Abstract
Tumor‐associated macrophages (TAM) of M2 phenotype promote tumor proliferation and are associated with a poor prognosis in patients with glioblastoma. We screened the natural compounds possessing an inhibitory effect on M2 polarization in human monocyte‐derived macrophages. Among 130 purified natural compounds examined, corosolic acid significantly inhibited the expression of CD163, one of the phenotype markers of M2 macrophages, and also suppressed the secretion of IL‐10, one of the anti‐inflammatory cytokines preferentially produced by M2 macrophages, thus suggesting that corosolic acid suppresses M2 polarization of macrophages. Furthermore, corosolic acid inhibited the proliferation of glioblastoma cells, U373 and T98G, and the activation of signal transducer and activator of transcription‐3 (STAT3) and nuclear factor‐kappa B (NF‐κB) in both human macrophages and glioblastoma cells. These results indicate that corosolic acid suppresses the M2 polarization of macrophages and tumor cell proliferation by inhibiting both STAT3 and NF‐κB activation. Therefore, corosolic acid might be a potential new tool for tumor prevention and therapy. (Cancer Sci 2011; 102: 206–211)
Macrophages infiltrating in cancer tissues are referred to as tumor‐associated macrophages (TAM) and they are closely involved in the development of the tumor microenvironment.( 1 , 2 , 3 ) Tumor‐associated macrophages are considered to belong to alternatively activated macrophages (M2) because of their anti‐inflammatory functions.( 4 , 5 ) In many kinds of tumors, the presence of TAM is associated with a poor prognosis for patients.( 3 , 6 , 7 )
Macrophage subpopulations have a different type of receptor expression and cytokine production.( 5 , 8 , 9 , 10 ) Classically activated macrophages (M1 macrophages) have the IL‐12high, IL‐23high and IL‐10low phenotypes and produce nitrogen intermediates and inflammatory cytokines, such as IL‐1β, tumor necrosis factor‐α (TNF‐α) and IL‐6.( 5 , 8 , 9 , 10 , 11 ) In contrast, alternatively activated macrophages (M2 macrophages) have the IL‐12low, IL‐23low and IL‐10high phenotypes and also show high expression of several receptors such as the class A scavenger receptor (SR‐A, CD204), the mannose receptor, CD163, dectin‐1 and DC‐SIGN.( 5 , 8 , 9 , 10 , 11 ) Furthermore, it is well known that M1 macrophages are potent effecter cells integrated in Th1 responses, which kill microorganisms and tumor cells and produce copious amounts of proinflammatory cytokines. In contrast, M2 macrophages regulate inflammatory responses and adaptive type I immunity, scavenge debris and promote angiogenesis, tumor progression, tissue remodeling and repair.
We previously demonstrated CD163 to be a useful marker for detecting M2 cells on paraffin‐embedded surgical specimens.( 12 ) In human glioblastoma, the proportion of CD163‐positive M2 TAM are closely involved in tumor cell proliferation and are also associated with a poor prognosis, whereas the total number of macrophages is not.( 13 ) These observations therefore indicate the significance of macrophage differentiation in tumor development.
Signal transducer and activator of transcription 3 (STAT3) is involved in the tumor microenvironment and tumor development due to its association with immunosuppression, angiogenesis and cancer cell proliferation.( 14 ) Therefore, STAT3 is considered to be an important target molecule for anti‐cancer therapy, and many researchers have so far reported the importance of various STAT3 inhibitors in anti‐cancer therapy.( 15 ) STAT3 signaling in macrophages is well known to be involved in the regulation of immune responses in the murine model,( 16 , 17 ) and STAT3 activation is essential for macrophage differentiation toward the M2 phenotype.( 18 ) Furthermore, NF‐κB activation plays an important role in macrophage differentiation toward the M2 phenotype( 19 ) and cancer cell proliferation.( 20 , 21 )
In this study, we prepared 130 purified compounds from natural products and measured their inhibitory effect on M2 polarization in human monocyte‐derived macrophages (HMDM) in order to identify potentially useful candidate agents for cancer immunotherapy.
