUP | IV | Tx N2a M0 | G3 | + | + | – | 28 | 65 |
| 10 | CUP | IV | Tx N2b M0 | G3 | + | + | – | 48 | 48 |
| 11 | CUP | IV | Tx N2a Mx | G3 | + | + | – | 36 | 36 |
| 12 | Oropharynx | IV | T4 N0 M0 | G3 | + | + | + | 20 | 48 |
| 13 | Oropharynx | II | T2 N0 Mx | G2 | + | + | + | 58 | 58 |
| 14 | Oropharynx | IV | T4 N2 c M0 | G3 | + | + | + | 30 | 30 |
| 15 | Oropharynx | IV | T4 N2 c M0 | G3 | + | + | + | 12 | 68 |
| 16 | Oropharynx | III | T3 N1 M0 | G2 | + | + | – | 190 | 190 |
| 17 | Oropharynx | IV | T2 N2b M0 | G2–3 | + | + | + | 32 | 100 |
| 18 | Oropharynx | II | T2 N0 M0 | G3 | + | + | – | 33 | 64 |
| 19 | Oropharynx | IV | T4 N2b M0 | G3 | + | + | – | 28 | 48 |
| 20 | Oropharynx | IV | T1 N2b M0 | G3 | + | + | + | 240 | 240 † |
| 21 | Oropharynx | III | T3 N2b M0 | G2 | + | + | + | 72 | 140 |
| 22 | Larynx | IV | T4 N0 M0 | G2 | + | + | – | 264 | 264 † |
| 23 | Larynx | IV | T4 N0 M0 | G3 | + | + | – | 176 | 176 † |
| 24 | Larynx | IV | T4 N0 M0 | G3 | + | + | – | 156 | 156 † |
| 25 | Larynx | IV | T4 N2b M0 | G2 | + | + | + | 44 | 44 |
| 26 | Larynx | IV | T4 N2 c Mx | G3 | + | – | – | 57 | 57 |
| 27 | Larynx | IV | T4 N0 M0 | G2 | + | + | – | 304 | 304 † |
| 28 | Larynx | IV | T4 N0 M0 | G3 | + | + | – | 264 | 264 † |
| 29 | Larynx | III | T3 N1 M0 | G3 | + | + | – | 7 | 7 |
| 30 | Larynx | IV | T4 N2b M0 | G3 | + | + | + | 128 | 128 † |
| 31 | Larynx | IV | T4 N0 M0 | G3 | + | + | – | 32 | 32 |
| 32 | Hypopharynx | IV | T1 N2b M0 | G2 | + | + | – | 72 | 72 |
| 33 | Hypopharynx | III | T1 N1 M0 | G3 | + | + | + | 26 | 96 |
| 34 | Hypopharynx | IV | T2 N2 c M0 | G3 | + | + | – | 85 | 85 |
| 35 | Hypopharynx | IV | T3 N2 c M0 | G3 | + | + | – | 123 | 123 |
| 36 | Hypopharynx | IV | T4 N2 c M0 | G3 | + | + | + | 54 | 54 |
| 37 | Hypopharynx | IV | T4 N2b M0 | G3 | + | + | – | 54 | 54 |
| 38 | Hypopharynx | IV | T3 N2 c M0 | G3 | + | + | + | 36 | 100 |
| 39 | Hypopharynx | IV | T4 N2 c M0 | G3 | + | + | – | 40 | 40 |
| 40 | Hypopharynx | IV | T2 N2 c M0 | G3 | + | + | – | 60 | 63 |
| 41 | Hypopharynx | IV | T4 N2b M0 | G3 | + | + | – | 40 | 40 |
Indicates patients, still living at cut‐off date of evaluation; OP, radical surgery; RTx, radiotherapy (pts. 6 and 25 rejected RTx); CTx: chemotherapy concomitant with adjuvant RTx or in palliative setting; CUP, carcinoma of unknown primary.
Protein quantification. Frozen tumor pieces of approximately 0.2 g weight were homogenized in 4.9 mL extraction buffer containing 20 mM Tris buffer at pH 7.5, 500 mM NaCl, 100 mM proteinase inhibitor PMSF (Roche Diagnostics GmbH, Mannheim, Germany), 10 µg/mL leupeptin proteinase inhibitor (Roche Diagnostics GmbH, Mannheim, Germany) dissolved in distilled water. Lysates were centrifuged for 3 min at full speed to eliminate cell residues. Subsequently, lysate supernatant passed several passages of centrifugation (10 min, 13.000 g), until no further impurity was recognized. Protein quantification was performed using the DC protein assay, which is based on a modified Lowry Assay( 18 ) and was applied according to the manufacturer's instructions (Bio‐Rad, Munich, Germany). Tumor lysates were used to measure amounts of angiogenic growth factors HGF, bFGF, VEGF‐A, VEGF‐D, PDGF‐AB, PDGF‐BB, G‐CSF, and GM‐CSF by ELISA (R & D Systems, Wiesbaden, Germany). Assays were performed in duplicates according to the manufacturer's instructions. Final data are means of two independent measurements.
Immunohistochemistry. Staining of tumor sections was carried out as described.( 16 ) Primary antibodies used were mouse antihuman CD34 (clone QBEnd10, Dako, Hamburg, Germany), VEGF‐A (PromoCell, Heidelberg, Germany), rabbit antihuman PDGF (recognizing all PDGF subtypes), mouse antihuman bFGF, GM‐CSF, G‐CSF (all Dianova, Hamburg, Germany), mouse antihuman HGF (R & D Systems Wiesbaden, Germany), and mouse antihuman c‐Met (abcam, Cambridge, UK). Isotype controls in equal concentrations as primary antibodies served as negative controls.
