Abstract
Chicken ovalbumin upstream promoter transcription factors (COUP‐TF) are orphan members of the nuclear receptor superfamily and consist of COUP‐TFI and COUP‐TFII. COUP‐TFI was reported to be overexpressed in human breast cancer and to promote estrogen‐independent transcriptional activity of estrogen receptor α. COUP‐TFII, however, has not been examined in the breast. Therefore, we carried out immunohistochemical analysis of COUP‐TFII in human breast cancer in order to clarify its biological and clinical significance. We immunolocalized COUP‐TFII in 119 human breast cancers and correlated the findings with various clinicopathological parameters. Fifty‐nine percent of the cases were immunohistochemically positive for COUP‐TFII. COUP‐TFII positivity was correlated with poor clinical outcome, and a statistically significant correlation was detected between COUP‐TFII and the following clinicopathological parameters: clinical stage, lymph node status, histological grade, and estrogen receptor α status. In addition, short interfering RNA‐mediated knockdown of COUP‐TFII in the breast carcinoma cell line MCF‐7 decreased the level of vascular endothelial growth factor‐C mRNA expression, which is a known inducer of lymphangiogenesis and lymph node metastasis. These results suggest that COUP‐TFII is involved in the development of advanced human breast cancer. (Cancer Sci 2009; 100: 639–645)
Abbreviations:
- ANG
angiopoietin
- COUP‐TF
chicken ovalbumin upstream promoter transcription factor
- DFS
disease‐free survival
- ER
estrogen receptor
- HER
human epidermal growth factor receptor
- LI
labeling index
- OS
overall survival
- PR
progesterone receptor
- RT
reverse transcription
- PCR
polymerase chain reaction
- SDS‐PAGE
sodium dodecylsulfate–polyacrylamide gel electrophoresis
- siRNA
short interfering RNA
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Chicken ovalbumin upstream promoter transcription factors are orphan members of the nuclear receptor superfamily.( 1 ) They belong to the steroid and thyroid hormone superfamily of nuclear receptors and are involved in regulating the expression of various genes.( 1 ) COUP‐TF were initially characterized in chick oviduct( 2 ) and HeLa extracts,( 3 ) where they bind as dimers to the COUP element of the ovalbumin gene promoter to activate transcription.( 2 ) COUP‐TF were therefore initially characterized as transcriptional activators of the chicken ovalbumin gene but are currently also considered as repressors of the transcriptional activity of other nuclear hormone receptors( 4 ) such as retinoic acid receptor, thyroid hormone receptor, peroxisome proliferator‐activated receptor, and vitamin D receptor, via direct interaction, competing for their DNA binding sites, or by heterodimerization with retinoid X receptor.( 5 , 6 ) There are two COUP‐TF genes reported in mammals, COUP‐TFI and COUP‐TFII. These two COUP‐TF genes are closely correlated, with an overall amino acid identity of 87%. They are evolutionarily conserved in the DNA binding domain as well as the ligand‐binding domain, which suggests that they are relatively primordial members of the nuclear receptor family and have important biological functions.( 7 )
In human breast cancer, the expression of COUP‐TFI was reported to be increased in carcinoma cells compared with normal cells and to promote estrogen‐independent transcriptional activity of ERα.( 8 , 9 ) Therefore, the expression of COUP‐TFI in ERα‐positive breast cancer is reasonably postulated to be involved in its progression. In addition, high amounts of COUP‐TFII expression were reported in invasive lung carcinoma cell lines( 10 ) and in dedifferentiated endometrial( 11 ) and ovarian cancer cell lines.( 12 ) All of these reporteds findings suggest that COUP‐TFII expression may affect breast cancer cell progression through modulation of other nuclear receptors such as ERα, as well as COUP‐TFI, or through other pathways. However, the clinical significance of COUP‐TFII in breast carcinoma remains virtually unknown.
Therefore, in the present study, we examined the immunolocalization of COU‐TFII in 119 cases of human breast carcinoma, and correlated these findings with various clinicopathological parameters in order to clarify the biological and clinical significance of this orphan nuclear receptor in human breast cancer progression. In addition, we examined the potential regulation of VEGF‐C mRNA expression by COUP‐TFII using the breast carcinoma cell line MCF‐7 and the siRNA‐mediated knockdown method.
Materials and Methods
Patients and tissue preparation. 119 specimens of invasive ductal carcinoma of the breast were retrieved from the pathology archives of the Department of Surgery, Tohoku University Hospital, Sendai, Japan. Breast tissue specimens were obtained from female patients with a mean age of 53.2 years (range 22–81 years) who underwent mastectomy from 1988 to 2000. The patients did not receive chemotherapy or irradiation prior to surgery. 67 of the patients received tamoxifen therapy after surgery. The median follow‐up time was 94 months (range 3–151 months). The histological grade of each specimen was evaluated based on the method of Elston and Ellis.( 13 ) All of the specimens had been fixed with 10% formalin and embedded in paraffin wax. The research protocols for this study were approved by the Ethics Committee at Tohoku University School of Medicine (approval number 2004‐146).