Materials and Methods
Cells and cell culture conditions. The human glioblastoma cell lines, T98G and U373‐MG (U373), THP‐1 macrophages and human dermal fibroblasts were purchased from American Type Culture Collection (Manassas, VA, USA) and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 u/mL penicillin, 100 μg/mL streptomycin and 0.1 mg/mL sodium pyruvate. Tumor culture supernatants (TCS) were prepared, as described previously.( 13 )
Peripheral blood mononuclear cells were obtained from healthy volunteer donors. Informed written consent was obtained from all healthy donors. CD14+ monocytes were purified from peripheral blood mononuclear cells by positive selection via magnetic‐activated cell sorting technology (Miltenyi Biotec, Bergisch Gladbach, Germany). The monocytes were cultured in DMEM supplemented with 10% FBS and 10 ng/mL granulocyte macrophage colony‐stimulating factor (GM‐CSF, Wako, Tokyo, Japan) for 5 days in order to differentiate them from macrophages.
Preparation of natural compounds. We selected 130 purified natural compounds having famous bioactive structure, such as flavonoid compounds, triterpenoid compounds and steroid compounds, from our natural compound library. The purified natural compounds were dissolved in DMSO to a 10 mM stock solution.
Extraction and isolation of corosolic acid from apple pomace. Apple pomace was extracted with mixed solution of MeOH and CHCl3 (1:1) by refluxing for 2 h, and the extract was concentrated in vacuo to obtain residues. The residues were loaded onto a Diaion HP‐20 column (Mitsubishi Chemical, Tokyo, Japan) and eluted with H2O and MeOH. The MeOH eluate was separated by silica gel column (Kantochemical Co. Inc.; Tokyo, Japan) and eluted with mixed solution of hexane and ethyl acetate to give a corosolic acid containing fraction and other compounds containing fractions. The corosolic acid containing fraction was further purified by silica gel column and eluted with a mixed solution of CHCl3 and ethyl acetate to give pure corosolic acid. The corosolic acid was dissolved in DMSO to a 100 mM stock solution.
Determination of the inhibitory effect of natural compounds on CD163 expression. The HMDM (5 × 104 cells per well of a 96‐well plate) were incubated with natural compounds (30 μM) for 24 h after treatment with IL‐10 (20 nM) or TCS for 2 days, followed by the determination of CD163 expression by cell enzyme‐linked immunosorbent assay (Cell‐ELISA).
Cell‐ELISA. Expression of CD163 on HMDM was evaluated using Cell‐ELISA, as described previously.( 12 ) Briefly, each well of a 96‐well plate was blocked with Block Ace (DS Pharma Biomedical, Osaka, Japan) and washed three times with PBS containing 0.05% Tween 20 (washing buffer). The wells were incubated with anti‐CD163 antibody, AM‐3K, (2 μg/mL) dissolved in washing buffer for 1 h. The wells were then washed with washing buffer three times and reacted with horseradish peroxidase (HRP) conjugated with anti‐mouse IgG antibody, followed by a reaction with ULTRASENSITIVE TMB (Moss INC., Pasadena, MD, USA). The reaction was then terminated by the addition of 1 M sulfuric acid, and the absorbance at 450 nm was read by a micro‐ELISA plate reader.
Determination of the inhibitory effect of natural compounds on IL‐10, IL‐6 and IL‐12 secretion. The HMDM and THP‐1 macrophages (5 × 104 cells per well of 96‐well plate) were stimulated with LPS (100 ng/mL) for 24 h after incubation with corosolic acid (30 μM) for 24 h in the presence of TCS, followed by the determination of IL‐10, IL‐6 and IL‐12 secretion by means of an ELISA kit (eBioscience, San Diego, CA, USA).
Immunohistochemistry. Cell block specimens were fixed in 10% neutral‐buffered formalin and then embedded in paraffin. The sections were deparaffinized in xylene and rehydrated in a graded ethanol series. After the reaction of anti‐phosphorylated STAT3 antibody (D3A7), the samples were incubated with HRP‐labeled goat anti‐mouse antibody (Nichirei, Tokyo, Japan). The reaction was visualized with the use of the diaminobenzidine substrate system (Vector, Burlingame, CA, USA).
STAT3 and JAK activation assay. STAT3 and JAK activation were determined by measuring the increased expression of the phosphorylated STAT3 and phosphorylated JAK by western blot analysis. Human monocyte‐derived macrophages were solubilized with Triton X‐100, and the protein concentration was determined using the BCA protein assay reagent, followed by pretreatment by boiling for 5 min in 2% SDS and 2‐mercaptoethanol. The protein (10 μg) was run on a 10% SDS‐polyacrylamide gel and transferred to a polyvinylidine fluoride (PVDF) transfer membrane (Millipore, Bedford, MA, USA). To detect phosphorylated STAT3, the membranes were exposed to an anti‐phosphorylated STAT3 antibody (D3A7)( 22 ) and visualized by HRP‐conjugated anti‐rabbit IgG antibody with ECL western blotting detection reagent (GE Healthcare Japan, Tokyo, Japan). The molecular size of phospho STAT3 detected by this immunoblot was approximately 80 kDa.