Microvessel density. To determine microvessel density (MVD), three sections per tumor were stained with an antibody to CD34. Sections were scanned at a low magnification (40×) to identify tumor areas with highest vascularity (hot spots). Then, in each tumor section three images were taken from different hot spots at 200× total magnification. Stained microvessels were counted using the AnalySIS image analysis software allowing an automatic assessment of vessel number and vessel area (Soft Imaging System, Muenster, Germany). Counting was performed in a blinded manner. Finally, mean values for each tumor were calculated.
Statistical analysis. Correlation between quantitative growth factor expression and patient prognosis was determined by log‐rank test and presented as a Kaplan–Meier curve. Furthermore, multivariate Cox regression with forward selection was used to evaluate the influence of growth factors on patient survival. Differences in expression pattern of a single angiogenic growth factor dependent on tumor localization were calculated by Kruskal‐Wallis test, which allows comparison of more than two independent variables, in this case comparison of the four tumor localizations with regard to one single growth factor. The U‐test by Mann–Whitney and Wilcoxon was chosen to compare two independent variables, for example, to find significant correlations between two growth factors. In each test P‐values <0.05 were considered to be statistically significant.
Results
Expression of angiogenic growth factors in native HNSCC tissues and MVD. Quantitative growth factor analysis for eight potent proangiogenic growth factors was performed on 41 native HNSCC tissues (Table 1). Obtained protein values as well as MVD data of the same tissues are listed in Table 2. Independent of tumor localization, HGF and bFGF reached highest concentrations with mean values of 2865 pg/mg (±1779) and 1104 pg/mg (±695), respectively, followed by VEGF‐A with a mean of 565 pg/mg (±964). The very same growth factors were detectable in all (HGF and bFGF) or almost all tumor tissues (VEGF‐A in 98%). PDGF‐AB and PDGF‐BB were still detectable in approximately two‐thirds (68%) of the samples but with markedly lower mean values of 30 pg/mg (±40) and 17 pg/mg (±22), respectively. Similarly, GM‐CSF and G‐CSF were found with 56% and 51% in almost the same frequencies and with mean growth factor amounts of 10 pg/mg (±29) and 48 pg/mg (±113) in similar amounts. In contrast, VEGF‐D was only detectable in very low amounts in not more than three tissues. Altogether, our observations point to a major role of at least HGF, bFGF and VEGF‐A in HNSCC, while the importance of VEGF‐D seems to be negligible in this tumor type.
Table 2.
Quantitative growth factors levels in head and neck squamous cell carcinoma (HNSCC) tissues
| Localization | Tissue code | PDGF‐AB* | PDGF‐BB* | VEGF‐A* | VEGF‐D* | GM‐CSF* | G‐CSF* | bFGF* | HGF* | MVD |
|---|---|---|---|---|---|---|---|---|---|---|
| CUP | 1 | 99 | 23 | 1102 | 0 | 16 | 21 | 703 | 3469 | 10 |
| CUP | 2 | 0 | 6.5 | 514 | 8.4 | 2.5 | 0 | 544 | 1959 | 38 |
| CUP | 3 | 0 | 8.7 | 16 | 6 | 0.9 | 0 | 335 | 710 | 37 |
| CUP | 4 | 0 | 0 | 0 | 0 | 5.9 | 0 | 544 | 1084 | 18 |
| CUP | 5 | 0 | 22.5 | 3930 | 0 | 9.5 | 0 | 375 | 3046 | n.a. |
| CUP | 6 | 36.5 | 17.5 | 340 | 0 | 3.5 | 11 | 261 | 542 | 72 |
| CUP | 7 | 35 | 12.5 | 1606 | 0 | 0 | 0 | 1516 | 1692 | 36 |
| CUP | 8 | 18 | 12.5 | 72.5 | 0 | 0 | 0 | 310 | 1747 | 41 |
| CUP | 9 | 13.5 | 10.5 | 74 | 0 | 8 | 8 | 424 | 1516 | 25 |
| CUP | 10 | 34 | 15 | 1738 | 0 | 3 | 45 | 1710 | 1920 | 14 |
| CUP | 11 | 34 | 0 | 160 | 0 | 0 | 0 | 541 | 3600 | 18 |
| Oropharynx | 12 | 232 | 98 | 280 | 20 | 10 | 35 | 975 | 3785 | n.a. |
