Abstract
Theaflavins (TF) and thearubigins (TR) are the major polyphenols of black tea. Our previous study revealed that TF‐ and TR‐induced apoptosis of human malignant melanoma cells (A375) is executed via a mitochondria‐mediated pathway. In our present study we observed the role of the three most important MAPK (ERK, JNK, and p38) in TF‐ and TR‐induced apoptosis. TF and TR treatment of A375 cells led to sustained activation of JNK and p38 MAPK but not ERK, suggesting that JNK and p38 are the effector molecules in this polyphenol‐induced cell death. This idea was further supported by subsequent studies in which JNK and p38 activation was inhibited by specific inhibitors. Significant inhibition was found in TF‐ and TR‐treated A375 cell death pretreated with JNK‐ or p38‐specific inhibitors only. Further, we have found that TF and TR treatment induces a time‐dependent increase in intracellular reactive oxygen species generation in A375 cells. Interestingly, treatment with the antioxidant N‐acetyl cystein inhibits TF‐ and TR‐induced JNK and p38 activation as well as induction of cell death in A375 cells. We also provide evidence demonstrating the critical role of apoptosis signal‐regulating kinase 1 in TF‐ and TR‐induced apoptosis in A375 cells. Taken together our results strongly suggest that TF and TR induce apoptotic death of A375 cells through apoptosis signal‐regulating kinase 1, MAPK kinase, and the JNK–p38 cascade, which is triggered by N‐acetyl cystein intracellular oxidative stress. (Cancer Sci 2009; 100: 1971–1978)
The water extract of the dry leaves of the plant Camellia sinensis is popularly known as tea. According to its processing, tea can be classified into green, oolong, and black tea, among which black tea is the most popular. TF and TR are the two most important and abundant polyphenols of black tea. Black tea has been shown to be potent in inhibiting tumorigenesis in animal models including lung, colon, and skin.( 1 , 2 , 3 ) Some reports provide evidence that black tea and its polyphenols, especially TF, significantly inhibit proliferation and enhance apoptosis in some cancer cells.( 4 , 5 , 6 )
Apoptosis is a form of programmed cell death in multicellular organisms. The process of apoptosis is controlled mainly by two cascades, namely the kinase cascade( 7 , 8 ) and the protease cascade.( 8 , 9 ) The MAPK cascade, one of the very important members of the kinase cascade, plays an important role in apoptosis induction especially when the apoptotic signal is initiated by various types of stresses such as intracellular oxidative stresses.( 10 ) It has been reported that ROS are important mediators of apoptosis.( 11 ) Oxidative stress induces multiple signal transduction pathways, including the MAPK pathway.( 12 , 13 ) The extent and duration of MAPK activation plays a key role in controlling different cell functions.( 14 , 15 )
MAPK are members of a family of serine/threonine kinases that are involved in both the stress response and apoptosis. To date, three MAPK cascades have been extensively characterized: ERK, JNK, and p38. The biological effects of MAPK signaling are mainly executed by phosphorylation of downstream substrates including transcription factors such as c‐Jun and c‐Fos.( 15 , 16 ) ERK is predominantly activated by mitogens leading to cell differentiation, growth, and survival.( 16 , 17 ) On the other hand, JNK and p38 are preferentially activated by oxidative stress and cytokines resulting in inflammation and apoptosis.( 7 , 17 )
JNK and p38 MAPK, as well as their upstream kinases such as MKK and MKKK, are the key regulators of stress‐activated apoptosis in various cells.( 7 , 18 , 19 , 20 ) ASK1 is a MKKK family member that is activated in response to various cellular stresses such as ROS, activates stress‐activated MAPK such as JNK and p38, and induces apoptosis.( 7 , 18 , 20 , 21 ) Many recent reports indicate that EGCG can produce intracellular ROS in various cell types.( 22 , 23 ) ROS act as the signaling intermediates and can trigger downstream cellular events, like mitochondrial dysfunction and activation of JNK and p38 MAPK and other signaling pathways that ultimately lead to apoptotic cell death.( 11 )
Previously we determined that TF and TR induce apoptosis in A375 cells, and the IC50 values of both TF and TR are approximately 50 µg/mL at 48 h.( 24 ) Several reports indicate that EGCG can modulate the activation of stress‐activated MAPK and apoptosis( 25 , 26 ) but the effect of black tea polyphenols on the apoptosis‐inducing effect of MAPK is hardly reported. In the present study, we have attempted to explore the role of MAPK and oxidative stress in TF‐ and TR‐induced apoptotic death in A375 cells.
Materials and Methods
Reagents. Primary antibodies (JNK1/2, ERK1/2, p‐38, ASK1, MKK4, MKK3, p‐JNK, p‐ERK, p‐p38, p‐ASK1, p‐MKK4, p‐MKK3/6, NF‐κB [p65], and β‐actin) and polyclonal secondary antibody were obtained from Cell Signalling Technology (Danvers, MA, USA). Bromophenol blue, EGTA, NaF, Na3VO4, Nonidet P‐40 (NP‐40), PMSF, aprotinin, leupeptin, pepstatin A, NAC, HEPES, MTT, H2O2, molecular‐grade BSA, Tween‐20, NBT/BCIP, Tris‐HCl, and DMSO were procured from Sigma‐Aldrich (St Louis, MO, USA). DCFH‐DA was from Molecular Probes (Eugene, OR, USA). The nucleus/cytosol fractionation kit was from BioVision (Mountain View, CA, USA). Caspase fluoremetric assay kits were obtained from Chemicon International Corporation (Temecula, CA, USA). All MAPK inhibitors were purchased from Calbiochem (La Jolla, CA, USA). The Bio‐Rad Protein Assay Kit was from Bio‐Rad Laboratories (Hercules, CA, USA).
Cell culture. A375 cells (human malignant melanoma) were purchased from the National Centre for Cell Science (Pune, India) and maintained in DMEM containing 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin (all from Invitrogen Corporation, Grand Island, NY, USA). The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 inside a CO2 incubator.
Extraction of TF and TR from black tea. Both TF and TR were extracted from the black tea (Tata Tea Ltd., Kolkata, India) as described previously.( 24 , 27 , 28 ) Extraction was carried out in the Department of Chemistry of our institute. In short, black tea (10 g) was initially extracted with boiling water (250 mL), and then the aqueous fraction was further extracted with chloroform to remove caffeine and successively with ethyl acetate and n‐butanol by liquid–liquid partitioning.( 29 ) The ethyl acetate fraction contained TF whereas the n‐butanol fraction contained TR.( 30 ) The total TF and TR contents of our samples were calculated to be 2.3 and 12.5%, respectively, of the dry weight of the sample.