Antibodies. Mouse monoclonal antibody for COUP‐TFII (H7147), ERα (1D5), PR (MAB429), and Ki‐67 (MIB1) were purchased from Perseus Proteomics (Tokyo, Japan), Immunotech (Marseille, France), Chemicon (Temecula, CA, USA) and Dako (Copenhagen, Denmark), respectively. Rabbit polyclonal antibody for HER‐2/neu (A0485) was purchased from Dako.
Immunohistochemistry. A Histofine Kit (Nichirei, Tokyo, Japan), which uses the streptavidin–biotin amplification method, was used for immunostaining in this study. Antigen retrieval for COUP‐TFII, ERα, PR, Ki‐67, and HER‐2/neu was carried out by heating the slides in an autoclave at 120°C for 5 min in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate, pH 6.0). The dilutions of the primary antibodies used in this study were as follows: COUP‐TFII, 1/250; ERα, 1/50; PR, 1/30; Ki‐67, 1/100; and HER2/neu, 1/200. The antigen–antibody complexes were visualized with 3,3′‐diaminobenzidine solution (1 mM, in 50 mM Tris‐HCl buffer, pH 7.6, and 0.006% H2O2), and counterstained with hematoxylin. Adrenal gland was used as a positive control for COUP‐TFII. As a negative control, normal, rabbit, or mouse IgG (subclass‐matched) was used instead of the primary antibodies.
Scoring of immunoreactivity. Immunoreactivity for COUP‐TFII, ERα, PR, and Ki‐67 was detected in the nucleus. Immunoreactivity was evaluated in more than 1000 carcinoma cells, and moderately or highly immunostained cells were counted for all of the antigens examined. The percentage of immunoreactivity (i.e. LI) was subsequently obtained. For ERα, PR, and Ki‐67, cases with a LI of more than 10% were determined to be positive.( 14 , 15 , 16 ) For the analysis evaluating the possible correlation between COUP‐TFII status and clinical outcome, the cases were classified into three different groups according to COUP‐TFII LI (++, >50% LI; +, 10–49% LI; and –, 0–9% LI). Subsequently, survival curve analysis according to the Kaplan–Meier method and univariate or multivariate analyses were carried out. HER2 score was also evaluated as follows: 0, none; 1+, faint and focal membrane staining of the tumor; 2+, moderate membrane staining of >10% of the tumor; and 3+, intense membrane staining of >10% of the tumor. Cases of 3+ were considered positive in the present study.
Cell culture. The human breast carcinoma cell line MCF‐7 was provided by the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan). MCF‐7 cells were cultured in RPMI‐1640 (Sigma‐Aldrich, St Louis, MO, USA) with 10% fetal bovine serum (JRH Bioscience, Lenexa, KS, USA).
RNA interference‐mediated knockdown of endogenous COUP‐TFII. siRNA oligonucleotides of COUP‐TFII were used for the knockdown of endogenous protein expression using Silencer Pre‐designed siRNA (Ambion, Austin, TX, USA), and Silencer Negative Control#1 siRNA (Ambion) was used as a negative control. The siRNA sequences against COUP‐TFII are summarized as follows: ID 5922, sense 5′‐GGCCAUAGUCCUGUUCACCtt‐3′ and antisense 5′‐GGUGAACAGGACUAUGGCCtt‐3′; ID 139844, sense 5′‐GGAACCACAUAUAACACUUtt‐3′ and antisense 5′‐AAGUGUUAUAUGUGGUUCCtc‐3′. siRNA (10 nM) was transfected using HiperFect transfection reagent (Qiagen, Hilden, Germany) according to the instruction manual.
Immunoblotting. The cell protein was extracted using M‐PER Mammalian Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL, USA) with Halt Protease Inhibitor Cocktail (Pierce Biotechnology) 3 days after siRNA transfection. The protein concentrations were determined using a protein assay kit (Wako Pure Chemical Industries, Osaka, Japan). The whole‐cell extracts (20 µg of the protein) were subjected to SDS‐PAGE (10% acrylamide gel). Following SDS‐PAGE, proteins were transferred onto Hybond P polyvinylidene difluoride membranes (GE Healthcare, Buckinghamshire, UK). The blots were then blocked in 5% non‐fat dry skim milk for 1 h at room temperature, and were subsequently incubated with a primary antibody for COUP‐TFII or β‐actin (Sigma‐Aldrich) for 24 h at 4°C. The dilutions of primary antibodies used were as follows: COUP‐TFII, 1/1000; β‐actin, 1/1000. After incubation with anti‐mouse IgG–horseradish peroxidase (GE Healthcare) for 1 h at room temperature, antibody–protein complexes on the blots were detected using ECL‐plus western blotting detection reagents (GE Healthcare). The protein bands were then visualized using an LAS‐1000 image analyzer (Fuji Photo Film, Tokyo, Japan).