| Oropharynx | 13 | 0 | 12.7 | 2 | n.a. | 3 | 0 | 244 | n.a. | 45 |
| Oropharynx | 14 | 37 | 32 | 140 | 0 | 31 | 99 | 1182 | 2603 | 9 |
| Oropharynx | 15 | 57.5 | 64 | 956 | 0 | 13.5 | 0 | 243 | 1795 | 60 |
| Oropharynx | 16 | 31 | 14.5 | 42 | 0 | 0 | 14 | 2024 | 3132 | 126 |
| Oropharynx | 17 | 18.5 | 10.5 | 218 | 0 | 0 | 0 | 626 | 1515 | n.a. |
| Oropharynx | 18 | 0 | 0 | 177 | 0 | 1 | 0 | 277 | 2224 | 7 |
| Oropharynx | 19 | 71.5 | 30 | 100 | 0 | 0 | 33 | 1007 | 4421 | 62 |
| Oropharynx | 20 | 0 | 0 | 311 | 0 | 0 | 0 | 1233 | 1354 | 18 |
| Oropharynx | 21 | 36 | 10.5 | 36 | 0 | 0 | 0 | 1224 | 890 | 120 |
| Larynx | 22 | 19 | 16 | 57 | 0 | 0 | 15 | 1854 | 3927 | 13 |
| Larynx | 23 | 60 | 61 | 530 | 0 | 177.5 | 213 | 1027 | 2099 | 14 |
| Larynx | 24 | 0 | 43 | 143 | 0 | 6 | 30 | 1519 | 6188 | 11 |
| Larynx | 25 | 0 | 0 | 105 | 0 | 0 | 0 | 747 | 1805 | 28 |
| Larynx | 26 | 0 | 52 | 369 | 0 | 0 | 0 | 1701 | 2504 | 21 |
| Larynx | 27 | 17.5 | 9.5 | 332 | 0 | 0 | 35 | 566 | 2938 | 42 |
| Larynx | 28 | 64 | 48.5 | 93 | 0 | 40.5 | 116 | 1021 | 1843 | 25 |
| Larynx | 29 | 27 | 13.5 | 114 | 0 | 9.5 | 327 | 2184 | 1537 | 24 |
| Larynx | 30 | 49 | 17.5 | 264 | 0 | 0 | 0 | 642 | 1793 | 29 |
| Larynx | 31 | 0 | 0 | 66 | 0 | 6 | 164 | 2574 | 7108 | 28 |
| Hypopharynx | 32 | 0 | 0 | 67 | 0 | 4 | 0 | 457 | 1417 | 14 |
| Hypopharynx | 33 | 22.5 | 0 | 60 | 0 | 3.5 | 23 | 1902 | 3468 | 13 |
| Hypopharynx | 34 | 24.5 | 0 | 464 | 0 | 10 | 52 | 1760 | 962 | 30 |
| Hypopharynx | 35 | 52 | 0 | 3877 | 0 | 0 | 0 | 1814 | 3186 | n.a. |
| Hypopharynx | 36 | 30 | 30 | 789 | 0 | 0 | 0 | 959 | 6476 | 20 |
| Hypopharynx | 37 | 57 | 0 | 3088 | 0 | 0 | 0 | 1201 | 4654 | 58 |
| Hypopharynx | 38 | 17.5 | 12 | 127 | 0 | 4 | 41 | 2954 | 6235 | 25 |
| Hypopharynx | 39 | 14 | 0 | 169 | 0 | 39 | 621 | 2046 | 6459 | 87 |
| Hypopharynx | 40 | 23.5 | 14.5 | 479 | 0 | 0 | 15 | 885 | 5322 | 62 |
| Hypopharynx | 41 | 0 | 0 | 140 | 0 | 0 | 36 | 1160 | 1688 | 50 |
| Mean | 30 | 17.3 | 564.6 | 0.9 | 9.9 | 47.7 | 1104.2 | 2865.3 | 36.5 |
Concentrations of angiogenic growth factors (pg/mg total protein); n.a., not assessed; MVD, microvessel density, mean number of microvessels per field; PDGF‐AB, platelet derived growth factor AB; PDGF‐BB, platelet derived growth factor BB; VEGF‐A, vascular endothelial growth factor A; VEGF‐D, vascular endothelial growth factor D; GM‐CSF, granulocyte macrophage colony‐stimulating factor; G‐CSF,granulocyte colony‐stimulating factor; bFGF, basic fibroblast growth factor; HGF, hepatocyte growth factor.
To determine protein localization of growth factors analyzed, immunohistochemical staining was performed on tumor sections (Fig. 1A‐F). Except for HGF which was primarily concentrated in the tumor stroma, we found a considerable staining of all growth factors analyzed on tumor cells as well as on blood vessels.
Figure 1.
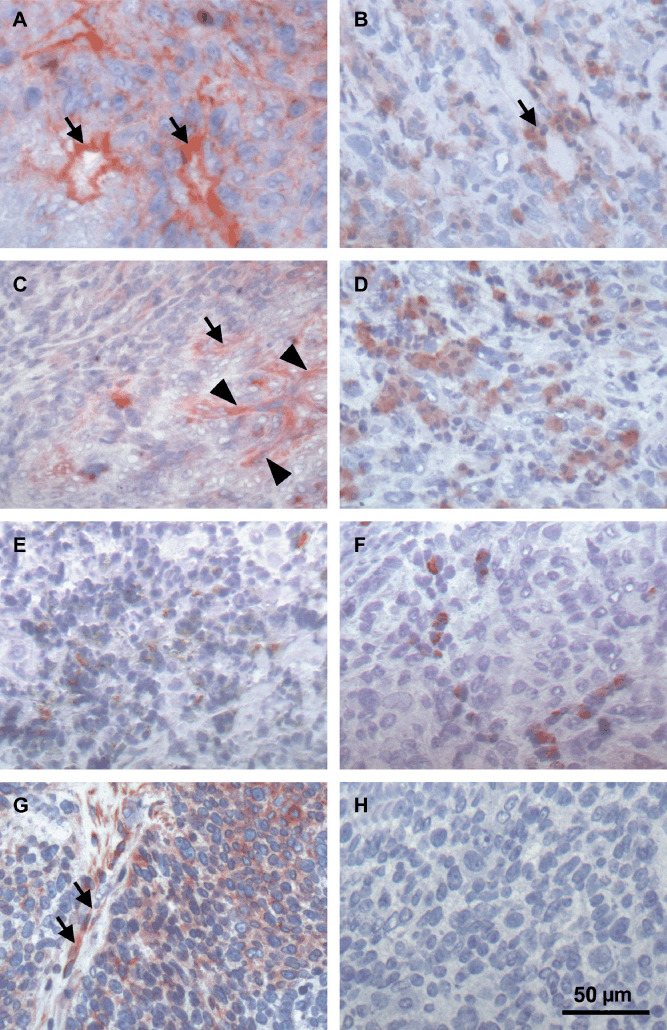
Immunohistochemical analysis of different angiogenic growth factors and the hepatocyte growth factor (HGF) receptor c‐MET in head and neck squamous cell carcinoma (HNSCC). Tumor sections of a hypopharynx carcinoma (#38, Table 1) stained positive for (A) basic fibroblast growth factor (bFGF), (B) vascular endothelial growth factor (VEGF), (C) HGF, (D) platelet derived growth factor (PDGF), (E) granulocyte colony‐stimulating factor (G‐CSF), and (F) granulocyte macrophage colony‐stimulating factor (GM‐CSF). Except for HGF, all growth factors analyzed were found on tumor cells and accumulated around blood vessels (arrows). Arrowheads in (C) point to HGF‐positive stroma cells. (G) Analysis of c‐MET in a larynx carcinoma (#26) reveals expression on endothelial (arrows) and tumor cells. (H) Negative control corresponding to (G).