To detect STAT3, the membranes were exposed to an anti‐STAT3 antibody (sc‐8019; Santa Cruz Biotech, Santa Cruz, CA, USA)( 23 ) and visualized by HRP‐conjugated anti‐mouse IgG antibody with ECL western blotting detection reagent. The molecular size of STAT3 detected by this immunoblot was approximately 80 kDa.
To detect phosphorylated JAK2, the membranes were exposed to an anti‐phosphorylated JAK2 antibody (sc‐16566‐R; Santa Cruz Biotech.)( 24 ) and visualized by HRP‐conjugated anti‐rabbit IgG antibody with ECL western blotting detection reagent (GE Healthcare Japan). The molecular size of phospho JAK2 detected by this immunoblot was approximately 130 kDa. These membranes were re‐blotted with an anti‐β‐actin antibody as an internal calibration control.
NF‐κB activation assay. NF‐κB activation was determined by measuring the increased expression of the p65 subunit of NF‐κB by western blot analysis. The cells were homogenized in 30 mM Tris‐HCl buffer containing 10 mM EGTA, 5 mM EDTA, 1% Triton X‐100, 250 mM sucrose, 1 mM NaF, 1 mM Phenylmethylsulfonyl fluoride, 15 μg/mL aprotinin, 5 μg/mL leupeptin, 5 μg/mL pepstatin and 1 mM Na2VO4. The homogenate was centrifuged at 5000 g for 5 min at 4°C to remove cell debris, and the resulting supernatant (40 μg protein) was separated on a 10% SDS‐polyacrylamide gel and transferred to a PVDF transfer membrane (Millipore). The membranes were exposed to an anti‐phosphorylated NF‐κB antibody (sc‐101748; Santa Cruz Biotech.)( 25 ) and visualized by HRP‐conjugated anti‐rabbit IgG antibody with ECL western blotting detection reagent (GE Healthcare Japan). The molecular size of phospho NF‐κB detected by this immunoblot was approximately 65 kDa.
Cell proliferation and cytotoxic assay. Briefly, 1 × 104 T98G and U373 cells were cultured in a 96‐well plate in quadruplicate before treatment. The cells were then cultured in the presence of corosolic acid. Cell viability was determined using the WST assay (WST‐8 cell counting kit; Dojin Chemical, Kumamoto, Japan) according to the manufacturer’s protocol. For analysis of cytotoxic activity, the amount of lactase dehydrogenase (LDH) released into the culture supernatant was calculated by means of the LDH release assay (LDH‐cytotoxic test kit; Wako).
Flow cytometry. For analysis of cleaved caspase‐3 in cell lines, the cells were fixed with 4% paraformaldehyde and permeabilized with 1% saponin. Thereafter, the cells were stained with anti‐cleaved caspase‐3 antibody (Cell Signaling Tech, Danvers, MA, USA).
Statistics. All data are representative of two or three independent experiments. Data are expressed as means ± SD. Mann–Whitney’s U‐test was used for two‐group comparison. A value of P < 0.05 was considered statistically significant.