MTT assay. The effect of TF and TR on the viability of A375 cells in the presence or absence of different inhibitors was determined by MTT assay following the method of Mosmann.( 31 ) Briefly, approximately 5 × 104 cells per well were plated in 96‐well plates and treated with TF or TR (0, 25, 50, 75, 100 µg/mL) for 48 h either with or without inhibitors. At the end of the stipulated time the medium was aspirated and MTT (50 µL from 5 mg/mL stock solution in PBS) was added into each well and incubated at 37°C for 2 h. The purple‐colored precipitate of formazan was dissolved in 150 µL DMSO. The color absorbance was recorded at 540 nm with a microplate reader with a reference serving as blank. The IC50 value was also determined.( 24 , 32 )
Preparation of cytosolic and nuclear fractions. A375 cells (5 × 106) were harvested after treatment with TF or TR for 1, 3, 6, 12, and 24 h. Isolation of the nuclear and cytosolic cell fractions was carried out using a nucleus/cytosol fractionation kit according to the manufacturer's protocol.
Measurement of intracellular ROS level. A375 cells were seeded in 90‐mm dishes (5 × 107 cells per dish) overnight. Cells were treated with 50 µg/mL TF or TR for 0.5, 1, 3, 6, 12, and 24 h and then collected by trypsinization. The cell suspension (200 µL, containing 2 × 106 cells/mL) was added to PBS (800 µL) and incubated with DCFH‐DA (10 µM) for 15 min at 37°C. The production of intracellular DCF‐DA due to the oxidation of DCFH‐DA by H2O2 was measured using a fluoremeter (excitation at 488 nm and emission at 515 nm). Another set of experiments was carried out simultaneously, with the same treatments and conditions, where NAC (5 mM) was added to the cell culture medium 30 min before the addition of TF and TR.
Activity of caspases. Caspase‐3 and caspase‐9 were assayed using the fluoremetric caspase assay kits designed for the respective caspases, as per the manufacturer's protocol. Briefly, A375 cells (2 × 105 per well) were plated in triplicate in a 24‐well tissue culture plate and treated with TF or TR (50 µg/mL) in the absence or presence of different MAPK inhibitors for 12 and 24 h. The caspase activity was determined fluorimetrically (excitation at 400 nm and emission at 505 nm).
Western blot analysis. Western blot analysis was done to determine the expression of different proteins. A375 cells were treated with the optimal (50 µg/mL) concentration of TF or TR for 1, 3, 6, 12 and 24 h, or with different concentrations of TF or TR (0, 25, 50, 75, 100 µg/mL) for 6 or 12 h. Cells were harvested, washed with cold PBS (pH 7.4), and lysed with ice‐cold lysis buffer (50 mM Tris‐HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 1% NP‐40, 1 mM PMSF, 10 µg/mL aprotinin, and 10 µg/mL leupeptin, pH 7.4) for 30 min and centrifuged at 12 000 g for 30 min at 4°C. Protein was evaluated using the Bio‐Rad Protein Assay Kit. Equal amounts of protein from each treatment were subjected to SDS‐PAGE. Thereafter, proteins were electrophoretically transferred to nitrocellulose membrane and non‐specific sites were blocked with 5% skim milk in 20 mM TBS (pH 7.5) containing 0.1% Tween‐20 and reacted with primary polyclonal antibodies (JNK1/2, ERK1/2, p38, ASK1, MKK4, MKK3, p‐JNK, p‐ERK, p‐p38, p‐ASK1, p‐MKK4, p‐MKK3/6, NF‐κB [p65], and β‐actin) for 4 h at room temperature. After washing with TBS containing 0.1% Tween‐20 the membrane was then incubated with alkaline phosphatase‐conjugated goat antirabbit secondary antibody. The protein bands were visualized using NBT–BCIP mixture.
Statistical analyses. GraphPad Instat software (Graphpad Software Inc., La Jolla, CA, USA) was used for statistical analyses. All data are expressed as the mean ± SD of three independent experiments. The differences between the control and the experimental groups were determined by one‐way analysis of variance (ANOVA) and post tests were done using Dunnett's multiple comparison test to determine the levels of significance. P < 0.05 and P < 0.01 were considered to be significant.
Results
JNK and p38, but not ERK, phosphorylation was upregulated by TF and TR treatment in A375 cells. MAPK are activated during the course of apoptosis induced by a number of compounds. Hence we monitored the activation of three members of the MAPK family (p38, JNK1/2, and ERK1/2) using western blot analysis with antibodies that recognize the phosphorylated forms of the three kinases. In the time‐dependent study, both TF (Fig. 1AI) and TR (Fig. 1AII) treatments induced sustained phosphorylation of JNK and p38 MAPK whereas expression of p‐ERK was maintained at a constant level in A375 cells. The expressions of total p38, JNK, and ERK MAPK were unaltered throughout the time course (Fig. 1AI,AII). We also studied the dose‐dependent changes of the phosphorylated forms of these MAPK proteins 12 h after TF or TR treatment (Fig. 1BI,BII). The expression of p‐p38 and p‐JNK was increased dose dependently but p‐ERK did not show a dose‐dependent increase in expression upon TF (Fig. 1BI) or TR (Fig. 1BII) treatment.
Figure 1.