With regard to MVD, we observed a marked heterogeneity in the number of endothelial vessels ranging from 7 to 126 per hotspot analyzed (Table 2). Highest vessel counts were found in tissues of oropharynx carcinomas (mean 56 ± 46.6) followed by hypopharynx tumors (mean 40 ± 26), CUP (mean 31 ± 18.2), and larynx carcinomas (mean 24 ± 7.5). A significant correlation between protein levels of growth factors and MVD was not detected, which might indicate that tumor‐induced angiogenesis is not caused by expression of one single growth factor.
Simultaneous growth factor expression. In order to identify typical growth factor profiles in HNSCC tissues, we focused on the simultaneous expression of angiogenic growth factors (Fig. 2). In all tumors at least three of eight growth factors tested were detectable. Approximately half (46.3%) of the study sample expressed 3–5 growth factors at the same time, while 53.7% of the tumors presented with 6–8 simultaneously expressed growth factors. The most common growth factor pattern in almost all HNSCCs (98%) consisted of a simultaneous expression of HGF, bFGF, and VEGF complemented in 92% of the cases by at least one other growth factor (Table 2). While for bFGF and VEGF a predominant proangiogenic response is well‐known,( 14 ) HGF further seems to play an important role in invasion and spreading of HNSCC cells beside a growth‐promoting effect on endothelial cells.( 16 , 19 , 20 ) To get more insight in HGF‐responsive cells in our study sample, we stained tissue sections of 33 tumors for the expression of the HGF receptor c‐Met (Fig. 1G,H). In all tumor samples c‐Met was found on endothelial cells and to a more variable extent on tumor cells supporting previous observations that both endothelial and tumor cells are targets of HGF‐induced signaling.
Figure 2.
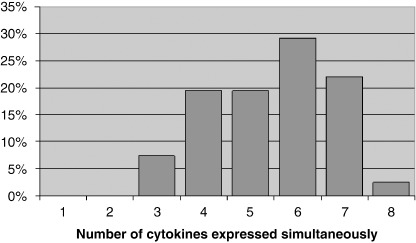
Simultaneous expression of angiogenic factors as determined by ELISA in HNSCC tumor tissue homogenates. Graph depicts how many of eight growth factors analyzed are detectable at the same time in a set of 41 head and neck squamous cell carcinoma (HNSCC) tissues.
Correlation between growth factor expression and patient prognosis. To assess whether detection of a specific growth factor is associated with a poorer clinical prognosis, we compared patients’ overall survival times with quantitative growth factor levels measured in the tumor tissues. Results as determined by log‐rank test revealed that HGF, bFGF and G‐CSF expression levels were significantly related to a worse patient survival (Fig. 3A–C). For HGF, concentrations above a critical threshold of 4000 pg/mg total protein negatively influenced patient outcome (P = 0.05), while bFGF amounts of more than 2000 pg/mg and a G‐CSF threshold of 30 pg/mg total protein had a severe impact on survival (P = 0.008 and P = 0.034, respectively). In addition, multivariate analysis by Cox regression revealed that bFGF amounts of more than 2000 pg/mg total protein were significantly related to an adverse patient outcome independent of HGF and G‐CSF levels (P = 0.013). Altogether, our data demonstrate that elevated protein amounts of HGF, bFGF and G‐CSF in HNSCC tumors result in a significant poorer prognosis of HNSCC patients.
Figure 3.
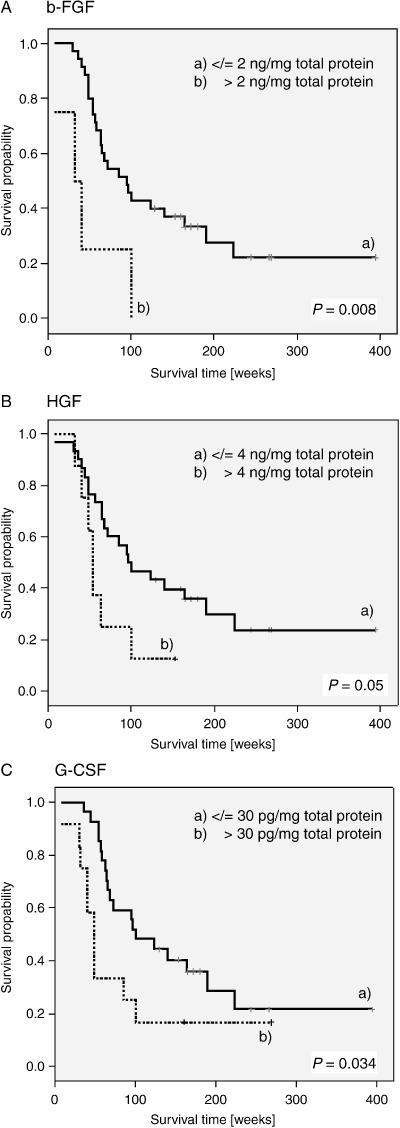
Correlation of growth factor levels and patient survival. Kaplan–Meier survival estimates for (A) basic fibroblast growth factor (bFGF), (B) hepatocyte growth factor (HGF) and (C) granulocyte colony‐stimulating factor (G‐CSF), showing significant differences in patients’ outcomes, depending on high or low protein levels.
