Abstract
Adenovirus‐mediated gene therapy combined with chemotherapeutic agents is expected to represent a new approach for treating pancreatic cancer. However, there have been no reports of definitive effects of chemotherapeutic agents on adenovirus‐mediated gene therapies. In the present study, we investigated the effects of chemotherapeutic agents on the transduction efficiency of an adenovirus‐based gene therapy. Adenovirus (Ad‐NK4) expressing NK4, which acts as a hepatocyte growth factor antagonist, was used as a representative gene therapy. Pancreatic cancer cells infected with Ad‐NK4 were treated with chemotherapeutic agents (5‐fluorouracil [5FU], cisplatin or etoposide), and the NK4 levels in their culture media were measured. To examine the effects of chemotherapeutic agents in vivo, Ad‐NK4 was administered to subcutaneous tumors in mice after treatment with the agents, and the tumor NK4 levels were measured. The NK4 levels in culture media from cells treated with 5FU, cisplatin and etoposide were 5.2‐fold (P = 0.026), 6‐fold (P < 0.001) and 4.3‐fold (P < 0.001) higher than those of untreated cells, respectively. The chemotherapeutic agents also increased Ad‐NK4 uptake. The NK4 levels in tumors treated with 5FU, cisplatin and etoposide were 5.4‐fold (P = 0.006), 11.8‐fold (P < 0.001) and 4.9‐fold (P = 0.017) higher than those in untreated tumors, respectively. The present findings suggest that chemotherapeutic agents significantly improve the efficiency of adenovirus‐mediated gene transfer in pancreatic cancer. Furthermore, they will contribute to decreases in the adenovirus doses required for gene transfer, thereby controlling the side‐effects of adenovirus infection in normal tissues. (Cancer Sci 2009; 100: 722–729)
On the basis of recent advances in our understanding of the molecular biology of a variety of cancers( 1 , 2 , 3 ) molecular therapies that target tumor‐specific pathways and interfere with key regulatory cellular functions, such as cancer cell proliferation, differentiation, metastasis and survival, have been extensively studied.( 4 , 5 ) Many researchers have used monoclonal antibodies, specific antagonists or specific small‐molecule inhibitors as antitumor agents against cancer‐associated genes. However, monoclonal antibodies are expensive and small‐molecule inhibitors have low specificity. These agents also induce allergic reactions, such as skin rashes.( 4 ) The use of viral vectors, which have high gene transfer efficiencies, is one molecular therapy approach that is useful for expressing an antagonist of a target protein. Adenovirus‐based vectors are often used owing to their high transduction efficiency and high levels of transient expression of the transfected gene.( 6 )
Pancreatic cancer is a leading cause of cancer‐related death in industrialized countries.( 7 , 8 ) Most patients with pancreatic cancer have poor outcomes because early diagnosis is difficult and conventional therapies have limited effectiveness.( 9 ) Recently, advances in our understanding of the genetics and epigenetics of pancreatic cancer have revealed that alterations in several tumor‐related genes, such as K‐ras, p53, MMP, HGF and EGFR ( 10 , 11 , 12 , 13 , 14 , 15 ) may underlie the aggressiveness of this neoplasm and its resistance to conventional therapies.( 5 ) Therefore, molecular therapies for pancreatic cancer are promising new approaches for treating this often fatal disease. Previous studies have used adenovirus‐mediated gene transfer to treat pancreatic cancer and have shown that adenovirus‐mediated gene therapy can inhibit the progression of pancreatic cancer in vivo and in vitro. ( 16 , 17 ) However, clinical trials have shown that it is difficult to eradicate pancreatic tumors with adenovirus‐mediated gene therapy alone.( 18 , 19 ) There is also a concern that the adenovirus doses necessary to achieve therapeutic effectiveness may be associated with significant toxicity. Therefore, adenovirus administration alone may not be an effective treatment for pancreatic cancer, and it may be necessary to combine adenovirus‐mediated gene therapies with conventional treatments to maximize the antitumor effects of such therapies for pancreatic cancer.
Recently, combinations of chemotherapy and adenovirus‐mediated gene therapy have been reported to be effective for cancer treatment. Topf et al.( 20 ) reported that administration of an adenoviral vector expressing the cytosine deaminase gene with 5‐fluorocytosine suppressed the growth of colon cancer. Similarly, Shieh et al.( 21 ) reported that low‐dose etoposide enhanced telomerase‐dependent adenovirus‐mediated cytosine deaminase gene therapy for bladder cancer, and Lee et al.( 22 ) reported that adenovirus‐Iκ Bα transduction restored the chemosensitivity of lung cancers showing resistance to cisplatin. These reports suggest that synergistic effects occur with such combination therapies. Furthermore, chemotherapeutic agents such as 5‐fluorouracil (5FU) or S‐1, cisplatin and etoposide have been used as second‐line chemotherapies for pancreatic cancer.( 23 , 24 , 25 , 26 , 27 )
Accumulating evidences have shown that hepatocyte growth factor (HGF) accelerates the invasion of pancreatic cancer cells.( 12 , 28 , 29 ) Previously, we reported that gene therapy with an adenovirus vector (Ad‐NK4) expressing NK4, which acts as an HGF antagonist, showed significant inhibitory effects on the invasion of pancreatic cancer cells.( 30 , 31 , 32 ) In the present study, we investigated the effects of three chemotherapeutic agents, cisplatin, etoposide, and 5FU instead of S‐1 that is an oral anticancer drug based on biochemical modulation of 5FU( 33 , 34 ) which are accepted as promising drugs for the treatment of pancreatic cancer( 23 , 24 , 25 , 26 , 27 ) on the efficiency of transfer and expression of a target gene. To achieve this, we examined their effects on NK4 expression by Ad‐NK4 as a representative gene therapy, and found that all three agents enhanced adenoviral gene transfer in vitro and in vivo.