Results
Effect of corosolic acid on M2 macrophage polarization and glioblastoma cell proliferation. First, we measured the effect of 130 natural compounds on IL‐10‐induced CD163 expression as a M2 phenotype marker in HMDM. Under the assay conditions used, some natural compounds such as corosolic acid, tigogenin, aucubin, timosaponin AIII and neoaspidistrin suppressed CD163 expression (Fig. 1). Among these compounds, corosolic acid (Fig. 2A), one of the triterpenoid compounds, significantly suppressed IL‐10‐induced CD163 expression (Fig. 1A). Therefore, we chose corosolic acid for further evaluation. Next, we measured the effect of corosolic acid on the expression of CD163 and secretion of IL‐10, IL‐6 and IL‐12 in HMDM and THP‐1 macrophages induced by TCS of the glioblastoma cell line, U373 cells. As shown in Figure 2, TCS increased CD163 expression (Fig. 2B), IL‐10 secretion (Fig. 2C,D) and M2 phenotype markers, and decreased IL‐6 and IL‐12 secretion (Fig. 2E,F) and M1 phenotype markers in the HMDM and THP‐1 macrophages. Under the assay conditions used, corosolic acid significantly suppressed TCS‐induced CD163 expression (Fig. 2B) and IL‐10 secretion (Fig. 2C,D), and enhanced IL‐6 and IL‐12 secretion reduced by the TCS treatment (Fig. 2E,F). These data strongly indicate that corosolic acid changes M2 polarization to M1 polarization in human macrophages. Furthermore, we measured the effect of corosolic acid on the cell proliferation of glioblastoma cells, because the proportion of CD163‐positive M2 TAM are closely involved in tumor cell proliferation and are also associated with a poor prognosis in human glioblastoma,( 13 ) and corosolic acid has also been reported to induce apoptosis in human cervical carcinoma HeLa cells.( 26 ) As a result, corosolic acid inhibited the proliferation of U373 cells (Fig. 3A,B) and T98G cells (Fig. 3C,D) in a dose‐dependent manner, whereas corosolic acid did not affect cell survival in human peripheral lymphocyte (HPL) HMDM and human dermal fibroblast (HDF) (Fig. 3E). These data suggest that effective concentration of corosolic acid (30–100 μM) on glioblastoma cell death does not affect normal cell viability. Therefore, we then examined whether corosolic acid activates caspase‐3 or not. As shown in Figure 3F, corosolic acid activated caspase‐3 in U373 cells, thus indicating that corosolic acid also induces apoptosis in glioblastoma cells.
Figure 1.
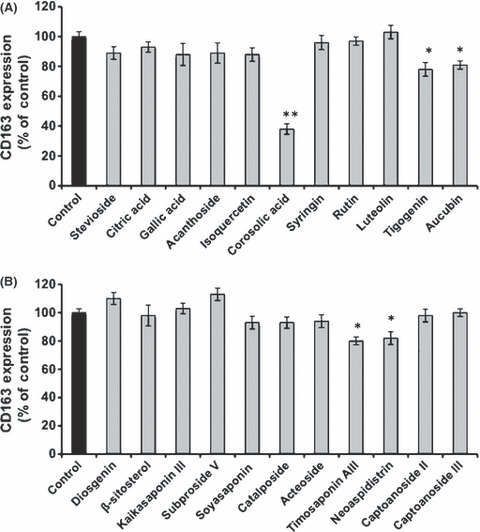
Effect of natural compounds on CD163 expression. Human monocyte‐derived macrophages (5 × 104 cells per well of a 96‐well plate) were incubated with natural compounds (30 μM) for 24 h after treatment with IL‐10 (20 nM) for 2 days, followed by the determination of CD163 expression by Cell‐ELISA, as described in the Materials and Methods. The data are presented as mean ± SD. *P < 0.01, **P < 0.001 vs control.
Figure 2.
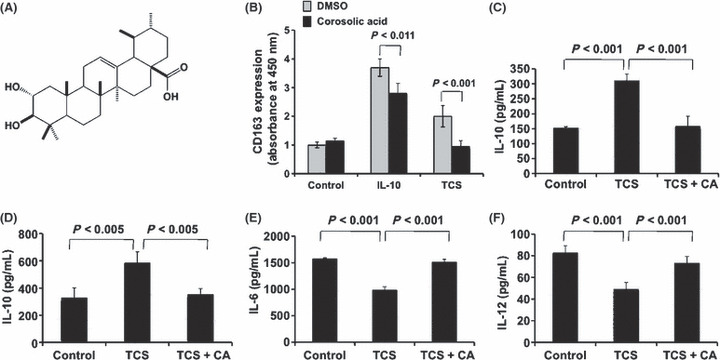
Effect of corosolic acid on CD163 expression and IL‐10, IL‐6 and IL‐12 secretion. (A) Chemical structure of corosolic acid. (B) Human monocyte‐derived macrophages (HMDM) (5 × 104 cells per well of a 96‐well plate) were incubated with corosolic acid (30 μM) for 24 h after treatment with IL‐10 (20 nM) or tumor culture supernatants (TCS) for 2 days, followed by determination of CD163 expression by cell enzyme‐linked immunosorbent assay (Cell‐ELISA), as described in the Materials and Methods. The HMDM (C) and THP‐1 macrophages (D) were stimulated with LPS (100 ng/mL) for 24 h after incubation with corosolic acid (30 μM) for 24 h in the presence of TCS, followed by the determination of IL‐10 secretion by ELISA, as described in the Materials and Methods. The HMDM were stimulated with LPS (100 ng/mL) for 24 h after incubation with corosolic acid (30 μM) for 24 h in the presence of TCS, followed by the determination of IL‐6 (E) and IL‐12 (F) secretion by ELISA, as described in the Materials and Methods.