Tumor localization‐dependent growth factor profiles. Following the idea of a multi‐target therapy, it is essential to interrupt the interactions of several key factors. Although we have shown that HGF, bFGF and VEGF‐A are compulsive elements of the angiogenic HNSCC profile, it is unclear whether the additional expression of other growth factors in HNSCC is restricted to certain tumor sites or if there are different localization‐dependent expression levels. To answer this question, we compared growth factor amounts of different tumor localizations by Kruskal–Wallis test. Our results demonstrate significantly lower bFGF levels in CUPs compared to that observed in larynx and hypopharynx carcinomas (Fig. 4A), while PDGF‐BB amounts were significantly higher in oropharynx and larynx carcinomas compared to hypopharynx carcinomas (Fig. 4B). Finally, highest G‐CSF levels were seen in larynx carcinomas with a significant difference from CUP tumors (Fig. 4C). Thus, our observations corroborate the hypothesis that in spite of a pattern of growth factors common to almost all HNSCC there are some tumor site‐dependent differences in the expression of angiogenic growth factors.
Figure 4.
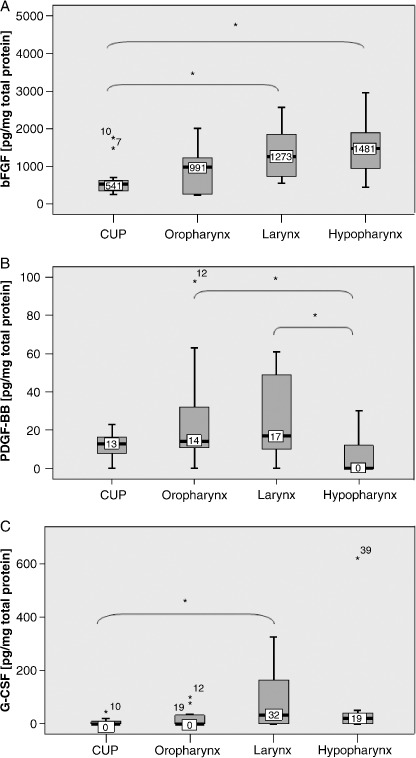
Differences in growth factor amounts depending on tumor localization. Boxplots for (A) basic fibroblast growth factor (bFGF), (B) platelet derived growth factor BB (PDGF‐BB) and (C) granulocyte colony‐stimulating factor (G‐CSF) demonstrating significantly different growth factor amounts depending on tumor localization. *Depicts significant differences between two tumor subgroups.
Interaction between angiogenic growth factors. Interaction between growth factors as well as compensating mechanisms when blocking only one single factor belong to the major obstacles of anti‐angiogenic therapy. To learn more about possible interactions of angiogenic growth factors, we studied whether protein amounts of specific growth factors are related with that of others by Mann–Whitney and Wilcoxon tests. Our analysis revealed significant associations of protein levels for bFGF with HGF (P = 0.012) and G‐CSF (P = 0.001), for G‐CSF with GM‐CSF (P = 0.001) as well as for PDGF‐AB with PDGF‐BB (P = 0.001) and VEGF‐A (P = 0.031). The only growth factor without detectable relation to one of the other molecules was VEGF‐D. These interactions allow an allocation of proteins tested into two groups: on the one hand bFGF links HGF and the G‐CSF and GM‐CSF families. On the other hand there is a significant axis of PDGF‐AB to PDGF‐BB and to VEGF‐A (Fig. 5).
Figure 5.
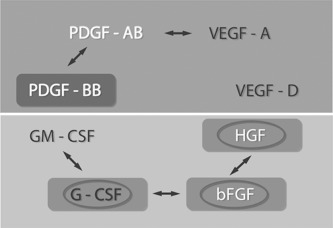
Clinical relevance and interrelation between angiogenic growth factors in head and neck squamous cell carcinoma (HNSCC) tumors:  significant correlations between expression levels of two growth factors;
significant correlations between expression levels of two growth factors;  significant correlation between growth factor amount and patient's prognosis;
significant correlation between growth factor amount and patient's prognosis;  significant difference in growth factor levels depending on tumor localization.
significant difference in growth factor levels depending on tumor localization.
Discussion
Malignant growth and metastasis of solid tumors are dependent on tumor angiogenesis.( 21 ) During the last 30 years a vast number of biomolecules have been identified which are involved in the regulation of tumor angiogenesis.( 22 ) When studying the quantitative expression of eight of these angiogenic growth factors in native HNSCC tissues, independent of tumor localization, the most common pattern we observed in almost all cases (98%) consisted of a simultaneous expression of HGF, bFGF, and VEGF complemented in 92% of the cases by at least one additional growth factor. It is noteworthy that highest protein levels were found for the very same growth factors descending from HGF to bFGF to VEGF. The importance of these growth factors for HNSCC was further corroborated by the observation that growth factor amounts of HGF and bFGF were significantly related to an adverse overall survival. Finally, sub‐analysis of our data regarding HNSCC tumor localization revealed that especially protein levels of CUP tumor tissues seemed to be different from that of the other anatomical sites analyzed.