Materials and methods
Cells and reagents. The human pancreatic cancer cell lines SUIT‐2, AsPC‐1, KP‐1 N and KP‐2 were generously donated by Dr H. Iguchi (National Shikoku Cancer Center, Matsuyama, Japan) and cultured as described previously.( 32 ) Human recombinant HGF was purified as described previously.( 35 , 36 ) 5FU was kindly provided by Kyowa Hakko Kogyo Company (Tokyo, Japan), while cisplatin and etoposide were both donated by Nippon Kayaku Company (Tokyo, Japan).
Construction of recombinant adenoviruses. Adenovirus (Ad)‐NK4 was constructed as described previously.( 37 , 38 , 39 , 40 ) A control vector expressing the bacterial β‐galactosidase (β‐gal) gene (lacZ) was constructed by the same procedure. Recombinant Ad‐NK4 and Ad‐lacZ were propagated in HEK293 cells.
Adenovirus infection of cells. Cells were infected with Ad‐NK4 or Ad‐lacZ at a multiplicity of infection (MOI) of 10 or 50 as described previously.( 32 ) The culture medium was replaced with fresh medium at 1.5 h after the transfection.
Extraction of proteins from cells infected with Ad‐NK4. SUIT‐2 cells were treated with chemotherapeutic agents and infected with Ad‐NK4 as described above. At 2 days after the infection, the cells were lyzed in 500 µL of ice‐cold lysis buffer.( 32 ) The supernatants were collected, and adjusted to 1.0 mg/mL.
NK4 expression by Ad‐NK4‐infected cancer cells. NK4 levels were measured by enzyme‐linked immunosorbent assay (ELISA) with a Human HGF ELISA Kit (Immunis HGF EIA; Institute of Immunology, Tokyo, Japan) according to the manufacturer's protocol.
Invasion assay. The invasiveness of pancreatic cancer cells was quantified as the number of cells invading through Matrigel‐coated transwell inserts (Becton Dickinson, Franklin Lakes, NJ, US) as described previously.( 41 ) SUIT‐2 cells( 1 ) × 107 were left untreated or treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) for 24 h. After removing culture media containing these agents, the cells were infected with Ad‐lacZ or Ad‐NK4 at an MOI of 50, and the culture media were collected on postinfection day 3. New SUIT‐2 cells were seeded in 24‐well plates at a density of 1 × 105 cells/cm2 in the upper chamber and cultured with 750 µL of conditioned media from the SUIT‐2 cells treated with the chemotherapeutic agents or left untreated and infected with Ad‐lacZ or Ad‐NK4. After 72 h of incubation in the presence of 0.01 ng/mL HGF, cells that had invaded to the lower surface of each Matrigel‐coated membrane were fixed with 70% ethanol, stained with hematoxylin and eosin (H&E), and counted in five randomly selected fields under a light microscope.
Cell proliferation assay. Cell proliferation was evaluated based on the fluorescence intensity of propidium iodide (PI), as described previously.( 42 ) All experiments were performed in triplicate wells.
Assessment of transgene distribution by evaluation of b‐gal expression. At 48 h after Ad‐lacZ infection, β‐gal activity was detected as described previously.( 32 )
Real‐time polymerase chain reaction (PCR) and reverse transcription‐PCR (RT‐PCR) assays. The Ad‐lacZ DNA contents in infected cells were determined by real‐time PCR analysis as described previously( 43 ) with primers for the β‐gal gene (5′‐CACGGCAGAT ACACTTGCTG‐3′ and 3′‐ATCGCCATTTGACCACTACC‐5′).( 44 ) The copy numbers of the viral DNA were calculated from a standard curve of the purified adenovirus vector (cytomegalo virus (CMV)‐β‐gal) and further adjusted relative to the protein concentration of each lysate. The dynamin 2, coxsackie virus and adenovirus receptor (CAR) and β3‐integrin mRNA levels were quantified from 100 ng of total RNA by real‐time RT‐PCR amplification with a QuantiTect SYBR Green RT‐PCR Kit (Qiagen, Valencia, CA, US) and primers specific for dynamin 2 (5′‐AGGAGTACTGGTTTGTGCTGACTG‐3′ and 3′‐GTGCATGATGGTCTTTGGCATGAG‐5′)( 44 ) CAR (5′‐GGCGCTCCTGCTGTGC‐3′ and 3′‐CTTCTCTACTAACTTTTTCGGTTTC‐5′) and β3‐integrin (5′‐GAGGATGACTGTGTCGTCAG‐3′ and 3′‐AAACTCCTTCTTGCGCGGTC‐5′). The dynamin 2, CAR andβ3‐integrin mRNA levels were normalized to the corresponding levels of 18S rRNA amplified with specific primers (5′‐GTAACCCGTTGAACCCCATT‐3′ and 3′‐GCGATGATGGCTAACCTACC‐5′)( 45 ) and expressed as ratios compared with untreated controls.