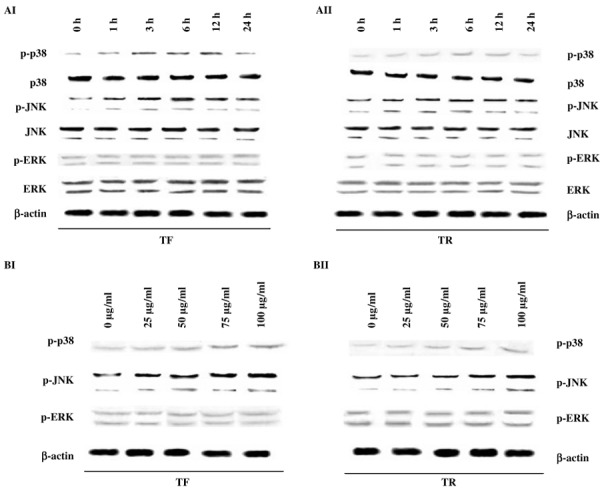
Time‐ and dose‐dependent effect of theaflavins (TF) and thearubigins (TR) on the expression of MAPK proteins. (AI,AII) Time‐dependent study of the effect of (AI) TF or (AII) TR on MAPK proteins. A375 cells (5 × 107 per treatment) were treated with TF or TR at 50 µg/mL concentration and the expression patterns of ERK, phosphorylated (p)‐ERK, JNK, p‐JNK, p38, and p‐p38 were observed at 0, 1, 3, 6, 12, and 24 h of treatment. Protein from the total cell lysate was subjected to SDS‐PAGE and western blotting using p‐p38, p38, p‐JNK, JNK, p‐ERK, ERK, and β‐actin antibodies. Representative blots from three independent experiments gave identical results. The relative intensity of each band after normalization with the intensity of β‐actin in a blot (below each western blot) was measured. (BI,BII) Dose‐dependent study of (BI) TF or (BII) TR on MAPK proteins after 12 h of incubation. A375 cells (5 × 107 per treatment) were treated with TF or TR at 0 (control), 25, 50, 75, and 100 µg/mL concentrations and the expression patterns of p‐ERK, p‐JNK, and p‐p38 were observed at 12 h of treatment. Protein from the total cell lysate was subjected to SDS‐PAGE and western blotting using p‐JNK, p‐p38, p‐ERK, and β‐actin antibodies. Representative blots from three independent experiments gave identical results. The relative intensity of each band was measured after normalization with the intensity of β‐actin in a blot (below each western blot).
Inhibition of JNK and p38 confers resistance to TF‐ and TR‐induced caspase activation and induction of cell death in A375 cells. To assess the role of the sustained activation of p38 and JNK during TF‐ and TR‐induced apoptosis, we used the specific cell‐permeable inhibitors SB203580 for p38 and SP600125 for JNK, and both inhibitors attenuated TF‐induced (Fig. 2AI) and TR‐induced (Fig. 2AII) cell death significantly. Blocking ERK with the MEK inhibitor PD98059 did not show any change in the rate of cell death induction by TF and TR (Fig. 2AI,AII). The inhibitory effects of SB203580 and SP600125 on TF‐ and TR‐induced caspase‐3 (Fig. 2BI) and caspase‐9 (Fig. 2BII) activities in A375 cells were also observed. Both of the inhibitors markedly reduced their activities as measured by the fluoremetric process. But PD98059 did not show any significant changes in caspase‐3 or caspase‐9 activity (Fig. 2BI,BII).
Figure 2.
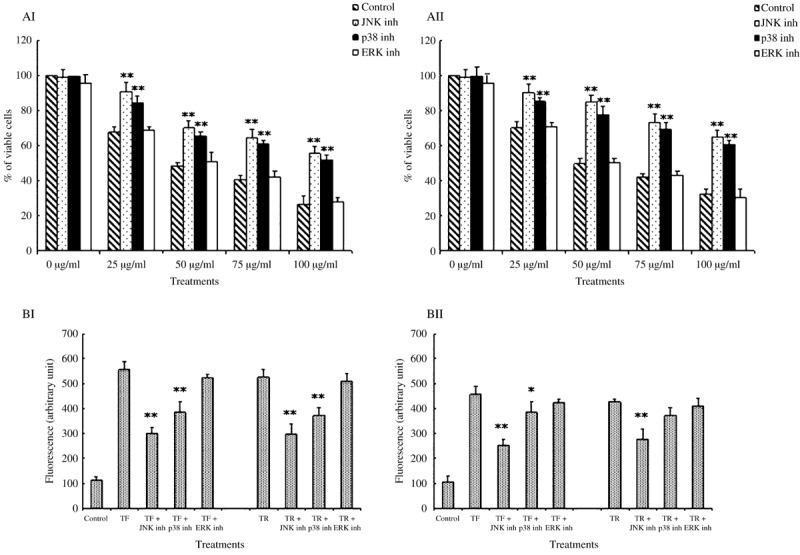
Effect of theaflavins (TF) and thearubigins (TR) on the viability and activity of caspase‐3 and caspase‐9 in A375 cells in the presence of MAPK inhibitors. (AI,AII) Effect of (AI) TF and (AII) TR on A375 cell viability in the presence of different MAPK inhibitors. A375 cells (5 × 104 per well) were pretreated with JNK‐specific inhibitor, p38‐specific inhibitor, or ERK‐specific inhibitor for 30 min at 37°C and then treated with (AI) TF or (AII) TR at different concentrations (0, 25, 50, 75, and 100 µg/mL). Then the cells were grown in a 96‐well plate for 48 h. The viability of the cells was observed by MTT assay. Each value is expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 are the TF plus inhibitor or TR plus inhibitor groups compared with the TF‐ or TR‐treated groups at different concentrations (0, 25, 50, 75, 100 µg/mL). (BI,BII) Effect of TF and TR on the activity of (BI) caspase‐3 and (BII) caspase‐9 in A375 cells in the presence of different MAPK inhibitors. Cells were preincubated with JNK‐specific inhibitor SP600125 (20 µM), p38‐specific inhibitor SB203580 (10 µM), or ERK‐specific inhibitor PD98059 (5 µM) for 30 min at 37°C. Then cells were treated with (BI) TF or (BII) TR at 50 µg/mL for 12 h and subjected to measurement of caspase activities. Each value is expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 are the TF plus inhibitor or TR plus inhibitor groups compared with TF‐ or TR‐treated cells.
Effect of TF and TR treatment on NF‐κB in A375 cells. TF and TR did not alter the expression level of NF‐κB as determined by western blot analysis of the nuclear and cytosolic fractions of TF‐ or TR‐treated A375 cells (Fig. 3).
Figure 3.