Reverse transcription–polymerase chain reaction analysis. Total RNA was extracted using TRIzol Reagent (Invitrogen Carlsbad, CA, USA) 3 days after siRNA transfection, and cDNA was synthesized using a QuantiTect reverse transcription kit (Qiagen). Real‐time PCR was carried out using the LightCycler System and FastStart DNA Master SYBR Green I (Roche Diagnostics, Mannheim Germany). The PCR primer sequence of VEGF‐C and the ribosomal protein L13A (RPL13A) used in the present study were as follows: VEGF‐C (NM_005429), forward 5′‐CAAGGCCCCAAACCAGTA‐3′ and reverse 5′‐GTCTTGTTCGCTGCCTGA‐3′; RPL13A (NM_012423), forward 5′‐CCTGGAGGAGAAGAGGAAAGAGA‐3′ and reverse 5′‐TTGAGGACCTCTGTGTATTTGTCAA‐3′. cDNA of known VEGF‐C concentration, and the housekeeping gene RPL13A were used to generate standard curves for real‐time quantitative PCR in order to determine the quantity of target cDNA transcript. The mRNA level in each case was represented as a ratio of RLP13A and was evaluated as a ratio (%) compared with the control.
Statistical analysis. Statistical differences were examined using StatView 5.0 J software (SAS Institute, Cary, NC, USA). In the comparison study between COUP‐TFII immunoreactivity and several clinicopathological parameters, COUP‐TFII LI was used because no rational thresholds were presented so far, and the value was demonstrated as the mean ± SEM. The statistical analyses of COUP‐TFII LI between dichotomous groups, such as menopausal status, tumor size, lymph node status, ERα status, PR status, Ki‐67 status, and HER2 status, were carried out using the Mann–Whitney U‐test. anova and Fisher's Protected Least Significant Difference (PLSD) test were also used for multiple comparisons of COUP‐TFII LI among each group of different clinical stages and histological grades, and the P‐values obtained were subjected to Bonferroni's correction. OS and DFS curves were generated according to the Kaplan–Meier method by classifying all of the patients into ‘++’ and ‘– or +’ groups, and statistical significance was calculated using the log‐rank test. Cox's proportional hazards model was used for univariate and multivariate analyses using the dichotomous groups of COUP‐TFII LI described above. A value of P < 0.05 was considered statistically significant.
Results
Immunohistochemical analysis of COUP‐TFII in 119 human breast carcinomas. COUP‐TFII immunoreactivity was detected in the nuclei of carcinoma cells (Fig. 1c,d). COUP‐TFII immunoreactivity was also detected in scattered foci of normal epithelial cells adjacent to the carcinoma but its relative immunointensity was markedly low compared to in carcinoma cells (Fig. 1b). The number of cases with immunopositive COUP‐TFII in each group was summarized as follows: –, 49 cases (41.2%); +, 44 cases (37.0%); and ++, 26 cases (21.8%).
Figure 1.
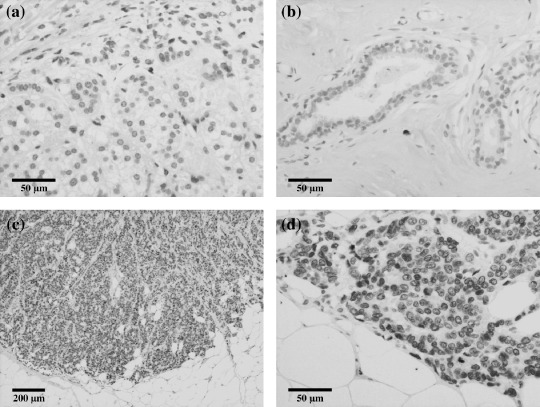
Representative illustrations of chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II immunohistochemistry in invasive ductal carcinomas of the breast. (a) Adrenal gland was used as a positive control for immunohistochemistry. (b) Immunoreactivity for COUP‐TFII was detected in a few normal epithelial cells but its relative immunointensity was low compared to that in carcinoma cells. (c,d) Marked COUP‐TFII immunoreactivity was detected in carcinoma cells of invasive ductal carcinoma. Low (c) and High (d) magnification.
Correlation between COUP‐TFII immunoreactivity and clinical outcome in the 119 patients. DFS and OS curves for the three groups of patients according to COUP‐TFII status (–, +, and ++) demonstrated a positive correlation between COUP‐TFII status and adverse clinical outcomes (Supporting Information Fig. 1). However, the tendency of the two groups ‘–’ and ‘+’ were relatively similar. The data from these two groups were subsequently merged and the prognostic analysis above was carried out using dichotomous data on the basis of classification into two patient groups, ‘– or +’ and ‘++’. The DFS and OS curves in these analyses are shown in Figure 2. The statistical analysis demonstrated that high COUP‐TFII status (++) in breast carcinoma cases was significantly associated with poor survival or adverse clinical outcome (log‐rank test: DFS, P = 0.0224; OS, P = 0.0183). The results of univariate analysis (1, 2) demonstrated that lymph node status (DFS, P < 0.0001; OS, P = 0.0001), histological grade (DFS, P = 0.0043; OS, P = 0.0019), tumor size (DFS, P = 0.0048; OS, P = 0.0143), COUP‐TFII status (DFS, P = 0.0266; OS, P = 0.0234), and HER2 status (DFS, P = 0.0172) were all significant prognostic factors for DFS and OS in the 119 patients examined. A subsequent multivariate analysis, however, revealed that lymph node status (DFS, P < 0.0001; OS, P = 0.0044), histological grade (OS, P = 0.0161), and HER2 status (DFS, P = 0.0231) were independent prognostic factors for DFS and OS in these patients, but COUP‐TFII status was not (DFS, P = 0.8974; OS, P = 0.4020) (1, 2).