Besides a confirmation of the expected central role for the angiogenic key regulators VEGF and bFGF,( 23 , 24 ) to our knowledge this is the first report describing a significant correlation of intratumoral HGF levels and a worse clinical outcome in HNSCC patients. In agreement with our data, an important role for HGF was previously assumed based on immunohistochemical analyses of HNSCC tissues demonstrating an overexpression of HGF and its receptor c‐MET.( 25 , 26 ) Concordantly, both c‐MET and HGF were found to be expressed invariably in all tumor tissues independent of tumor localization and reaching highest concentrations of all growth factors analyzed. Similarly, Alexandrakis identified HGF and bFGF levels being significant predictors of survival in myelodysplastic syndromes.( 27 )
In addition, analysis of the hematopoietic growth factors G‐CSF and GM‐CSF was included due to their angiogenesis‐inducing properties.( 28 , 29 ) In line with others,( 30 ) both factors were found in about half the samples analyzed and confirming our former results obtained from HNSCC cell lines, G‐CSF level had a negative impact on patient prognosis. This has particular therapeutic relevance as G‐ and GM‐CSF are used as adjuvants to ameliorate chemo‐ and radiotherapy‐induced neutropenia and mucositis.( 31 ) Consistently, with growing evidence that both factors contribute to the progression of HNSCC and impair long‐term prognosis,( 11 , 31 ) our data suggest further evaluation of their therapeutic use.
Although in a former in vitro study, we reported a significant correlation between the amount of VEGF‐A in HNSCC cell culture supernatant and outcome,( 11 ) surprisingly the correlation of VEGF‐A levels in native tumor tissues with overall survival did not reach statistical significance. These contradictory results fit to a large body of studies on VEGF‐A in HNSCC yielding inconclusive and conflicting results( 32 , 33 ) and might at least in part be attributed to the missing tumor stroma containing additional VEGF‐producing normal cells.( 34 )
In the emerging era of targeted therapy, PDGF‐AB has gained new interest because of the option of a highly selective targeted therapy for tumors such as gastrointestinal stromal tumors (GISTs) with, for example, imatinib, a small molecule and a selective inhibitor of platelet derived growth factor receptor (PDGF‐R).( 35 , 36 , 37 ) The observation that PDGF‐AB and PDGF‐BB were detectable in two‐thirds of the tumors not only underlines their significance as major growth factors in HNSCC but also suggests the application of specific inhibitors of the PDGF pathway in combination with additional antiangiogenic drugs targeting the other angiogenic key players identified in our study.
However, not all of the factors analyzed seem to have the same importance. VEGF‐D exerting growth‐promoting properties in a number of epithelial malignancies only played a subordinate role in HNSCC, as it was found in very low amounts in no more than three tissues.
Except for VEGF‐D, in a previous study we demonstrated a marked angiogenesis‐inducing effect of all growth factors analyzed.( 16 ) However, since c‐MET like the corresponding receptors for VEGF, G‐CSF( 38 ) and GM‐CSF( 31 ) are co‐expressed together with their ligands on HNSCC tumor cells, an additional autocrine function has to be considered. This dual role might explain why we could not relate quantitative growth factor levels to MVD. Lack of correlation might also mirror that angiogenic growth is caused by a mixture of cooperating angiogenic growth factors and is not dependent on one single molecule.
It is supposed that growth factor alterations in regulatory routes within the HNSCC microenvironment play a critical role in tumor aggressiveness.( 39 ) Therefore, a better understanding of inter‐relationships between growth factors is essential to improve application of antiangiogenic as well as antitumor drugs in order to increase clinical perspectives. Statistical analysis revealed significant correlations between five pairs of growth factors. Not very surprisingly, there was an association between the closely related molecules G‐CSF and GM‐CSF on the one hand and PDGF‐AB and PDGF‐BB on the other. In line with this, an upregulated synthesis of G‐CSF caused by GM‐CSF has been described earlier.( 40 ) Most interestingly, there was a similar correlation between the three factors which have shown prognostic relevance in our study: protein levels of bFGF and HGF as well as of bFGF and G‐CSF were significantly related with one another. Lending further support to our findings, upregulation of HGF expression in fibroblasts by tumor‐secreted bFGF has been described by Nakamura and coauthors.( 17 ) Whether bFGF also directly influences G‐CSF levels needs to be analyzed in future studies. However, results of our multivariate analysis showing bFGF to be related to an adverse survival independent of HGF and G‐CSF suggests bFGF to be upstream of HGF and G‐CSF signaling. Finally, for VEGF we only identified a direct correlation with PDGF‐AB which is in agreement with observations on PDGF‐overexpressing in non‐small cell lung cancer cells responding with increased VEGF‐A levels.( 41 ) Altogether, this allows us to define two subgroups of inter‐relating growth factors in our series: VEGF‐A forming one group with the two PDGFs while the three prognostically relevant factors HGF, bFGF, and G‐CSF together with GM‐CSF belong to the second group. Also our data indicate that in particular the members of the latter group might be important candidates for targeted therapeutic strategies.