Figure 3.
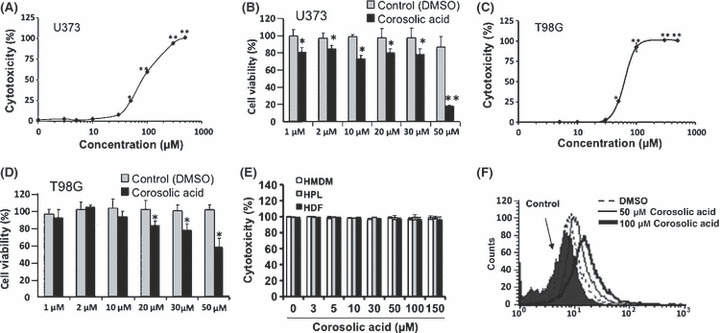
Effect of corosolic acid on cell proliferation in glioblastoma cells and normal cells. U373 cells were incubated with the indicated concentrations of corosolic acid for 24 h, followed by the determination of cell proliferation by both WST‐8 assay (A) and LDH assay (B), as described in the Materials and Methods. The T98G cells were incubated with the indicated concentrations of corosolic acid for 24 h, followed by the determination of cell proliferation by both WST‐8 assay (C) and LDH assay (D), as described in the Materials and Methods. Human monocyte‐derived macrophages (HMDM), human peripheral lymphocyte (HPL) and human dermal fibroblast (HDF) were incubated with the indicated concentrations of corosolic acid for 24 h, followed by the determination of cell proliferation by LDH assay (E), as described in the Materials and Methods. The U373 cells were incubated with corosolic acid for 4 h, and cleaved caspase‐3 was detected by flow cytometry, as described in the Materials and Methods (F). The data are presented as mean ± SD. *P < 0.01, **P < 0.001 vs control.
Effect of corosolic acid on the JAK‐STAT signaling pathway and NF‐κB activation in human macrophages. Since activation of STAT3 and NF‐κB contributes to the M2 polarization of macrophages( 18 , 19 ) and TCS of glioblastoma induces STAT3 and NF‐κB activation, we next investigated the effect of corosolic acid on activation of STAT3 and NF‐κB in human macrophages. As shown in Figure 4A,B, TCS of glioblastoma induced STAT3 activation in THP‐1 macrophages. Under the assay conditions used, corosolic acid inhibited TCS‐induced STAT3 activation in a dose dependent manner. Furthermore, corosolic acid also inhibited the IL‐10‐ and TCS‐induced activation of JAK‐STAT3 and NF‐κB in HMDM (Fig. 4C). These results suggest that corosolic acid inhibits M2 polarization of human macrophages by suppressing the JAK‐STAT and NF‐κB signaling pathway.
Figure 4.
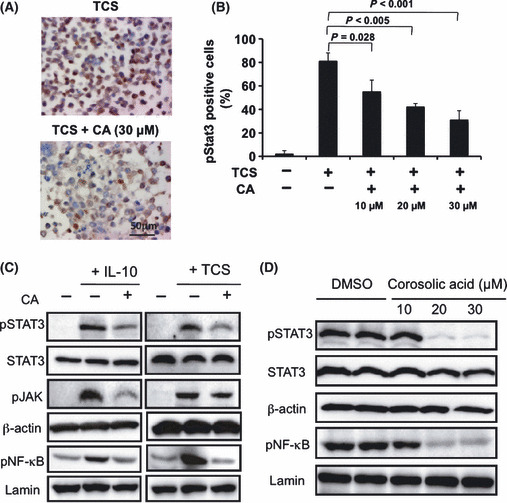
Effect of corosolic acid on signal transducer and activator of transcription‐3 (STAT3) and nuclear factor‐kappa B (NF‐κB) activation in human monocyte‐derived macrophages (HMDM) and glioblastoma cells. (A) The THP‐1 macrophages were incubated with the indicated concentration of corosolic acid for 1 h after treatment with tumor culture supernatants (TCS) for 24 h, followed by determination of phosphorylated STAT3 expression by immunohistochemistry, as described in the Materials and Methods. (B) The number of macrophages positive for phosphorylated STAT3. (C) The HMDM were incubated with corosolic acid (30 μM) for 1 h after treatment with IL‐10 (20 nM) or TCS for 24 h, followed by the determination of phosphorylated STAT3, STAT3, phosphorylated JAK, phosphorylated NF‐κB, β‐actin and lamin expression by western blot analysis, as described in the Materials and Methods. (D) The U373 cells were incubated with the indicated concentrations of corosolic acid for 1 h, followed by determination of phosphorylated STAT3, STAT3, phosphorylated NF‐κB, β‐actin and lamin expression by western blot analysis, as described in the Materials and Methods.