Figure 2.
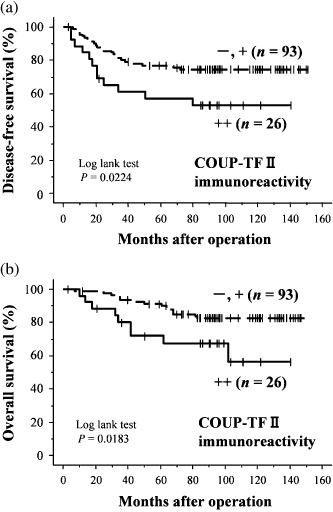
(a) Disease‐free and (b) overall survival of 119 patients with breast carcinoma according to the status of chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II immunoreactivity of carcinoma cells (Kaplan–Meier method). Cases were classified into two groups according to COUP‐TFII labeling index (LI): ++, >50% LI; +, 10–49% LI; and –, 0–9% LI. COUP‐TFII status was significantly associated with an increased risk of adverse clinical outcome.
Table 1.
Univariate and multivariate analysis of disease‐free survival in 119 breast cancer patients examined
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| P‐value | Relative risk (95% CI) | P‐value | Relative risk (95% CI) | |
| Lymph node status (positive/negative) | <0.0001 | 9.561 (4.162–21.963) | <0.0001 | 7.934 (3.272–19.241) |
| Histological grade (3/1,2) | 0.0043 | 2.640 (1.357–5.139) | 0.0708 | 1.919 (0.946–3.893) |
| Tumor size (≥2.5 cm/<2.5 cm) | 0.0048 | 3.117 (1.415–6.869) | 0.2603 | 1.621 (0.699–3.758) |
| COUP‐TF status (++/–, +) | 0.0266 | 2.205 (1.096–4.436) | 0.8974 | 1.051(0.491–2.254) |
| Estrogen receptor α status (positive/negative) | 0.2571 | 0.673 (0.339–1.336) | ||
| Ki‐67 status (positive/negative) | 0.7724 | 0.906 (0.464–1.770) | ||
| HER2 status (positive/negative) | 0.0172 | 2.306 (1.160–4.585) | 0.0231 | 2.278 (1.119–4.634) |
Data considered significant (P < 0.05) in the univariate analysis are shown in bold, and were examined in the multivariate analysis. CI, confidence interval; COUP‐TF, chicken ovalbumin upstream promoter transcription factor; HER, human epidermal growth factor receptor.
Table 2.
Univariate and multivariate analysis of over‐all survival in 119 breast cancer patients examined
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| P‐value | Relative risk (95% CI) | P‐value | Relative risk (95% CI) | |
| Lymph node status (positive/negative) | 0.0001 | 6.204 (2.458–15.660) | 0.0044 | 4.338 (1.582–11.897) |
| Histological grade (3/1,2) | 0.0019 | 3.719 (1.623–8.521) | 0.0161 | 2.888 (1.217–6.850) |
| Tumor size (≥2.5 cm/<2.5 cm) | 0.0143 | 3.427 (1.278–9.187) | 0.2413 | 1.860 (0.659–5.254) |
| COUP‐TF status (++/–, +) | 0.0234 | 2.605 (1.138–5.964) | 0.4020 | 1.467 (0.599–3.596) |
| Estrogen receptor α status (positive/negative) | 0.1679 | 0.565 (0.251–1.272) | ||
| Ki‐67 status (positive/negative) | 0.2209 | 1.734 (0.718–4.184) | ||
| HER2 status (positive/negative) | 0.0644 | 2.184 (0.954–4.996) | ||
Data considered significant (P < 0.05) in the univariate analysis are shown in bold, and were examined in the multivariate analysis. CI, confidence interval; COUP‐TF, chicken ovalbumin upstream promoter transcription factor; HER, human epidermal growth factor receptor.
Correlation between COUP‐TFII LI and clinicopathological variables in 119 breast carcinoma patients. The associations between COUP‐TFII LI and the clinicopathological variables in 119 breast carcinomas are summarized in Table 3. COUP‐TFII LI was significantly associated with clinical stage (I vs IV, P = 0.0222), lymph node status (P = 0.0369), histological grade (1 vs 3, P = 0.0222), and ERα status (P = 0.0050). No significant correlations were detected between COUP‐TFII LI and menopausal status (P = 0.7616), tumor size (P = 0.582), PR status (P = 0.2964), Ki‐67 status (P = 0.1157), or HER2 status (P = 0.8364).
Table 3.