Electroporation. pcDNA3‐NK4 (2.5 µg, NK4‐expressing plasmid) or pcDNA3 (2.5 µg, empty vector) was mixed with 5 × 106 SUIT‐2 cells and electroporated with a Nucleofector (Amaxa Biosystems GmbH, Koln, Germany) according to the manufacturer's instructions.
Evaluation of chemotherapeutic agent‐induced NK4 expression in vivo in xenografts in severe combined immunodeficient (SCID) mice. To investigate the chemotherapeutic agent‐enhanced NK4 expression induced by Ad‐NK4 in vivo, subcutaneous tumors were established in SCID mice by injection of 5 × 106 SUIT‐2 cells into back and both flanks. After 7 days, two mice were, respectively, treated by intraperitoneal (i.p.) administration of 5FU (10 mg/kg), cisplatin (5 mg/kg) or etoposide (10 mg/kg) and two mice were left untreated as controls. At 24 h after the treatment, 5 × 107 plaque forming unit (pfu) of Ad‐NK4 (100 µL) was injected into the tumors. To examine the expression levels of NK4 protein in the subcutaneous tumors, the mice were killed at 48 h after the administration of Ad‐NK4 and the tumors were excised. Each tumor was homogenized in 300 µL of protein lysis buffer.( 32 ) The protein concentrations in the tumors were adjusted to 10.0 mg/mL with lysis buffer. The NK4 concentrations in each extract were analyzed by ELISA (Immunis HGF EIA).
Statistical analysis. Values are expressed as the mean ± standard deviation (SD). All differences among sets of two groups were analyzed by Student's t‐test. The level of statistical significance was set at P < 0.05. To confirm the induction results, the experiments were repeated at least three times.
Results
Effects of 5FU, cisplatin and etoposide on the expression of a target gene delivered by an adenoviral vector. To investigate the effects of 5FU, cisplatin and etoposide on the expression of a target gene delivered by an adenoviral vector, we measured the NK4 levels in culture media from pancreatic cancer cells infected with Ad‐NK4 with or without treatment with these agents. SUIT‐2 cells( 2 ) × 105 were treated with 5FU (2, 5 or 10 µM), cisplatin (1, 2 or 5 µM) or etoposide (2, 5 or 10 µM) for 24 h, and then infected with Ad‐NK4 at an MOI of 10. Culture media were collected on postinfection days 1, 2 and 3. NK4 expression by Ad‐NK4‐infected cells peaked on day 2 after the transfection (data not shown). As shown in Fig. 1(A) and 5U, cisplatin and etoposide increased the NK4 expression levels at day 2 in dose‐dependent manners (P < 0.05). NK4 expression was not detected in cells that were not infected with Ad‐NK4 (data not shown). Furthermore, AsPC‐1, KP‐1 N and KP‐2 cells (2 × 105 cells for each cell line) were treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) and infected with Ad‐NK4 as described above. As shown in Fig. 1(B), pretreatment of these three cell lines with all three agents increased adenovirus‐induced NK4 expression, consistent with the results for SUIT‐2 cells. To investigate the intracellular NK4 protein levels, we extracted proteins from SUIT‐2 cells treated with the three agents and infected with Ad‐NK4, and measured the levels of NK4. As shown in Fig. 1(C) and 5U and cisplatin significantly increased the intracellular NK4 protein levels in dose‐dependent manners, while etoposide tended to be associated with increased intracellular NK4 protein levels (P = 0.124). These data suggest that 5FU, cisplatin and etoposide can enhance the expression of a target gene delivered by an adenovirus vector.
Figure 1.