HNSCCs are heterogeneous tumors arising from different anatomical sites of the upper aero‐digestive tract with different prognoses relying on differences in aggressiveness, rate of metastasis, and also in responsiveness to chemo‐ and radiation therapy.( 42 , 43 , 44 ) As a pilot study, we examined whether there were localization‐dependent differences in growth factor expression by analyzing HNSCC tumors derived from four major localizations including oropharynx, hypopharynx, larynx, as well as CUP. On the one hand, with the expression of HGF, bFGF, and VEGF in almost all tumor tissues we were surprised to find a growth factor pattern common to all anatomical sites. On the other hand, in CUP tissues we found significant lower levels of the prognostically relevant molecules bFGF and G‐CSF compared to hypopharynx and larynx, respectively. This might be attributed to the fact that CUPs are lymph node metastases which are derived from small and therefore undetected primary tumors.( 45 ) However, since our case number was limited this observation deserves further investigation to establish the role of such differences in medical decision making.
In summary, HNSCC are obviously strongly angiogenesis‐dependent emphasizing their suitability for anti‐angiogenic strategies. According to our results, HGF, bFGF, and G‐CSF may serve as prognostic markers in native tissue homogenates, and besides the almost constitutively expressed VEGF‐A, might be interesting candidates for targeted therapies. Significant interactions of certain growth factors point at common regulatory pathways. Their clinical significance and their possible therapeutic use warrant further evaluation.
Acknowledgments
We gratefully acknowledge the help of all medical colleagues in gathering the tumor biopsies. We thank Ms Heike Westphal, Ms Melanie Greibich, and Ms Renate Steinle for expert technical assistance as well as Rolf Warta for critical reading of the manuscript. This work was supported by the Tumorzentrum Heidelberg‐Mannheim (CHM).
References
- 1. Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res 2007; 86: 104–14. [DOI] [PubMed] [Google Scholar]
- 2. Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer 2005; 5: 127–35. [DOI] [PubMed] [Google Scholar]
- 3. Chin D, Boyle GM, Theile DR, Parsons PG, Coman WB. Molecular introduction to head and neck cancer (HNSCC) carcinogenesis. Br J Plast Surg 2004; 57: 595–602. [DOI] [PubMed] [Google Scholar]
- 4. Maula SM, Luukkaa M, Grenman R, Jackson D, Jalkanen S, Ristamaki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res 2003; 63: 1920–6. [PubMed] [Google Scholar]
- 5. St John MA, Abemayor E, Wong DT. Recent new approaches to the treatment of head and neck cancer. Anti-Cancer Drugs 2006; 17: 365–75. [DOI] [PubMed] [Google Scholar]
- 6. Pries R, Nitsch S, Wollenberg B. Role of cytokines in head and neck squamous cell carcinoma. Expert Rev Anticancer Ther 2006; 6: 1195–203. [DOI] [PubMed] [Google Scholar]
- 7. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 8. Folkman J. Tumor angiogensis: role in regulation of tumor growth. The Symp Soc for Dev Biol Soc for Dev Biol 1974; 30: 43–52. [PubMed] [Google Scholar]
- 9. Folkman J, Hanahan D. Switch to the angiogenic phenotype during tumorigenesis. Princess Takamatsu Symp 1991; 22: 339–47. [PubMed] [Google Scholar]
- 10. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3: 401–10. [DOI] [PubMed] [Google Scholar]
- 11. Ninck S, Reisser C, Dyckhoff G, Helmke B, Bauer H, Herold‐Mende C. Expression profiles of angiogenic growth factors in squamous cell carcinomas of the head and neck. Int J Cancer 2003; 106: 34–44. [DOI] [PubMed] [Google Scholar]
- 12. Gospodarowicz D, Cheng J. Growth of myoblasts in lipoprotein‐supplemented, serum‐free medium: regulation of proliferation by acidic and basic fibroblast growth factor. In Vitro Cell Dev Biol 1987; 23: 507–14. [DOI] [PubMed] [Google Scholar]
- 13. Mattern J, Koomagi R, Volm M. Coexpression of VEGF and bFGF in human epidermoid lung carcinoma is associated with increased vessel density. Anticancer Res 1997; 17: 2249–52. [PubMed] [Google Scholar]
- 14. Cao R, Brakenhielm E, Pawliuk R et al . Angiogenic synergism, vascular stability and improvement of hind‐limb ischemia by a combination of PDGF‐BB and FGF‐2. Nat Med 2003; 9: 604–13. [DOI] [PubMed] [Google Scholar]
- 15. Strauss L, Volland D, Kunkel M, Reichert TE. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): possible link between angiogenesis and immune tolerance. Med Sci Monit 2005; 11: BR280–92. [PubMed] [Google Scholar]
- 16. Vasvari GP, Dyckhoff G, Kashfi F et al . Combination of thalidomide and cisplatin in an head and neck squamous cell carcinomas model results in an enhanced antiangiogenic activity in vitro and in vivo . Int J Cancer 2007; 121: 1697–704. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T. Induction of hepatocyte growth factor in fibroblasts by tumor‐derived factors affects invasive growth of tumor cells: in vitro analysis of tumor‐stromal interactions. Cancer Res 1997; 57: 3305–13. [PubMed] [Google Scholar]
- 18. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193: 265–75. [PubMed] [Google Scholar]