Association between chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II immunoreactivity and clinicopathological parameters in 119 breast carcinomas
| Variable | Patient number | COUP‐TFII labeling index (%) | P‐value | P‐value adjusted by Bonferroni |
|---|---|---|---|---|
| Menopausal status | ||||
| pre‐ | 51 | 24.9 ± 3.4 | ||
| post‐ | 68 | 27.5 ± 3.3 | 0.7616 | |
| Stage | ||||
| I | 32 | 21.9 ± 4.5 | ||
| II | 58 | 22.6 ± 3.0 | 0.9071 (I vs II) | |
| III | 13 | 26.0 ± 7.6 | 0.6247 (I vs III) | |
| IV | 16 | 44.8 ± 7.4 | 0.0037 (I vs IV) | 0.0222 |
| Tumor size (cm) | ||||
| ≥2.5 | 65 | 26.6 ± 3.1 | ||
| <2.5 | 54 | 24.8 ± 3.6 | 0.582 | |
| Lymph node status | ||||
| Positive | 46 | 34.1 ± 4.3 | ||
| Negative | 73 | 20.5 ± 2.6 | 0.0369 | |
| Histological grade | ||||
| 1 (well) | 34 | 15.3 ± 3.3 | ||
| 2 (moderate) | 44 | 28.6 ± 4.0 | 0.0231 (1 vs 2) | 0.0693 |
| 3 (poor) | 41 | 31.3 ± 4.4 | 0.0074 (1 vs 3) | 0.0222 |
| Estrogen receptor α status | ||||
| Positive | 83 | 29.8 ± 3.0 | ||
| Negative | 36 | 16.5 ± 3.6 | 0.0050 | |
| Progesterone receptor status | ||||
| Positive | 78 | 26.7 ± 2.9 | ||
| Negative | 41 | 24.0 ± 4.2 | 0.2964 | |
| Ki‐67 status | ||||
| Positive | 72 | 28.8 ± 3.1 | ||
| Negative | 47 | 21.1 ± 3.6 | 0.1157 | |
| HER2 status | ||||
| Positive | 27 | 28.2 ± 5.5 | ||
| Negative | 92 | 25.4 ± 2.6 | 0.8364 | |
Data are presented as means ± SEM. All other values represent the number of cases. P‐values less than 0.05 were considered significant, and are shown in bold. HER, XXX.
Knockdown of COUP‐TFII protein and downregulation of VEGF‐C mRNA expression. Decreased amounts of COUP‐TFII protein were also detected by immunoblotting analysis 3 days following the transfection of specific siRNA (Fig. 3a). VEGF‐C mRNA expression was decreased (Fig. 3b) in both COUP‐TFII‐knockdown cell lines compared to the cells transfected with non‐specific siRNA.
Figure 3.
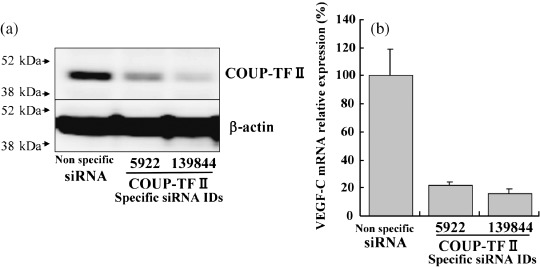
Short interfering RNA (siRNA)‐mediated knockdown of endogenous chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II protein in MCF‐7 cells. Knockdown cells were subjected to immunoblotting and reverse transcription–polymerase chain reaction analysis 3 days after transfection of siRNA. (a) COUP‐TFII immunoreactivity was detected in the protein extracts of cells transfected with non‐specific siRNA (lane 1). COUP‐TFII immunoreactivity was decreased in the cells treated with COUP‐TFII‐specific siRNA (lanes 2 and 3). Vascular endothelial growth factor (VEGF)‐C mRNA expression was also repressed in the cells treated with COUP‐TFII‐specific siRNA compared to cells treated with non‐specific siRNA in the reverse transcription–polymerase chain reaction analysis.
Discussion
To the best of our knowledge, this is the first report to demonstrate immunolocalization of COUP‐TFII in human breast carcinoma. Our results demonstrated that 59% of the cases examined had moderate (+, 37.0%) or high (++, 21.8%) positivity in the nuclei of carcinoma cells. The rate of COUP‐TFII‐positive cases was relatively high compared with the number of cases positive for ERα (69.7%) and PR (65.5%) in our present study. The prognostic analyses in our present study indicated that a relatively high abundance of COUP‐TFII in breast carcinoma cells was significantly associated with poor or adverse clinical outcome. The results of multivariate analysis demonstrated that a relatively high abundance of COUP‐TFII was not necessarily an independent prognostic factor, but in the multivariate analysis without including the ‘lymph node’ factor, COUP‐TFII status reached statistical significance in terms of an independent prognostic factor (DFS, P = 00359; OS, P = 0.0356). Therefore, the COUP‐TFII status of carcinoma cells is postulated, at least partly, to be involved in the process of lymph node metastasis.