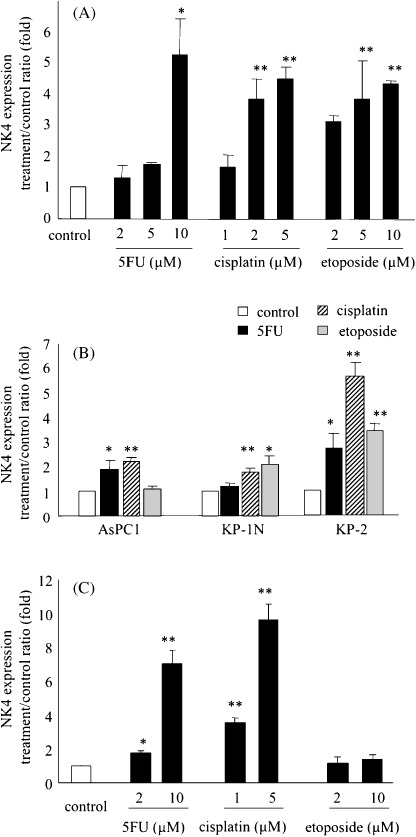
5‐fluorouracil (5FU), cisplatin and etoposide significantly increase NK4 expression in Ad‐NK4‐infected cells. (A) SUIT‐2 cells were treated with 5FU, cisplatin or etoposide for 24 h and then infected with Ad‐NK4 at a multiplicity of infection (MOI) of 10. The NK4 levels in the culture media were measured by enzyme‐linked immunosorbent assay (ELISA) on postinfection day 2. Each value represents the mean ± SD of three independent samples. **P < 0.01. *P < 0.05, compared with control cells. (B) AsPC‐1, KP‐1 N and KP‐2 cells were treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) and then infected with Ad‐NK4 as described in (A). The NK4 levels in the culture media were measured by ELISA on postinfection day 2. **P < 0.01. *P < 0.05, compared with each control cells. (C), Proteins were isolated from SUIT‐2 cells treated with 5FU, cisplatin or etoposide and infected with Ad‐NK4 as described above on postinfection day 2, and the NK4 concentrations were determined by ELISA. Each value represents the mean ± SD of three independent samples. **P < 0.01. *P < 0.05, compared with control cells.
Effects of 5FU, cisplatin and etoposide on β‐gal expression by Ad‐lacZ‐infected cells. To investigate the effects of the chemotherapeutic agents on the expression of another gene delivered by an adenoviral vector, we used Ad‐lacZ instead of Ad‐NK4 and examined the expression of β‐galactosidase by the transfected cells. SUIT‐2 cells (2 × 105) were treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) for 24 h, and then infected with Ad‐lacZ at an MOI of 10. At 48 h after infection, the cells were stained for β‐gal. As shown in Fig. 2(A), large numbers of cells treated with 5FU, cisplatin or etoposide showed the characteristic blue staining indicative of β‐gal activity, while only small numbers of untreated cells were positive for β‐gal. The numbers of β‐gal‐positive cells in five independent fields were significantly higher for the treated cells than for the untreated cells (Fig. 2B, P < 0.001). These data are consistent with those of our Ad‐NK4 experiments.
Figure 2.
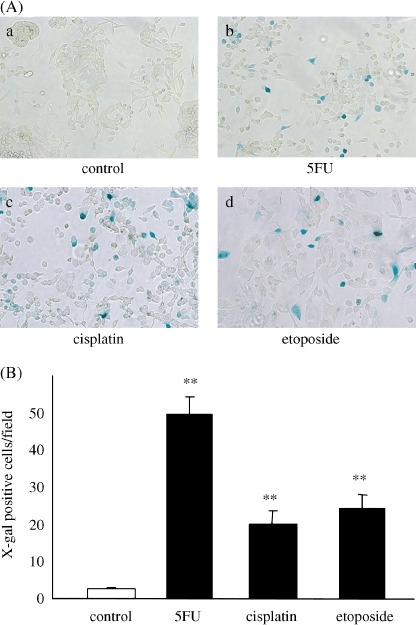
5‐fluorouracil (5FU), cisplatin and etoposide significantly increase β‐galactosidase (β‐gal) expression by Ad‐lacZ‐infected cells. SUIT‐2 cells were treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) for 24 h and then infected with Ad‐lacZ at a multiplicity of infection (MOI) of 10. β‐gal activity was assessed by X‐gal staining at 48 h after infection. (A) Photomicrographs of X‐gal‐stained control or treated cell cultures (×100). a, control; b, 5FU; c, cisplatin; d, etoposide. (B) Numbers of β‐gal‐positive control or treated cells per field. Each value represents the mean ± SD of five independent fields. **P < 0.01, compared with control cells.