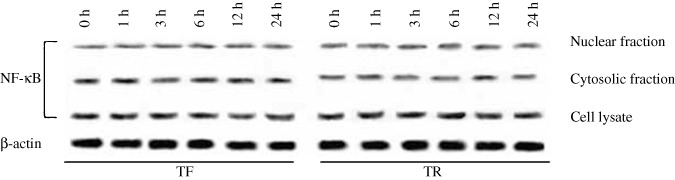
Effect of theaflavin (TF) or thearubigin (TR) treatment on the expression level of nuclear factor (NF)‐κB (p65) in the cytosolic fraction, nuclear fraction, and whole‐cell lysate of A375 cells at different time points. A375 cells (5 × 107 per treatment) were treated with TF or TR at 50 µg/mL for 0, 1, 3, 6, 12, and 24 h. Protein from the cytosolic fraction, nuclear fraction, and total‐cell lysate was subjected to SDS‐PAGE and western blotting using NF‐κB (p65) and β‐actin antibodies. Representative blots from three independent experiments gave identical results. The relative intensity of each band was measured after normalization with the intensity of β‐actin in a blot (given below each western blot).
TF and TR induced ROS generation time‐dependently. ROS are known to play an important role in the intracellular signaling pathway of apoptosis. We studied intracellular ROS production caused by TF (Fig. 4AI) and TR (Fig. 4AII) treatment at their optimal concentration (50 µg/mL) in A375 cells at different time points. The fluorescence of DCF‐DA increased gradually during the first 3 h of incubation and then decreased (Fig. 4AI,AII). Control or vehicle‐treated cells (0.05% DMSO) alone did not increase the fluorescence (data not shown). Next, the cells were pretreated with 5 mM NAC for 30 min prior to TF or TR treatment. We observed that the intracellular ROS level decreased upon NAC treatment (Fig. 4AI,AII).
Figure 4.
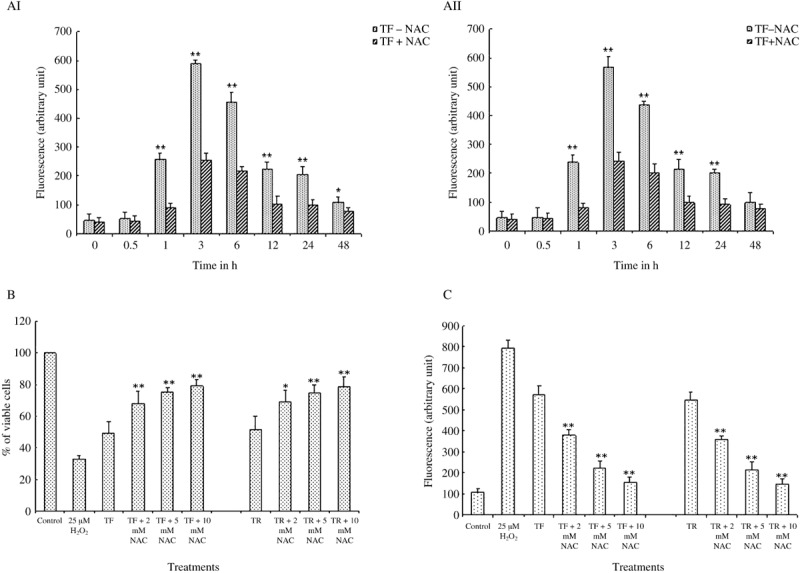
Effect of theaflavins (TF) and thearubigins (TR) on intracellular reactive oxygen species (ROS) generation, cell viability, and caspase‐3 activity in presence of N‐acetyl cystein (NAC) (ROS inhibitor) in A375 cells. (AI,AII) Time‐dependent effect of (AI) TF and (AII) TR on ROS production in A375 cells in the absence or presence of NAC. NAC was added at 5 mM, 30 min prior to the addition of TF and TR. Then the cells were treated with TF or TR at a concentration of 50 µg/mL for 0, 0.5, 1, 3, 6, 12, 24, and 48 h. Each value is expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 are the TF‐ or TR‐treated groups at 0 h of treatment, as compared with the TF‐ or TR‐treated groups at other time points (0.5, 1, 3, 6, 12, 24, 48 h). (B) Effect of TF and TR on A375 cell viability in the presence of NAC at different concentrations. NAC was added at different concentrations (2, 5, 10 mM) to the cell culture medium 30 min before the addition of TF and TR. Cells were then treated with TF or TR at a concentration of 50 µg/mL for 48 h. Each value is expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 are the TF plus different concentrations of NAC or TR plus different concentrations of NAC groups, compared with TF‐ or TR‐treated groups. (C) Effect of TF and TR on caspase‐3 activity in the presence of NAC at different concentrations. NAC was added at different concentrations (2, 5, 10 mM) to the cell culture medium 30 min before the addition of TF and TR. Then the cells were treated with TF or TR at a concentration of 50 µg/mL for 12 h. Each value is expressed as mean ± SD (n = 3). **P < 0.01 is the TF plus different concentrations of NAC or TR plus different concentrations of NAC groups compared with TF‐ or TR‐treated groups.
Effect of NAC on TF‐ and TR‐induced cell death induction and caspase‐3 activation. We also studied the effects of NAC, a known scavenger of ROS, on the extent of cell death induced by TF and TR in A375 cells. Treatment with NAC (2, 5, and 10 mM) prior to TF and TR treatment in A375 cells showed a significant reduction in black tea polyphenol‐induced cell death in the cancer cells (Fig. 4B). An inhibitory effect of NAC (2, 5, and 10 mM) on TF‐ and TR‐induced caspase‐3 activation was observed when NAC was added to A375 cells 30 min before treatment with TF and TR (Fig. 4C).
Effect of NAC on the TF‐ and TR‐induced activation of p38 and JNK MAPK in A375 cells. Our results showed that phosphorylation of JNK and p38 MAPK with different doses of TF and TR (25, 50, and 75 µg/mL) treatment in A375 cells was clearly reduced when the cells were pretreated with NAC (Fig. 5AI,AII). When we treated A375 cells with H2O2 alone as a known ROS inducer, it clearly upregulated the expression of p‐JNK and p‐p38 in a dose‐dependent manner but not p‐ERK (Fig. 5B).
Figure 5.