Effect of corosolic acid on STAT3 and NF‐κB activation in glioblastoma cells. It is clear that activation of STAT3 and NF‐κB is critically involved in tumorigenesis,( 27 , 28 ) and STAT3 is considered to be an important target molecule for anti‐cancer therapy including glioblastoma.( 29 , 30 ) Therefore, we also investigated the effect of corosolic acid on STAT3 and NF‐κB activation in glioblastoma cells. As shown in Figure 4D, STAT3 and NF‐κB were constantly activated in U373 glioblastoma cells. Under assay conditions, corosolic acid inhibited the constitutive activation of STAT3 and NF‐κB at concentrations of 20 μM and higher. These data suggest that corosolic acid inhibits the STAT3 and NF‐κB activation, not only in macrophages but also in glioblastoma cells, and corosolic acid suppresses glioblastoma cell proliferation by inhibiting STAT3 and NF‐κB activation.
Discussion
It is well known that TAM play an important role in cancer growth. The TAM release many proangiogenic cytokines and growth factors, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), colony stimulation factor‐1 (CSF‐1), platelet‐derived growth factor (PDGF) and basic fibroblast growth factor to promote tumor progression. They also produce arginase‐1, IL‐10 and transforming growth factor‐β (TGF‐β), which inhibit the antitumor function of T cells and natural killer cells.( 1 , 2 , 3 ) In our recent study, we revealed that macrophages account for a major share of the cells that infiltrate glioblastomas and their polarization toward an M2 phenotype is significantly associated with a poor prognosis in those patients.( 13 ) We also found that tumor‐derived factors induce STAT3 activation in TAM, thereby leading them to differentiate themselves into M2 macrophages (unpublished data). Therefore, it is speculated that the inhibition of macrophage polarization toward the M2 phenotype could thus possibly be a new strategy for anticancer therapy.
In the present study, we used CD163 as a marker of the M2 phenotype of macrophages. CD163, a member of the scavenger receptor cysteine‐rich protein superfamily, is a receptor for the hemoglobin–haptoglobin (Hb‐Hp) complex, erythroblasts, TNF‐like weak inducer of apoptosis (TWEAK), and porcine reproductive and respiratory syndrome virus.( 31 , 32 , 33 , 34 ) CD163 also binds bacteria and induces the production of proinflammatory cytokines.( 35 ) It is reported that binding of the Hb‐Hp complex to CD163‐bearing cells elicited potent interleukin‐10 secretion and this was inhibited by the anti‐CD163 antibody.( 36 ) It is also reported that CD163 upregulates HO‐1 expression.( 37 ) These data indicate that CD163 is actively involved in the anti‐inflammatory function of M2 macrophages, although the precise ligand–receptor–effector pathway is not clear yet.
In this study, we prepared 130 purified compounds from natural products and screened their inhibitory effect on the M2 polarization of human monocyte‐derived macrophages. In this screening, we identified several natural compounds, such as corosolic acid, tigogenin and aucubin, which all have an inhibitory effect on CD163 expression, which is a maker of the M2 phenotype (Fig. 1). Among these compounds, corosolic acid significantly inhibited CD163 expression (Fig. 1). Therefore, we chose corosolic acid as a candidate agent for anticancer therapy.
Corosolic acid (Fig. 2A), a triterpenoid compound, is contained in several plants such as banaba leaves, Eriobotrya japonica leaves and apples. The compound possesses various biological properties, including anti‐diabetic, anti‐obesity and anti‐oxidative activities.( 38 , 39 , 40 )
The current study demonstrated that corosolic acid inhibited IL‐10‐ and TCS‐induced CD163 expression and TCS‐induced IL‐10 secretion and enhanced IL‐6 and IL‐12 secretion reduced by TCS treatment in human macrophages (Fig. 2), whereas corosolic acid did not affect cell survival in HMDM (Fig. 3E), thus suggesting that corosolic acid changes M2 polarization to M1 polarization in human macrophages.