Effects of 5FU, cisplatin and etoposide on Ad‐NK4‐mediated inhibition of HGF‐induced invasion of pancreatic cancer cells. We previously reported that NK4 inhibits HGF‐induced invasion of pancreatic cancer cells.( 30 , 31 ) In the present study, we tested the biological effects of the 5FU‐, cisplatin‐ and etoposide‐induced increases in NK4 expression by Ad‐NK4 on the inhibition of invasion of pancreatic cancer cells. SUIT‐2 cells (1 × 107) were left untreated or treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) for 24 h. After removing media containing chemotherapeutic agents, the cells were then infected with Ad‐lacZ or Ad‐NK4 at an MOI of 50 in fresh media, and conditioned media collected on postinfection day 3 were used for the following invasion assay. We used an in vitro invasion assay to examine the inhibitory effects of the five different conditioned media derived from Ad‐lacZ‐infected cells, Ad‐NK4‐infected cells, Ad‐NK4‐infected cells pretreated with 5FU, Ad‐NK4‐infected cells pretreated with cisplatin, or Ad‐NK4‐infected cells pretreated with etoposide, on the HGF (0.01 ng/mL)‐induced invasiveness of new SUIT‐2 cells. The number of invading cells cultured with conditioned media from SUIT‐2 cells infected with Ad‐NK4 was less than that of cells cultured with conditioned media from SUIT‐2 cells infected with Ad‐lacZ (P = 0.011), consistent with a previous report.( 29 ) We further found that the conditioned media from SUIT‐2 cells treated with each of the three chemotherapeutic agents prior to infection with Ad‐NK4 significantly inhibited the invasiveness of pancreatic cancer cells compared with the conditioned media from untreated cells (P < 0.001) (Fig. 3A,B). To investigate the effects of Ad‐NK4‐induced NK4 expression enhanced by pretreatment of cells with 5FU‐, cisplatin‐ and etoposide on the proliferation of pancreatic cancer cells, we collected conditioned media of SUIT‐2 cells infected by Ad‐NK4 with or without pretreatment with chemotherapeutic agents, and evaluated the proliferation of SUIT‐2 cells cultured with these conditioned media 3 days after treatment. We found no differences among the groups examined (Fig. S1), consistent with previous reports.( 46 ) These data suggest that not only was the adenovirus‐delivered NK4 expression enhanced by the chemotherapeutic agents, but the increased NK4 also exerted biological effects as an antagonist of HGF.
Figure 3.
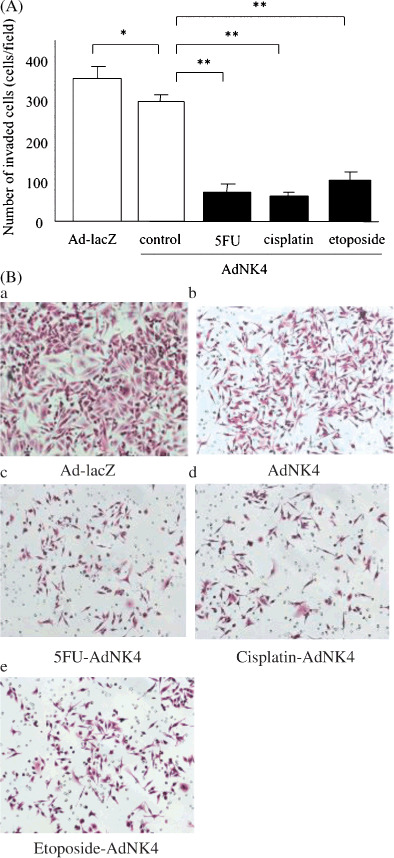
5‐fluorouracil (5FU), cisplatin and etoposide significantly enhance Ad‐NK4‐mediated inhibition of hepatocyte growth factor (HGF)‐induced invasion. SUIT‐2 cells (1 × 107) were left untreated or treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) for 24 h. After removing the media containing chemotherapeutic agents, the cells were then infected with Ad‐lacZ or Ad‐NK4 at a multiplicity of infection (MOI) of 50 in fresh media, and the conditioned media were collected on postinfection day 3. New SUIT‐2 cells were seeded into the upper chambers of 24‐well plates and then exposed to each of the five precollected conditioned media in the presence of 0.01 ng/mL HGF for 72 h. (A) Numbers of cells that invaded to the lower surface of the Matrigel‐coated membranes. Each value represents the mean ± SD of five randomly selected fields. **P < 0.01. *P < 0.05. (B) Photomicrographs of in vitro invasion assays with SUIT‐2 cells cultured with conditioned media derived from Ad‐lacZ‐infected cells (a), Ad‐NK4‐infected cells (b), Ad‐NK4‐infected cells pretreated with 5FU (c), Ad‐NK4‐infected cells pretreated with cisplatin (d) or Ad‐NK4‐infected cells pretreated with etoposide (e). Hematoxylin and eosin stain (×100).
Effects of 5FU, cisplatin and etoposide on adenovirus uptake. Next, we investigated the effects of 5FU, cisplatin and etoposide on adenovirus uptake by pancreatic cancer cells. SUIT‐2 cells were treated with 5FU (2, 5 or 10 µM), cisplatin (1, 2 or 5 µM) or etoposide (2, 5 or 10 µM) for 24 h, and then infected with Ad‐lacZ at an MOI of 10. At 24 h after infection, the viral DNA contents were quantified by real‐time PCR. As shown in Fig. 4(A), the viral DNA contents of the agent‐treated cells at 24 h were significantly higher than that of untreated cells (P < 0.01). These data suggest that 5FU, cisplatin and etoposide increase adenovirus uptake in dose‐dependent manners.
Figure 4.