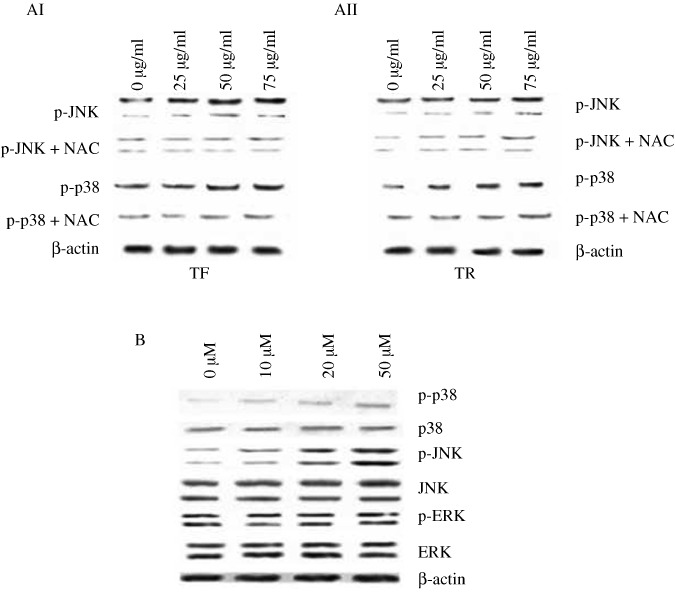
Effect of N‐acetyl cystein (NAC) and H2O2 on phosphorylated (p)‐JNK and p‐p38 expression in theaflavin (TF)‐ or thearubigin (TR)‐treated A375 cells. (AI,AII) Effect of NAC on the expression of phosphorylated forms of JNK and p38 after 12 h of (AI) TF or (AII) TR treatment at different concentrations. A375 cells (5 × 107 per treatment) were pretreated with NAC (5 mM) for 30 min at 37°C and then treated with TF or TR at 0 (control), 25, 50 and 75 µg/mL for 12 h. Protein from the total‐cell lysate was subjected to SDS‐PAGE and western blotting using p‐JNK, p‐p38, and β‐actin antibodies. Representative blots from three independent experiments gave identical results. The relative intensity of each band was measured after normalization with the intensity of β‐actin in a blot (below each western blot). (B) Effect of H2O2 at different concentrations (0, 10, 20, and 50 µM) on the expression of phosphorylated forms of p38, JNK, and ERK after 12 h of TF or TR treatment. A375 cells (5 × 107 per treatment) were treated with H2O2 at different concentrations for 30 min at 37°C and then treated with TF or TR at 50 µg/mL for 12 h. Protein from the total‐cell lysate was subjected to SDS‐PAGE and western blotting using p‐JNK, p‐p38, p‐ERK, and β‐actin antibodies. Representative blots from three independent experiments gave identical results. The relative intensity of each band was measured after normalization with the intensity of β‐actin in a blot (below each western blot).
Effect of TF and TR treatment on the upstream signaling molecules of JNK and p38 in A375 cells. Our time‐dependent study revealed that TF (Fig. 6AI) and TR (Fig. 6AII) treatments upregulated the level of p‐MKK3 and 6 and p‐MKK4, whereas the non‐phospho forms of MKK3 and MKK4 remained unaltered. Our result also indicates that ASK1 is phosphorylated during the early hours of TF and TR treatment (Fig. 6AI,AII). The expression of p‐ASK1 was also upregulated upon TF and TR treatment in a dose‐dependent manner (Fig. 6B).
Figure 6.
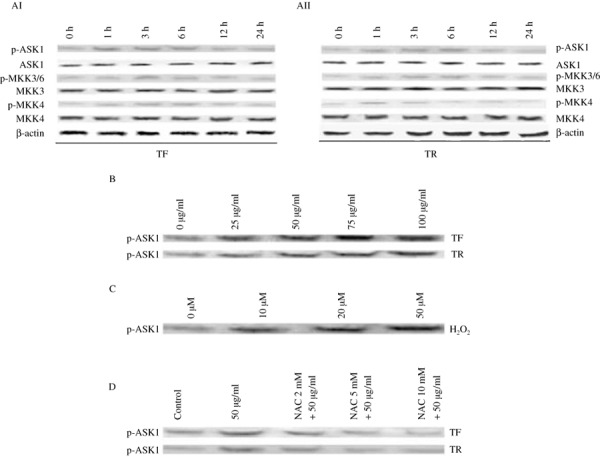
Study of the expression patterns of molecules upstream of JNK and p38 and there interrelationship with reactive oxygen species generation. (AI,AII) Time‐dependent study of the effect of (AI) theaflavins (TF) and (AII) thearubigins (TR) on the upstream kinases JNK and p38. A375 cells (5 × 107 per treatment) were treated with (AI) TF or (AII) TR at 50 µg/mL and the expression patterns of phosphorylated (p)‐apoptosis signal‐regulating kinase (ASK) 1, ASK1, p‐MAPK kinase (MKK) 3/6, MKK3, p‐MKK4, MKK4, and β‐actin were observed at 0, 1, 3, 6, 12, and 24 h of treatment. Protein from the total‐cell lysate was subjected to SDS‐PAGE and western blotting was carried out using p‐ASK1, ASK1, p‐MKK3/6, MKK3, p‐MKK4, MKK4, and β‐actin primary antibodies. Representative blots from three independent experiments gave identical results. The relative intensity of each band after normalization with the intensity of β‐actin in a blot (below each western blot) was measured. (B) Dose‐dependent study of the effect of TF and TR on p‐ASK1 protein expression. A375 cells (5 × 107 per treatment) were treated with TF or TR at 0, 25, 50, 75, and 100 µg/mL and the expression pattern of p‐ASK1 was observed at 6 h of treatment. Representative blots from three independent experiments gave identical results. The relative intensity of each band was measured after normalization with the intensity of β‐actin in a blot. (C) Effect of H2O2 at different concentrations (0, 10, 20, and 50 µM) on the expression of p‐ASK1 after 6 h of TF or TR treatment. A375 cells (5 × 107 per treatment) were treated with H2O2 at different concentrations for 30 min at 37°C and then treated with TF or TR at 50 µg/mL for 6 h. Protein from the total‐cell lysate was subjected to SDS‐PAGE and western blotting using p‐ASK1 antibody. Representative blots from three independent experiments gave identical results. The relative intensity of each band was measured after normalization with the intensity of β‐actin in a blot. (D) Effect of different concentrations of N‐acetyl cystein (NAC) on the expression of phosphorylated form of ASK1 at 6 h of TF or TR treatment. A375 cells (5 × 107 per treatment) were pretreated with NAC (0, 2, 5, 10 mM) for 30 min at 37°C and then treated with TF or TR at 50 µg/mL for 6 h. Protein from the total‐cell lysate was subjected to SDS‐PAGE and western blotting using p‐ASK1 antibody. Representative blots from three independent experiments gave identical results. The relative intensity of each band was measured after normalization with the intensity of β‐actin in a blot.