Chicken ovalbumin upstream promoter transcription factor II LI and lymph node status were significantly correlated, as expected based on the prognostic analysis described above. Schafer et al. reported that VEGF‐D gene expression is directly regulated by hepatocyte nuclear factor (HNF)‐4α, COUP‐TFI, and COUP‐TFII through binding of these orphan nuclear receptors to the response element existing in the VEGF‐D gene promoter.( 17 ) VEGF‐C and VEGF‐D were originally discovered as members of the VEGF family, which is involved in lymphangiogenesis as well as angiogenesis( 18 ) via binding to VEGFR‐2 and VEGFR‐3 expressed on the surface of vascular and lymphatic endothelial cells.( 18 ) Skobe et al. reported that VEGF‐C expression in breast tumor cells induces intratumoral lymphangiogenesis and enhances metastasis to the lymph nodes.( 19 ) Sun et al. also reported that migration of human lymphatic endothelial cells cocultured with human breast carcinoma cell line MCF‐7 transfected with siRNA against VEGF‐C was significantly suppressed.( 20 ) These findings above indicate the importance of evaluating VEGF‐C and VEGF‐D regulation by COUP‐TFII in human breast carcinoma cells. In our present study, the expression of VEGF‐C mRNA was markedly suppressed in the COUP‐TFII‐knockdown MCF‐7 cells, as expected. The expression of VEGF‐D in MCF‐7 cell was extremely low, but decreased expression of VEGF‐D was detected in these circumstances (data not shown). COUP‐TFII LI was also significantly higher in the tissue of carcinoma cases diagnosed with stage IV compared with other clinical stages. These findings strongly suggest that COUP‐TFII regulates the expression of VEGF‐C or VEGF‐D in breast carcinoma cells and contributes to lymph node or distant metastasis, which consequently results in poor patient prognosis.
Pereira et al. reported that targeted deletion of the COUP‐TFII gene results in embryonic lethality associated with defects in angiogenesis and decreased expression of the angiogenesis factor ANG‐1.( 21 ) This result suggests that COUP‐TFII regulates the organized process of angiogenesis during development and that COUP‐TFII accelerates angiogenesis in human breast carcinoma by inducing ANG‐1 expression. However, the results of the COUP‐TFII knockdown assay in our study demonstrated that decreased ANG‐1 mRNA expression was not necessarily detected in the RT‐PCR analysis because of extremely low ANG‐1 expression in MCF‐7 cells. Therefore, the regulatory mechanism of ANG‐1 by COUP‐TFII awaits further investigation for clarification.
Pereira et al. also reported that COUP‐TFII is markedly expressed in mesenchymal cells that undergo differentiation to epithelium, but not in terminally differentiated epithelium.( 22 ) We have previously reported that COUP‐TFII expression is not detected in the great majority of glandular epithelium of non‐pathological human adult breast tissue.( 23 ) These results are clearly consistent with our present results in which the expression of COUP‐TFII tended to be more abundant in poorly differentiated high histological‐grade carcinoma cases. COUP‐TFII is, therefore, considered to be involved in dedifferentiation of cancer cells in advanced breast cancer.
Riggs et al. demonstrated that treating MCF‐7 cells with estradiol increases COUP‐TFII mRNA expression, and transient transfection of MCF‐7 with siRNA against ERα decreases the expression of COUP‐TFII mRNA.( 24 ) These results are consistent with our finding in which the COUP‐TFII LI was significantly higher in the ERα‐positive group than in the ERα‐negative group. Therefore, ERα is also considered to be one of the regulatory factors for COUP‐TFII expression, which may explain why a correlation between COUP‐TFII LI and ERα status was detected in our present study. In this study, the prognostic analysis indicated that COUP‐TFII status was associated with a worse prognosis or clinical outcome for the patients, and a correlation between COUP‐TFII LI and ERα status was detected. However, it is also true that ERα is generally considered to be a modest but good prognostic factor( 25 ) in breast carcinoma patients because it is a potential target of hormone therapy. In fact, the results of the univariate analysis in our present study demonstrated that ERα status in carcinoma cells tended to be a good prognostic factor (ERα‐positive patients: DFS, relative risk 0.673; OS, relative risk 0.565) even though this association did not reach statistical significance (DFS, P = 0.2571; OS: P = 0.1679), possibly due to the relatively small number of cases examined. More et al. reported that COUP‐TFII is present in several ERα‐negative breast cancer cell lines, such as MDA‐MB‐231, HS‐578, MT‐SV1‐7, and SK‐BR‐3.( 26 ) In addition, they demonstrated that epidermal growth factor and transforming growth factor‐α, both of which are well‐known growth factors for breast carcinoma cells,( 27 ) induce COUP‐TFII mRNA expression in MCF‐7 cells.( 26 ) Therefore, the expression of COUP‐TFII may be considered to be regulated not only by ERα but also other factors in breast carcinoma cells. COUP‐TFII induced by factors other than ERα is reasonably postulated not to be affected by hormonal therapy and to induce aggressive events such as lymphangiogenesis, which bestows a poor prognosis regardless of the patients’ ERα status. Indeed, the expression of COUP‐TFII was associated with poor prognosis and adverse clinical outcome in each group of ERα‐positive (n = 83) and ERα‐negative (n = 36) patients in the same fashion in our study (Supporting information Fig. 2).