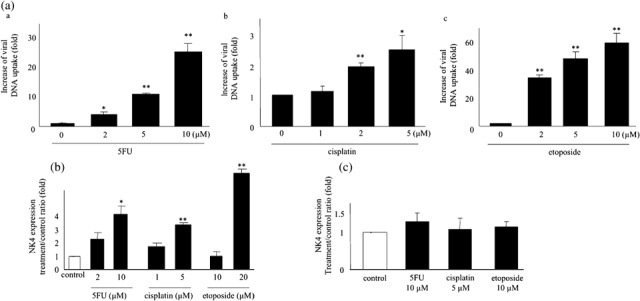
5‐fluorouracil (5FU), cisplatin and etoposide increase adenovirus infection but not CMV promoter activity. (A) SUIT‐2 cells were treated with 5FU (a), cisplatin (b) or etoposide (c) for 24 h and then infected with Ad‐lacZ at a multiplicity of infection (MOI) of 10. DNA was extracted at 24 h after the infection. The viral DNA contents were quantified by real‐time polymerase chain reaction and expressed as the fold increases relative to untreated cells. Each value represents the mean ± SD of triplicate measurements. **P < 0.01. *P < 0.05, compared with control cells. (B) Post‐treatment of cells with 5FU, cisplatin and etoposide increases NK4 expression in Ad‐NK4‐infected cells. SUIT‐2 cells were infected with Ad‐NK4 at an MOI of 10 prior to treatment with 5FU, cisplatin or etoposide. NK4 levels in the culture media were measured by enzyme‐linked immunosorbent assay on postinfection day 2. Each value represents the mean ± SD of three independent samples. **P < 0.01. *P < 0.05, compared with control cells. (C) SUIT‐2 cells were transfected with NK4‐expressing plasmids and then treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM) at 24 h after transfection. NK4 concentrations in culture media were measured on post‐treatment day 1. Each value represents the mean ± SD of three independent samples.
Effects of post‐treatment of cells with chemotherapeutic agents on Ad‐NK4‐induced NK4 expression. To investigate the effects of chemotherapeutic agents on adenovirus‐mediated gene transfer independently of alteration of adenovirus infection at early phase, we infected cells with Ad‐NK4 prior to treatments with 5FU, cisplatin, or etoposide. All three chemotherapeutic agents increased NK4 expression (Fig. 4B), which was similar to the results shown in Fig. 1, although the increases were relatively small.
Effects of post‐treatment of cells with chemotherapeutic agents on expression driven by the CMV promoter. To evaluate the effect of chemotherapeutic agents on the CMV promoter used by Ad‐NK4 and Ad‐lacZ to drive expression of the target gene, SUIT‐2 cells (5 × 106) were transfected with a plasmid expressing NK4 under the control of the CMV promoter, incubated for 24 h, and then left untreated or treated with 5FU (10 µM), cisplatin (5 µM) or etoposide (10 µM). Culture media were collected on day 1 after treatment, and NK4 expression was measured. We observed no significant differences in NK4 expression among the groups examined (Fig. 4C). We also measured NK4 expression in culture media on days 2 and 3 after treatment, and found similar results (data not shown).
Effects of 5FU, cisplatin and etoposide on the expression levels of CAR, dynamin 2 and β3‐integrin. For gene expression, adenoviruses require sequential steps of binding to a cell, endocytosis, endosomal escape, intracellular trafficking and nuclear delivery.( 47 ) Endocytosis of adenoviruses mediated by clathrin‐coated vesicles( 48 , 49 ) requires the action of the large guanosine triphosphatase (GTPase) dynamin as a constrictase.( 50 ) It was recently reported that radiation induces adenovirus infection via dynamin 2 in colon, brain, breast and pancreatic cancers( 43 , 44 , 51 ) and that etoposide acts via CAR.( 21 ) To investigate the effects of 5FU, cisplatin and etoposide on the expression levels of dynamin 2 and CAR in pancreatic cancer cells, we quantified the dynamin 2 and CAR mRNA levels in SUIT‐2 cells using real‐time RT‐PCR. We found that the dynamin 2 mRNA expression levels were significantly higher in cells treated with 5FU (10 µM) and cisplatin (10 µM) at 4 h after treatment (5FU, P = 0.033; cisplatin, P = 0.042), while cells treated with etoposide were not affected (Fig. 5A). On the other hand, CAR mRNA expression was only affected in cells treated with 5FU (P = 0.013) (Fig. 5B). We also examined the expression levels of clathrin mRNA after treatment and found no changes after treatment with all three chemotherapeutic agents (data not shown).
Figure 5.