Effect of H2O2 and NAC on phospho‐ASK1 expression in TF‐ or TR‐treated A375 cells. To find out the link between ROS generation and the activation of MAPK, we investigated the effect of H2O2 on ASK1 in A375 cells after TF or TR treatment using the specific antibody that recognizes the phosphorylated form of this upstream MKKK. We found that the p‐ASK1 level was increased dose dependently upon treatment with H2O2, a known ROS inducer (Fig. 6C).
As shown in Figure 6(D), TF‐ and TR‐induced activation of p‐ASK1 was effectively inhibited by treatment with NAC at different doses, suggesting that ASK1 is one of the most important upstream molecules that might influence the ROS‐dependent upregulation of JNK and p‐38 upon TF and TR treatment.
Discussion
The MAPK pathway is an important intracellular signaling cascade in the transduction of apoptotic signals initiated in response to various extracellular stimuli, including environmental stresses.( 7 , 12 ) This pathway is well conserved in cells from yeast to vertebrates and consists of MAPK, MKK, and MKKK.( 33 , 34 ) MKK, such as MKK3 and 6 or MKK4 and 7, in turn can be activated by several MKKK, including ASK1, ASK2, TGF‐β activated kinases 1, and Tumor progression locus 2.( 12 , 13 ) Of the MKKK molecules, ASK1, is ubiquitously expressed and activates the MKK4/7–JNK and MKK3/6–p38 signaling cascades.( 13 , 18 ) Overexpression of ASK1 also induces apoptosis in cultured cells,( 35 ) suggesting that ASK1 is a pivotal component in stress or cytokine‐induced apoptosis. Three distinct MAPK (ERK, p38, and JNK) have been characterized and reported to be involved in apoptosis in many different paradigms of cellular toxicity.( 7 )
Previously we have reported that TF and TR can induce apoptosis in A375 cells via a mitochondrial pathway.( 24 ) The objective of our present work was to investigate the involvement of all major MAPK, namely ERK, p38, and JNK, in induction of apoptosis of A375 cells upon TF and TR treatment.
The active form of the MAPK is the phosphorylated form of these proteins.( 14 ) Hence we observed the expression of the phosphorylated forms of these proteins upon TF and TR treatment in A375 cells. Our findings suggest that both p‐p38 and p‐JNK are upregulated by means of TF and TR treatment in a time‐ and dose‐dependent manner. But the p‐ERK and ERK levels were not modulated either time or dose dependently upon treatment with TF or TR, suggesting that ERK might not be affected by this treatment in A375 cells. To validate whether JNK and p38 have any significant role in death induction we used the JNK inhibitor SP600125 (20 µM) and p38 inhibitor SB203580 (10 µM) before treatment with TF or TR and found that the cell viability was increased significantly after 48 h of incubation. This indicates that these two proteins are important in transduction of the death signal upon TF and TR treatment in A375 cells. However, no significant change in the viability of cells was observed in presence of the ERK‐specific inhibitor PD98059 (5 µM) along with TF and TR treatment. These findings together suggest that TF and TR induce death of A375 cells via both p38 and JNK MAPK, without involving the ERK pathway.
The death of A375 cells upon TF and TR treatment occurs mainly by mitochondria‐mediated apoptosis that involves caspase‐9 and caspase‐3 activity.( 24 ) Therefore the effect of TF and TR on the activity of caspase‐3 and caspase‐9 in A375 cells was studied in the presence of different MAPK inhibitors and it was found that both the JNK and p38 inhibitors could reduce the activity of caspases significantly. ERK inhibitor did not alter the activity of the caspases upon TF or TR treatment. This result further supports our previous findings and suggests that JNK and p38, but not ERK, are involved in apoptosis induction of A375 cells by TF and TR via a caspase‐mediated pathway.
NF‐κB is a ubiquitously expressed, inducible transcription factor that has been implicated in ROS signaling and can induce cell death.( 36 , 37 , 38 , 39 , 40 , 41 , 42 ) Green tea polyphenols like EGCG can downregulate the NF‐κB activity and induce apoptosis.( 43 ) As NF‐κB might induce cell death, we studied the expression level of this protein in whole‐cell lysate as well as in nuclear and cytoplasmic fractions. However, we did not find any significant change in the occurrence of NF‐κB protein in either the nuclear fraction or the cytoplasmic fraction. This observation suggests that NF‐κB might not be a target of TF or TR in A375 cells.
In various cells, the apoptosis‐triggering effects of ROS were noted.( 44 , 45 ) A few reports show that green tea polyphenols can induce ROS‐dependent apoptosis in certain cancer cells.( 22 , 23 ) Accordingly we hypothesized that TF and TR might induce ROS in A375 cells. We found that both TF and TR can induce intracellular ROS generation time dependently, thereby supporting our hypothesis. The cell‐permeable stable antioxidant NAC inhibited induction of ROS by TF and TR. We also observed enhanced viability of the cells and reduced activity of caspase‐3 when the cells were incubated with NAC before the treatment with TF or TR. These observations signify that ROS could be an important factor in inducing caspase activity and apoptotic death of A375 cells upon TF and TR treatment.
There are several reports suggesting that ROS can induce activation of JNK and p‐38 MAPK and can also induce apoptosis.( 10 , 21 , 46 ) We therefore wanted to know whether activation of these two MAPK and induction of ROS are interlinked. When the A375 cells were pretreated with NAC prior to TF and TR treatment the expression of p‐JNK and p‐p38 were suppressed. This result suggests that the TF‐ and TR‐induced intracellular ROS might have upregulated the expression of p‐JNK and p‐p38. For further confirmation, when we treated the cells with different concentrations of H2O2, upregulation of p‐JNK and p‐p38 was observed dose dependently but the p‐ERK level was unaltered. These results imply that the activation of JNK as well as p38 and induction of ROS in TF‐ and TR‐treated A375 cells are interlinked. ROS can activate JNK and p38 via different proteins, the most important of which is ASK1. ASK1 is one of the MKKK that are activated by various types of stress such as ROS, tumor necrosis factor α, lipopolysaccharide, endoplasmic reticulum stress, and calcium influx. ASK1 in turn selectively activates the JNK and p38 MAPK pathways.( 18 , 46 , 47 , 48 ) Oxidative stress is one of the most potent activators of ASK1, which is essential for oxidative stress‐induced cell death.( 20 ) ASK1 can induce apoptosis, which also activates the JNK and p38 signaling pathways.( 18 ) As a result we hypothesized that ASK1 and its downstream MKK MKK3 and MKK4 could activate JNK and p38 after TF and TR treatment. Interestingly we found that p‐ASK1 was being upregulated time dependently upon TF and TR treatment prior to JNK and p38. MKK3 and MKK4 showed almost similar patterns of activation in a time‐dependent manner upon TF or TR treatment suggesting that ASK1, MKK3, and MKK4 are the upstream molecules that might play important roles in activation of JNK and p38 due to TF or TR treatment in A375 cells. Pretreatment of cells with NAC significantly inhibited the upregulation of p‐ASK1 upon TF or TR treatment as well. These observations further confirm our hypothesis that in A375 cells ASK1 is upregulated by intracellular ROS when treated with TF and TR, and subsequently this protein upregulates the stress‐activated MAPK together.