Riggs et al. reported that COUP‐TFII expression is required for growth inhibition induced by tamoxifen treatment,( 24 ) even though the status of COUP‐TFII in breast carcinoma tissue was not associated with the therapeutic effects of tamoxifene in our study (data not shown). In addition, Nakshatri et al. demonstrated that COUP‐TFII induces both p21 expression and growth inhibition in breast cancer cells.( 28 ) Therefore, together with our data, the expression of COUP‐TFII could be detrimental to cell growth in vitro, whereas its expression may affect the microenvironment of tumor tissue in which lymphangiogenesis or angiogenesis are induced. This effect is considered to overcome the detrimental effects described above and contributes to poor prognosis.
In summary, we demonstrated the immunolocalization of COUP‐TFII in human breast carcinoma. COUP‐TFII immunoreactivity was correlated with several adverse clinicopathological parameters associated with aggressive biological behavior and a worse prognosis for patients. Therefore, COUP‐TFII is considered to be involved in the development of advanced human breast carcinoma, possibly via lymphangiogenesis and angiogenesis.
Supporting information
Fig. 1. (a) Disease‐free and (b) overall survival of 119 patients with breast carcinoma according to the status of chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II immunoreactivity of carcinoma cells (Kaplan–Meier method). Cases were classified into three different groups according to COUP‐TFII labeling index (LI): ++, >50% LI; +, 10–49% LI; and –, 0–9% LI.
Fig. 2. Overall survival of patients with estrogen receptor (ER) α (a) negative and (b) positive breast carcinoma according to the status of chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II immunoreactivity (Kaplan–Meier method). Cases were classified into two groups according to COUP‐TFII LI: ++, >50% LI; +, 10–49% LI; and –, 0–9% LI.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Acknowledgments
We appreciate the skillful technical assistance of Mr Katsuhiko Ono, Ms Miki Mori, and Ms Ikumi Miura (Department of Pathology, Tohoku University School of Medicine). This work was partly supported by grants from the Japanese Ministry of Health, Labour, and Welfare for Research on Intractable Diseases, Risk Analysis Research on Food and Pharmaceuticals, and Development of Multidisciplinary Treatment Algorithm with Biomarkers and Modeling of the Decision‐making Process with Artificial Intelligence for Primary Breast Cancer. This work was also partly supported by a Grant‐in‐Aid for Scientific Research (18390109) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, and the Yasuda Medical Foundation.
References
- 1. Tsai SY, Tsai MJ. Chick ovalbumin upstream promoter‐transcription factors (COUP‐TFs): coming of age. Endocr Rev 1997; 18: 229–40. [DOI] [PubMed] [Google Scholar]
- 2. Sagami I, Tsai SY, Wang H, Tsai MJ, O'Malley BW. Identification of two factors required for transcription of the ovalbumin gene. Mol Cell Biol 1986; 6: 4259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang LH, Tsai SY, Sagami I, Tsai MJ, O'Malley BW. Purification and characterization of chicken ovalbumin upstream promoter transcription factor from HeLa cells. J Biol Chem 1987; 262: 16 080–6. [PubMed] [Google Scholar]
- 4. Park JI, Tsai SY, Tsai MJ. Molecular mechanism of chicken ovalbumin upstream promoter‐transcription factor (COUP‐TF) actions. Keio J Med 2003; 52: 174–81. [DOI] [PubMed] [Google Scholar]
- 5. Cooney AJ, Leng X, Tsai SY, O'Malley BW, Tsai MJ. Multiple mechanisms of chicken ovalbumin upstream promoter transcription factor‐dependent repression of transactivation by the vitamin D, thyroid hormone, and retinoic acid receptors. J Biol Chem 1993; 268: 4152–60. [PubMed] [Google Scholar]
- 6. Kliewer SA, Umesono K, Heyman RA, Mangelsdorf DJ, Dyck JA, Evans RM. Retinoid X receptor–COUP‐TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci USA 1992; 89: 1448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang LH, Ing NH, Tsai SY, O'Malley BW, Tsai MJ. The COUP‐TFs compose a family of functionally related transcription factors. Gene Expr 1991; 1: 207–16. [PMC free article] [PubMed] [Google Scholar]
- 8. Metivier R, Gay FA, Hubner MR et al . Formation of an hER alpha‐COUP‐TFI complex enhances hER alpha AF‐1 through Ser118 phosphorylation by MAPK. EMBO J 2002; 21: 3443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Dily F, Metivier R, Gueguen MM et al . COUP‐TFI modulates estrogen signaling and influences proliferation, survival and migration of breast cancer cells. Breast Cancer Res Treat 2008; 110: 69–83. [DOI] [PubMed] [Google Scholar]
- 10. Navab R, Gonzalez‐Santos JM, Johnston MR et al . Expression of chicken ovalbumin upstream promoter‐transcription factor II enhances invasiveness of human lung carcinoma cells. Cancer Res 2004; 64: 5097–105. [DOI] [PubMed] [Google Scholar]