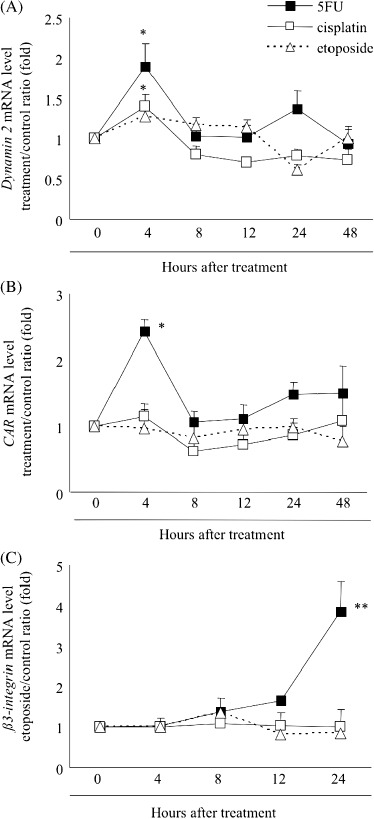
Effects of 5‐fluorouracil (5FU), cisplatin and etoposide on the expression levels of dynamin 2, coxsackie virus and adenovirus receptor (CAR) andβ3‐integrin. (A) Dynamin 2 mRNA was quantified by real‐time reverse transcription polymerase chain reaction from total RNA extracted from SUIT‐2 cells treated with 5FU, cisplatin or etoposide at 4, 8, 12, 24 and 48 h after treatment and expressed as the fold increases relative to untreated cells. (B) CAR mRNA was quantified and expressed as described in (a). (C) β3‐integrin mRNA was quantified and expressed as described in (A). Each value represents the mean ± SD of triplicate measurements. **P < 0.01. *P < 0.05, compared with 0 h cells.
Recently, other cell surface adenovirus receptors, namely av‐integrin, β3‐integrin and β5‐integrin, have been reported to trigger cytoskeletal changes for endocytosis and mediate endosomal escape.( 47 ) Therefore, we investigated the changes in the expression levels of these receptors. The results revealed that β3‐integrin mRNA expression was significantly enhanced by 5FU (P = 0.0019, Fig. 5C), while the expression levels of the other two receptors were not significantly affected (data not shown).
Effects of 5FU, cisplatin and etoposide on the expression of NK4 delivered by Ad‐NK4 in mice xenografts. To evaluate the effects of the chemotherapeutic agents on NK4 expression in pancreatic cancers infected with Ad‐NK4 in vivo, we established subcutaneous tumors treated with 5FU (10 mg/kg; n = 6), cisplatin (5 mg/kg; n = 6) or etoposide (5 mg/kg; n = 6) and untreated tumors (n = 5) in SCID mice. At 24 h after administration of the chemotherapeutic agents into the peritoneal cavity (i.p), 5 × 107 pfu of Ad‐NK4 (100 µL) was injected into each subcutaneous tumor. To examine the levels of NK4 protein expression in the subcutaneous tumors, the mice were killed at 48 h after the administration of Ad‐NK4, and the NK4 concentrations in the tumor lysates were measured by ELISA. The tumors treated with 5FU, cisplatin and etoposide expressed 5.4 ± 1.4‐fold (P = 0.006), 11.8 ± 2.6‐fold (P < 0.001) and 4.9 ± 1.8‐fold (P = 0.017) higher levels of NK4 than the untreated tumors, respectively (Fig. 6). These results are consistent with our in vitro data and suggest that chemotherapeutic agents can enhance the expression of a target gene delivered by an adenovirus vector in vivo.
Figure 6.
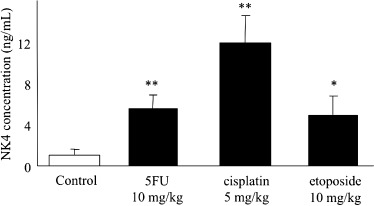
5‐fluorouracil (5FU), cisplatin and etoposide significantly increase NK4 expression in subcutaneous tumors in mice. Subcutaneous tumors were established by injecting 5 × 106 SUIT‐2 cells into back and both flanks of mice on day 0. At 7 days after the injection, six tumors were, respectively, treated with 5FU (10 mg/kg, i.p.), cisplatin (5 mg/kg, i.p.) or etoposide (10 mg/kg, i.p.), and five tumors were left untreated. At 24 h after intraperitoneal administration of chemotherapeutic agents, 5 × 107 pfu of Ad‐NK4 (100 µL) was injected into each tumor. The mice were killed at 48 h after administration of Ad‐NK4, and protein lysates of the tumors were adjusted to 10 mg/mL with lysis buffer. The NK4 concentrations in the tumor extracts were analyzed by enzyme‐linked immunosorbent assay. The tumors treated with 5FU, cisplatin and etoposide express 5.4 ± 1.4‐fold (P = 0.006), 11.8 ± 2.6‐fold (P < 0.001) and 4.9 ± 1.8‐fold (P = 0.017) higher levels of NK4 than the untreated tumors, respectively. Each value represents the mean ± SD of the NK4 expression in the six treated and five untreated tumors, respectively. **P < 0.01. *P < 0.05, compared with control tumors.
Discussion
In the present study, we found that the chemotherapeutic agents 5FU, cisplatin and etoposide could enhance the expression of target genes delivered to pancreatic cancer cells by adenovirus‐based vectors in dose‐dependent manners. Previously, many researchers have reported that adenoviruses enhance chemosensitivity.( 22 , 52 , 53 ) Despite these previous reports focused on the effects of adenovirus‐mediated gene therapy on chemotherapy, there are few reports regarding the effects of chemotherapy on adenovirus‐mediated gene therapy. Recently, Reddy et al.( 54 ) reported that cisplatin enhanced the apoptosis induced by an adenovirus expressing TNF related apotosis inducing ligand (TRAIL) in lung cancer, although the mechanism remained unclear. Furthermore, Shieh et al.( 21 ) reported that low‐dose etoposide enhanced adenoviral gene therapy through up‐regulation of the human telomerase reverse transcriptase (hTERT) promoter activity and adenoviral infection. These recent data suggest that chemotherapy can be a new approach to enhance adenoviral gene therapy. Hecht et al.( 55 ) reported a trial of intratumoral endoscopic ultrasound injection of ONYX‐015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Based on their results, endoscopic ultrasound injection of Ad‐NK4 combined with intravenous chemotherapeutic agents may be one of the promising clinical protocols.