In conclusion, our results strongly suggest that both TF and TR can induce JNK‐ and p38‐dependent apoptotic cell death in A375 cells and ROS might be one of the most important upstream substances that triggers this apoptotic stimulus. ROS‐mediated activation of MAPK is controlled or maintained by ASK1 protein. So ASK1 is one of the key regulator proteins in TF‐ and TR‐induced apoptotic death in A375 cells. These findings provide a rationale to explore the role of TF and TR as chemopreventive and perhaps as chemotherapeutic agents and may add some information to the existing knowledge that black tea is beneficial for health and prevents various diseases, including cancer.
Disclosure Statement
This is to certify that there is no personal or financial conflict of interest in any form whatsoever among the authors of this article.
Abbreviations
| ASK | apoptosis signal‐regulating kinase |
| BCIP | 5‐bromo‐4‐chloro‐3‐indolyl phospate, p‐toluidine |
| DCF‐DA | Dichlorofluorescein diacetate |
| DCFH‐DA | 2′,7′‐dichlorofluorescein diacetate |
| EGCG | Epigallocatechins gallate |
| ERK | extracellular signal‐regulated kinase |
| JNK | c‐Jun N‐terminal kinase |
| MAP | mitogen‐activated protein |
| MAPK | MAP kinase |
| MKK | MAP kinase kinase |
| MKKK | MAP kinase kinase kinase |
| NAC | N‐acetyl cystein |
| NBT | nitro blue tetrazolium |
| NF | nuclear factor |
| p | phosphorylated |
| ROS | reactive oxygen species |
| TBS | Tris‐buffered saline |
| TF | theaflavin |
| TR | thearubigin |
Acknowledgments
The authors are thankful to the National Tea Research Foundation, Kolkata, for providing the Junior Research Fellowship to Udayan Bhattacharya.
References
- 1. Yang GY, Liu Z, Seril DN et al . Black tea constituents, theaflavins, inhibit 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone (NNK)‐induced lung tumorigenesis in A/J mice. Carcinogenesis 1997; 18: 2361–5. [DOI] [PubMed] [Google Scholar]
- 2. Weisburger JH, Rivenson A, Reinhardt J et al . Effect of black tea on azoxymethane‐induced colon cancer. Carcinogenesis 1998; 19: 229–32. [DOI] [PubMed] [Google Scholar]
- 3. Javed S, Mehrotra NK, Shukla Y. Chemopreventive effects of black tea polyphenols in mouse skin model of carcinogenesis. Biomed Environ Sci 1998; 11: 307–13. [PubMed] [Google Scholar]
- 4. Prasad S, Kaur J, Roy P, Kalra N, Shukla Y. Theaflavins induce G2/M arrest by modulating expression of p21waf1/cip1, cdc25C and cyclin B in human prostate carcinoma PC‐3 cells. Life Sci 2007; 81: 1323–31. [DOI] [PubMed] [Google Scholar]
- 5. Kalra N, Seth K, Prasad S, Singh M, Pant AB, Shukla Y. Theaflavins induced apoptosis of LNCaP cells is mediated through induction of p53, down‐regulation of NF‐kappa B and mitogen‐activated protein kinases pathways. Life Sci 2007; 80: 2137–46. [DOI] [PubMed] [Google Scholar]
- 6. Kaur S, Greaves P, Cooke DN et al . Breast cancer prevention by green tea catechins and black tea theaflavins in the C3(1) SV40 T,t antigen transgenic mouse model is accompanied by increased apoptosis and a decrease in oxidative DNA adducts. J Agric Food Chem 2007; 55: 3378–85. [DOI] [PubMed] [Google Scholar]
- 7. Ichijo H. From receptors to stress‐activated MAP kinases. Oncogene 1999; 18: 6087–93. [DOI] [PubMed] [Google Scholar]
- 8. Yuo A. Differentiation, apoptosis, and function of human immature and mature myeloid cells: intracellular signaling mechanism. Int J Hematol 2001; 73: 438–52. [DOI] [PubMed] [Google Scholar]
- 9. Stennicke HR, Salvesen GS. Caspases – controlling intracellular signals by protease zymogen activation. Biochimica et Biophysica Acta 2000; 1477: 299–306. [DOI] [PubMed] [Google Scholar]
- 10. Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c‐Jun N‐terminal kinase and p38 mitogen‐activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol 2001; 21: 6913–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osone S, Hosoi H, Kuwahara Y, Matsumoto Y, Iehara T, Sugimoto T. Fenretinide induces sustained‐activation of JNK/p38 MAPK and apoptosis in a reactive oxygen species‐dependent manner in neuroblastoma cells. Int J Cancer 2004; 112: 219–24. [DOI] [PubMed] [Google Scholar]
- 12. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001; 410: 37–40. [DOI] [PubMed] [Google Scholar]
- 13. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–52. [DOI] [PubMed] [Google Scholar]
- 14. Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 2007; 26: 3100–12. [DOI] [PubMed] [Google Scholar]
- 15. Roux PP, Blenis J. ERK and p38 MAPK‐activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004; 68: 320–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pearson G, Robinson F, Beers Gibson T et al . Mitogen‐activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001; 22: 153–83. [DOI] [PubMed] [Google Scholar]
- 17. Kyriakis JM, Avruch J. Mammalian mitogen‐activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001; 81: 807–69. [DOI] [PubMed] [Google Scholar]
- 18. Ichijo H, Nishida E, Irie K et al . Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science (New York, NY) 1997; 275: 90–4. [DOI] [PubMed] [Google Scholar]
- 19. Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochimica et Biophysica Acta 1997; 1333: 85–104. [DOI] [PubMed] [Google Scholar]