- 19. Bussolino F, Di Renzo MF, Ziche M et al . Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 1992; 119: 629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsumoto K, Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem 1994; 269: 31807–13. [PubMed] [Google Scholar]
- 21. Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol 1992; 3: 65–71. [PubMed] [Google Scholar]
- 22. Lutsenko SV, Kiselev SM, Severin SE. Molecular mechanisms of tumor angiogenesis. Biochemistry (Mosc) 2003; 68: 286–300. [DOI] [PubMed] [Google Scholar]
- 23. Tabone MD, Landman‐Parker J, Arcil B et al . Are basic fibroblast growth factor and vascular endothelial growth factor prognostic indicators in pediatric patients with malignant solid tumors? Clin Cancer Res 2001; 7: 538–43. [PubMed] [Google Scholar]
- 24. Szabo S, Sandor Z. The diagnostic and prognostic value of tumor angiogenesis. Eur J Surg Suppl 1998: 99–103. [DOI] [PubMed] [Google Scholar]
- 25. Marshall DD, Kornberg LJ. Overexpression of scatter factor and its receptor (c‐met) in oral squamous cell carcinoma. Laryngoscope 1998; 108: 1413–7. [DOI] [PubMed] [Google Scholar]
- 26. Kim CH, Moon SK, Bae JH et al . Expression of hepatocyte growth factor and c‐Met in hypopharyngeal squamous cell carcinoma. Acta Otolaryngol 2006; 126: 88–94. [DOI] [PubMed] [Google Scholar]
- 27. Alexandrakis MG, Passam FH, Pappa CA et al . Serum evaluation of angiogenic cytokine basic fibroblast growth factor, hepatocyte growth factor and TNF‐alpha in patients with myelodysplastic syndromes. Correlation with Bone Marrow Microvascular Density Int J Immunopathol Pharmacol 2005; 18: 287–95. [DOI] [PubMed] [Google Scholar]
- 28. Bussolino F, Wang JM, Defilippi P et al . Granulocyte‐ and granulocyte‐macrophage‐colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 1989; 337: 471–3. [DOI] [PubMed] [Google Scholar]
- 29. Bussolino F, Ziche M, Wang JM et al . In vitro and in vivo activation of endothelial cells by colony‐stimulating factors. J Clin Invest 1991; 87: 986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsuzuki H, Fujieda S, Sunaga H, Noda I, Saito H. Expression of granulocyte colony‐stimulating factor receptor correlates with prognosis in oral and mesopharyngeal carcinoma. Cancer Res 1998; 58: 794–800. [PubMed] [Google Scholar]
- 31. Gutschalk CM, Herold‐Mende CC, Fusenig NE, Mueller MM. Granulocyte colony‐stimulating factor and granulocyte‐macrophage colony‐stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo . Cancer Res 2006; 66: 8026–36. [DOI] [PubMed] [Google Scholar]
- 32. Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 2005: 1–7. [DOI] [PubMed] [Google Scholar]
- 33. Tse GM, Chan AW, Yu KH et al . Strong immunohistochemical expression of vascular endothelial growth factor predicts overall survival in head and neck squamous cell carcinoma. Ann Surg Oncol 2007; 14: 3558–65. [DOI] [PubMed] [Google Scholar]
- 34. Rosenthal E, McCrory A, Talbert M, Young G, Murphy‐Ullrich J, Gladson C. Elevated expression of TGF‐beta1 in head and neck cancer‐associated fibroblasts. Mol Carcinog 2004; 40: 116–21. [DOI] [PubMed] [Google Scholar]
- 35. Dirnhofer S, Zimpfer A, Went P. [The diagnostic and predictive role of kit (CD117)]. Therapeutische Umschau 2006; 63: 273–8. [DOI] [PubMed] [Google Scholar]
- 36. Gross DJ, Munter G, Bitan M et al . The role imatinib mesylate (Glivec) for treatment patients with malignant endocrine tumors positive for C‐Kit PDGF‐R. Endocrine-Related Cancer 2006; 13: 535–40. [DOI] [PubMed] [Google Scholar]
- 37. Joensuu H, Dimitrijevic S. Tyrosine kinase inhibitor imatinib (STI571) as an anticancer agent for solid tumours. Ann Med 2001; 33: 451–5. [DOI] [PubMed] [Google Scholar]
- 38. Herold‐Mende C, Steiner HH, Andl T et al . Expression and functional significance of vascular endothelial growth factor receptors in human tumor cells. Lab Invest 1999; 79: 1573–82. [PubMed] [Google Scholar]
- 39. Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor Rev 2006; 17: 141–6. [DOI] [PubMed] [Google Scholar]
- 40. Vellenga E, Rambaldi A, Ernst TJ, Ostapovicz D, Griffin JD. Independent regulation of M‐CSF and G‐CSF gene expression in human monocytes. Blood 1988; 71: 1529–32. [PubMed] [Google Scholar]
- 41. Shikada Y, Yonemitsu Y, Koga T et al . Platelet‐derived growth factor‐AA is essential autocrine regulator vascular endothelial growth factor expression non‐small cell lung carcinomas. Cancer Res 2005; 65: 7241–8. [DOI] [PubMed] [Google Scholar]
- 42. Magnano M, Bongioannini G, Lerda W et al . Lymphnode metastasis in head and neck squamous cells carcinoma: multivariate analysis of prognostic variables. J Exp Clin Cancer Res 1999; 18: 79–83. [PubMed] [Google Scholar]
- 43. Singh B, Li R, Xu L et al . Prediction of survival in patients with head and neck cancer using the histoculture drug response assay. Head Neck 2002; 24: 437–42. [DOI] [PubMed] [Google Scholar]
- 44. Gotte K, Tremmel SC, Popp S et al . Intratumoral genomic heterogeneity in advanced head and neck cancer detected by comparative genomic hybridization. Adv Oto-Rhino-Laryngology 2005; 62: 38–48. [DOI] [PubMed] [Google Scholar]
- 45. Grosbach AB. Carcinoma of unknown primary site: a clinical enigma. Arch Intern Med 1982; 142: 357–9. [PubMed] [Google Scholar]