In a previous study, STAT3 and NF‐κB activation were revealed to contribute to the M2 polarization of macrophages.( 18 , 41 ) Corosolic acid significantly suppressed JAK‐STAT3 and NF‐κB activation in human macrophages (Fig. 4A–C), thus indicating that corosolic acid inhibits M2 polarization by suppressing the JAK‐STAT and NF‐κB signaling pathway. Recently, it is known that STAT3 and NF‐κB activation also correlate with tumor cell proliferation, and various STAT3 inhibitors have been reported by many researchers with the aim of identifying a new anticancer therapy.( 15 ) Corosolic acid inhibited cell proliferation in U373 glioblastoma cells by suppressing both STAT3 and NF‐κB activation (Fig. 4D). Furthermore, corosolic acid also suppresses STAT3 activity on osteosarcoma cells and ovarian carcinoma cells (data not shown, manuscript in preparation), and enhanced the anticancer effect of CDDP (Fig. S1), suggesting that the combination of corosolic acid and anticancer agents might be useful for anticancer therapy.
Recently, corosolic acid has been reported to induce apoptosis through the mitochondrial pathway and caspase activation in human cervical carcinoma HeLa cells.( 26 ) In the present study, corosolic acid also induced caspase activation in human glioblastoma cells (Fig. 3F). Therefore, corosolic acid might induce tumor apoptosis by both induction of caspase activation and inhibition of STAT3 and NF‐κB activation. Furthermore, corosolic acid also inhibits macrophage polarization of the M2 phenotype associated with tumor proliferation. These data suggest that corosolic acid might be a potentially useful new compound for anticancer therapy.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Combination effect of corosolic acid and CDDP on the proliferation of glioblastoma cells.
Supporting info item
Acknowledgments
The authors thank Mr Takenobu Nakagawa, Mrs Emi Kiyota, Mr Osamu Nakamura and Ms Yui Hayashida for their technical assistance. This study was supported in part by Grants‐in‐Aid for Scientific Research (B20390113) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Mantovani A, Schioppa T, Biswas SK, Marchesi F, Allavena P, Sica A. Tumor‐associated macrophages and dendritic cells as prototypic type II polarized myeloid populations. Tumori 2003; 89: 459–68. [DOI] [PubMed] [Google Scholar]
- 2. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour‐associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti‐cancer therapy. Eur J Cancer 2006; 42: 717–27. [DOI] [PubMed] [Google Scholar]
- 3. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006; 66: 605–12. [DOI] [PubMed] [Google Scholar]
- 4. Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF‐kappaB. Blood 2009; 113: 3139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 6. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9: 239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sica A, Larghi P, Mancino A et al. Macrophage polarization in tumour progression. Semin Cancer Biol 2008; 18: 349–55. [DOI] [PubMed] [Google Scholar]
- 8. Mosser DM. The many faces of macrophage activation. J Leukoc Biol 2003; 73: 209–12. [DOI] [PubMed] [Google Scholar]
- 9. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25: 677–86. [DOI] [PubMed] [Google Scholar]
- 10. Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen‐presenting cells. Immunity 1999; 10: 137–42. [DOI] [PubMed] [Google Scholar]
- 11. Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte‐to‐macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006; 177: 7303–11. [DOI] [PubMed] [Google Scholar]
- 12. Komohara Y, Hirahara J, Horikawa T et al. AM‐3K, an anti‐macrophage antibody, recognizes CD163, a molecule associated with an anti‐inflammatory macrophage phenotype. J Histochem Cytochem 2006; 54: 763–71. [DOI] [PubMed] [Google Scholar]
- 13. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti‐inflammatory macrophage phenotype in growth of human gliomas. J Pathol 2008; 216: 15–24. [DOI] [PubMed] [Google Scholar]
- 14. Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7: 41–51. [DOI] [PubMed] [Google Scholar]
- 15. Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs 2005; 16: 601–7. [DOI] [PubMed] [Google Scholar]
- 16. Matsukawa A, Takeda K, Kudo S, Maeda T, Kagayama M, Akira S. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J Immunol 2003; 171: 6198–205. [DOI] [PubMed] [Google Scholar]
- 17. Takeda K, Clausen BE, Kaisho T et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 1999; 10: 39–49. [DOI] [PubMed] [Google Scholar]