- 11. Kieback DG, Levi T, Kohlberger P et al . Chicken ovalbumin upstream promoter‐transcription factor (COUP‐TF) expression in human endometrial cancer cell lines. Anticancer Res 1996; 16: 3371–6. [PubMed] [Google Scholar]
- 12. Kieback DG, Runnebaum IB, Moebus VJ et al . Chicken ovalbumin upstream promoter transcription factor (COUP‐TF): an orphan steroid receptor with a specific pattern of differential expression in human ovarian cancer cell lines. Gynecol Oncol 1993; 51: 167–70. [DOI] [PubMed] [Google Scholar]
- 13. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long‐term follow‐up. Histopathology 1991; 19: 403–410 [DOI] [PubMed] [Google Scholar]
- 14. Suzuki T, Inoue A, Miki Y et al . Early growth responsive gene 3 in human breast carcinoma: a regulator of estrogen‐meditated invasion and a potent prognostic factor. Endocr Relat Cancer 2007; 14: 279–92. [DOI] [PubMed] [Google Scholar]
- 15. Honma N, Horii R, Iwase T et al . Clinical importance of estrogen receptor‐β evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol 2008; 26: 3727–34. [DOI] [PubMed] [Google Scholar]
- 16. Ogawa Y, Hai E, Matsumoto K et al . Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol 2008; 13: 431–5. [DOI] [PubMed] [Google Scholar]
- 17. Schafer G, Wissmann C, Hertel J, Lunyak V, Hocker M. Regulation of vascular endothelial growth factor D by orphan receptors hepatocyte nuclear factor‐4 alpha and chicken ovalbumin upstream promoter transcription factors 1 and 2. Cancer Res 2008; 68: 457–66. [DOI] [PubMed] [Google Scholar]
- 18. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002; 2: 573–83. [DOI] [PubMed] [Google Scholar]
- 19. Skobe M, Hawighorst T, Jackson DG et al . Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 20. Sun P, Gao J, Liu YL, Wei LW, Wu LP, Liu ZY. RNA interference (RNAi)‐mediated vascular endothelial growth factor‐C (VEGF‐C) reduction interferes with lymphangiogenesis and enhances epirubicin sensitivity of breast cancer cells. Mol Cell Biochem 2008; 308: 161–8. [DOI] [PubMed] [Google Scholar]
- 21. Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY. The orphan nuclear receptor COUP‐TFII is required for angiogenesis and heart development. Genes Dev 1999; 13: 1037–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pereira FA, Qiu Y, Tsai MJ, Tsai SY. Chicken ovalbumin upstream promoter transcription factor (COUP‐TF): expression during mouse embryogenesis. J Steroid Biochem Mol Biol 1995; 53: 503–8. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki T, Moriya T, Darnel AD, Takeyama J, Sasano H. Immunohistochemical distribution of chicken ovalbumin upstream promoter transcription factor II in human tissues. Mol Cell Endocrinol 2000; 164: 69–75. [DOI] [PubMed] [Google Scholar]
- 24. Riggs KA, Wickramasinghe NS, Cochrum RK, Watts MB, Klinge CM. Decreased chicken ovalbumin upstream promoter transcription factor II expression in tamoxifen‐resistant breast cancer cells. Cancer Res 2006; 66: 10 188–98. [DOI] [PubMed] [Google Scholar]
- 25. Pentheroudakis G, Kalogeras KT, Wirtz RM et al . Gene expression of estrogen receptor, progesterone receptor and microtubule‐associated protein Tau in high‐risk early breast cancer: a quest for molecular predictors of treatment benefit in the context of a Hellenic Cooperative Oncology Group trial. Breast Cancer Res Treat 2008. Available from URL: http://www.springerlink.com/content/t56x2545068w4320/. [DOI] [PubMed] [Google Scholar]
- 26. More E, Fellner T, Doppelmayr H et al . Activation of the MAP kinase pathway induces chicken ovalbumin upstream promoter‐transcription factor II (COUP‐TFII) expression in human breast cancer cell lines. J Endocrinol 2003; 176: 83–94. [DOI] [PubMed] [Google Scholar]
- 27. Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast Cancer Res Treat 2006; 95: 211–18. [DOI] [PubMed] [Google Scholar]
- 28. Nakshatri H, Mendonca MS, Bhat‐Nakshatri P, Patel NM, Goulet RJ Jr, Cornetta K. The orphan receptor COUP‐TFII regulates G2/M progression of breast cancer cells by modulating the expression/activity of p21 (WAF1/CIP1), cyclin D1, and cdk2. Biochem Biophys Res Commun 2000; 270: 1144–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. (a) Disease‐free and (b) overall survival of 119 patients with breast carcinoma according to the status of chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II immunoreactivity of carcinoma cells (Kaplan–Meier method). Cases were classified into three different groups according to COUP‐TFII labeling index (LI): ++, >50% LI; +, 10–49% LI; and –, 0–9% LI.
Fig. 2. Overall survival of patients with estrogen receptor (ER) α (a) negative and (b) positive breast carcinoma according to the status of chicken ovalbumin upstream promoter transcription factor (COUP‐TF) II immunoreactivity (Kaplan–Meier method). Cases were classified into two groups according to COUP‐TFII LI: ++, >50% LI; +, 10–49% LI; and –, 0–9% LI.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item