- 20. Tobiume K, Matsuzawa A, Takahashi T et al . ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001; 2: 222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishitoh H, Saitoh M, Mochida Y et al . ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell 1998; 2: 389–95. [DOI] [PubMed] [Google Scholar]
- 22. Chan MM, Soprano KJ, Weinstein K, Fong D. Epigallocatechin‐3‐gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J Cell Physiol 2006; 207: 389–96. [DOI] [PubMed] [Google Scholar]
- 23. Nakagawa H, Hasumi K, Woo JT, Nagai K, Wachi M. Generation of hydrogen peroxide primarily contributes to the induction of Fe (II)‐dependent apoptosis in Jurkat cells by (–)‐epigallocatechin gallate. Carcinogenesis 2004; 25: 1567–74. [DOI] [PubMed] [Google Scholar]
- 24. Halder B, Bhattacharya U, Mukhopadhyay S, Giri AK. Molecular mechanism of black tea polyphenols induced apoptosis in human skin cancer cells: involvement of Bax translocation and mitochondria mediated death cascade. Carcinogenesis 2008; 29: 129–38. [DOI] [PubMed] [Google Scholar]
- 25. Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin‐3‐gallate induces mitochondrial membrane depolarization and caspase‐dependent apoptosis in pancreatic cancer cells. Carcinogenesis 2005; 26: 958–67. [DOI] [PubMed] [Google Scholar]
- 26. Shankar S, Suthakar G, Srivastava RK. Epigallocatechin‐3‐gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front Biosci 2007; 12: 5039–51. [DOI] [PubMed] [Google Scholar]
- 27. Halder B, Pramanick S, Mukhopadhyay S, Giri AK. Inhibition of benzo[a]pyrene induced mutagenicity and genotoxicity by black tea polyphenols theaflavins and thearubigins in multiple test systems. Food Chem Toxicol 2005; 43: 591–7. [DOI] [PubMed] [Google Scholar]
- 28. Halder B, Pramanick S, Mukhopadhyay S, Giri AK. Anticlastogenic effects of black tea polyphenols theaflavins and thearubigins in human lymphocytes in vitro . Toxicol in Vitro 2006; 20: 608–13. [DOI] [PubMed] [Google Scholar]
- 29. Xie B, Shi H, Chen Q, Ho CT. Antioxidant properties of fractions and polyphenol constituents from green, oolong and black teas. Proc Natl Sci Council, Republic China 1993; 17: 77–84. [PubMed] [Google Scholar]
- 30. Roberts EAH. Economic importance of flavonoid substances: tea fermentation. In: Geissman TA, ed. The Chemistry of Flavonoid Compounds. Oxford: Pergamon Press, 1962; 468–512. [Google Scholar]
- 31. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 32. Muench HR, Reed LJ. A simple method of estimating 50 per cent end points. Am J Hyg 1938; 27: 493–7. [Google Scholar]
- 33. Nishida E, Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci 1993; 18: 128–31. [DOI] [PubMed] [Google Scholar]
- 34. Marshall CJ. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Current Opinion Genet Dev 1994; 4: 82–9. [DOI] [PubMed] [Google Scholar]
- 35. Kanamoto T, Mota M, Takeda K et al . Role of apoptosis signal‐regulating kinase in regulation of the c‐Jun N‐terminal kinase pathway and apoptosis in sympathetic neurons. Mol Cell Biol 2000; 20: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. The NF‐kappaB‐mediated control of ROS and JNK signaling. Histol Histopathol 2006; 21: 69–80. [DOI] [PubMed] [Google Scholar]
- 37. Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G. Linking JNK signaling to NF‐kappaB: a key to survival. J Cell Sci 2004; 117: 5197–208. [DOI] [PubMed] [Google Scholar]
- 38. Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF‐B transcription factor and HIV‐1. EMBO J 1991; 10: 2247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baeuerle PA, Henkel T. Function and activation of NF‐B in the immune system. Annu Rev Immunol 1994; 12: 141–79. [DOI] [PubMed] [Google Scholar]
- 40. Flohé L, Brigelius‐Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF‐kappa B activation. Free Radic Biol Med 1997; 22: 1115–26. [DOI] [PubMed] [Google Scholar]
- 41. Shou Y, Li N, Li L, Borowitz JL, Isom GEJ. NF‐kappaB‐mediated up‐regulation of Bcl‐X(S) and Bax contributes to cytochrome c release in cyanide‐induced apoptosis. J Neurochem 2002; 81: 842–52. [DOI] [PubMed] [Google Scholar]
- 42. Collett GP, Campbell FC. Overexpression of p65/RelA potentiates curcumin‐induced apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2006; 27: 1285–91. [DOI] [PubMed] [Google Scholar]
- 43. Kim SJ, Jeong HJ, Lee KM et al . Epigallocatechin‐3‐gallate suppresses NF‐kappaB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J Nutritional Biochem 2007; 18: 587–96. [DOI] [PubMed] [Google Scholar]
- 44. Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. Faseb J 2003; 17: 2202–8. [DOI] [PubMed] [Google Scholar]
- 45. Chen Q, Olashaw N, Wu J. Participation of reactive oxygen species in the lysophosphatidic acid‐stimulated mitogen‐activated protein kinase kinase activation pathway. J Biol Chem 1995; 270: 28499–502. [DOI] [PubMed] [Google Scholar]
- 46. Noguchi T, Ishii K, Fukutomi H et al . Requirement of reactive oxygen species‐dependent activation of ASK1‐p38 MAPK pathway for extracellular ATP‐induced apoptosis in macrophage. J Biol Chem 2008; 283: 7657–65. [DOI] [PubMed] [Google Scholar]
- 47. Takeda K, Matsuzawa A, Nishitoh H et al . Involvement of ASK1 in Ca2+‐induced p38 MAP kinase activation. EMBO Rep 2004; 5: 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsuzawa A, Saegusa K, Noguchi T et al . ROS‐dependent activation of the TRAF6–ASK1–p38 pathway is selectively required for TLR4‐mediated innate immunity. Nat Immunol 2005; 6: 587–92. [DOI] [PubMed] [Google Scholar]