- 18. Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007; 117: 1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–55. [DOI] [PubMed] [Google Scholar]
- 20. Haybaeck J, Zeller N, Wolf MJ et al. A lymphotoxin‐driven pathway to hepatocellular carcinoma. Cancer Cell 2009; 16: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pikarsky E, Porat RM, Stein I et al. NF‐kappaB functions as a tumour promoter in inflammation‐associated cancer. Nature 2004; 431: 461–6. [DOI] [PubMed] [Google Scholar]
- 22. Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol 2000; 13: 47–50. [DOI] [PubMed] [Google Scholar]
- 23. Duan HO, Simpson‐Haidaris PJ. Cell type‐specific differential induction of the human gamma‐fibrinogen promoter by interleukin‐6. J Biol Chem 2006; 281: 12451–7. [DOI] [PubMed] [Google Scholar]
- 24. Tang Y, Zheng S, Chen A. Curcumin eliminates leptin’s effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology 2009; 150: 3011–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vermeulen L, De WG, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF‐kappaB p65 subunit by mitogen‐ and stress‐activated protein kinase‐1 (MSK1). EMBO J 2003; 22: 1313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, Ge R, Du J et al. Corosolic acid induces apoptosis through mitochondrial pathway and caspase activation in human cervix adenocarcinoma HeLa cells. Cancer Lett 2009; 284: 229–37. [DOI] [PubMed] [Google Scholar]
- 27. Thoennissen NH, Iwanski GB, Doan NB et al. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res 2009; 69: 5876–84. [DOI] [PubMed] [Google Scholar]
- 28. Gurova KV, Hill JE, Guo C et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF‐kappaB‐dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA 2005; 102: 17448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fuh B, Sobo M, Cen L et al. LLL‐3 inhibits STAT3 activity, suppresses glioblastoma cell growth and prolongs survival in a mouse glioblastoma model. Br J Cancer 2009; 100: 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwamaru A, Szymanski S, Iwado E et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 2007; 26: 2435–44. [DOI] [PubMed] [Google Scholar]
- 31. Fabriek BO, Polfliet MM, Vloet RP et al. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood 2007; 109: 5223–9. [DOI] [PubMed] [Google Scholar]
- 32. Bover LC, Cardó‐Vila M, Kuniyasu A et al. A previously unrecognized protein‐protein interaction between TWEAK and CD163: potential biological implications. J Immunol 2007; 178: 8183–94. [DOI] [PubMed] [Google Scholar]
- 33. Kristiansen M, Graversen JH, Jacobsen C et al. Identification of the haemoglobin scavenger receptor. Nature 2001; 409: 198–201. [DOI] [PubMed] [Google Scholar]
- 34. Calvert JG, Slade DE, Shields SL et al. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J Virol 2007; 81: 7371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fabriek BO, van Bruggen R, Deng DM et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 2009; 113: 887–92. [DOI] [PubMed] [Google Scholar]
- 36. Philippidis P, Mason JC, Evans BJ et al. Hemoglobin scavenger receptor CD163 mediates interleukin‐10 release and heme oxygenase‐1 synthesis: antiinflammatory monocyte‐macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res 2004; 94: 119–26. [DOI] [PubMed] [Google Scholar]
- 37. Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. Constitutive endocytosis of CD163 mediates hemoglobin‐heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res 2006; 99: 943–50. [DOI] [PubMed] [Google Scholar]
- 38. Miura T, Ueda N, Yamada K et al. Antidiabetic effects of corosolic acid in KK‐Ay diabetic mice. Biol Pharm Bull 2006; 29: 585–7. [DOI] [PubMed] [Google Scholar]
- 39. Zong W, Zhao G. Corosolic acid isolation from the leaves of Eriobotrta japonica showing the effects on carbohydrate metabolism and differentiation of 3T3‐L1 adipocytes. Asia Pac J Clin Nutr 2007; 16 (Suppl 1): 346–52. [PubMed] [Google Scholar]
- 40. Yamaguchi Y, Yamada K, Yoshikawa N, Nakamura K, Haginaka J, Kunitomo M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr‐cp rats, a model of metabolic syndrome. Life Sci 2006; 79: 2474–9. [DOI] [PubMed] [Google Scholar]
- 41. Porta C, Rimoldi M, Raes G et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappa B. Proc Natl Acad Sci USA 2009; 106: 14978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Combination effect of corosolic acid and CDDP on the proliferation of glioblastoma cells.
Supporting info item