In the present study, we found that adenovirus‐specific gene uptake in pancreatic cancer cells was increased after chemotherapy, suggesting that chemotherapeutic agents enhance adenovirus infection. Zhang et al.( 43 ) reported that radiation improves viral gene uptake in human colon, breast and brain cancer cells, and indicated that the increased adenovirus uptake was mediated by up‐regulation of dynamin 2. On the other hand, Shieh et al.( 21 ) reported that increased adenovirus uptake was mediated by up‐regulation of CAR in bladder cancer. Therefore, chemotherapeutic agents may affect different targets in the adenoviral infection pathway depending on the agent or the types of target cells. To investigate the effects of 5FU, cisplatin and etoposide on the expression levels of dynamin 2 and CAR in pancreatic cancer cells, we quantified the dynamin 2 and CAR mRNA levels in SUIT‐2 cells using real‐time RT‐PCR. 5FU and cisplatin significantly enhanced the expression levels of dynamin 2 mRNA, while etoposide did not. Furthermore, 5FU significantly enhanced the expression of CAR mRNA, while the other two agents did not. 5FU alone enhanced the expression of β3‐integrin mRNA. Therefore, several other mechanisms may underlie chemotherapeutic agent‐induced adenovirus uptake.
In the present study, we found that chemotherapeutic agents enhanced adenovirus‐mediated gene transfer without alteration of adenovirus infection levels in the early phase. However, the effect of post‐treatment of cells with chemotherapeutic agents was relatively small compared with those of pretreatments. The data suggest that there are several mechanisms for chemotherapeutic agent‐induced enhancement of adenoviral gene transfer other than enhanced adenovirus infection in the early phase. Furthermore, we found no significant differences in activation of the CMV promoter that followed by a NK4 cDNA( 40 ) between cells treated with chemotherapeutic agents and untreated cells. We also found that expression of the β3‐integrin receptor, which mediates endocytosis or endosomal escape of adenoviruses( 47 ) was increased after treatment with 5FU. These data suggest that chemotherapy‐induced adenovirus gene transfer can be mediated by altered efficiency of active penetration of the endosomal membrane and escape to the cytosol.
The mortality rate of pancreatic cancer remains the highest among cancers.( 9 ) Although gene therapy with adenovirus vectors is a promising strategy for treatment of cancers, the antitumor effects of a single dose of adenovirus‐mediated gene therapy is often insufficient in clinics( 5 , 18 , 19 ) possibly due to limited transduction efficiency of the adenovirus vectors. In the present study, we found that chemotherapeutic agents, the dosages of which were similar to those used in the clinical setting( 56 , 57 , 58 ) dramatically enhanced adenovirus‐mediated gene expression, but were unable to clarify the mechanism for these effects. Further investigations of the underlying mechanism may lead to the discovery of new approaches that exert the same effects on adenoviral gene therapy as these agents. In conclusion, the present data suggest that chemotherapeutic agents significantly improve the efficiency of adenovirus‐mediated gene transfer in pancreatic tumors. Furthermore, they will probably contribute to decreases in the adenovirus doses required for gene transfer, thereby controlling the side‐effects of adenovirus infection in surrounding normal tissues.
Supporting information
Fig. S1. Cell proliferation assays with new SUIT‐2 cells cultured with precollected conditioned media derived from Ad‐NK4‐infected cells without chemotherapeutic agents (control), Ad‐NK4‐infected cells pretreated with 5‐fluorouracil (5FU) (10 µM), Ad‐NK4‐infected cells pretreated with cisplatin (5 µM) or Ad‐NK4‐infected cells pretreated with etoposide (5 µM). Each value represents the mean ± SD of proliferation ratio of triplicate wells to control at postseeding day 3.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supported in part by a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan
References
- 1. Sohn TA, Yeo CJ. The molecular genetics of pancreatic ductal carcinoma: a review. Surg Oncol 2000; 9: 95–101. [DOI] [PubMed] [Google Scholar]
- 2. Kountouras J, Zavos C, Chatzopoulos D. New concepts of molecular biology on gastric carcinogenesis. Hepato-Gastroenterology 2005; 52: 1305–12. [PubMed] [Google Scholar]
- 3. Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. Colorectal cancer: genetics of development and metastasis. J Gastroenterol 2006; 41: 185–92. [DOI] [PubMed] [Google Scholar]
- 4. Weiner LM, Borghaei H. Targeted therapies in solid tumors: monoclonal antibodies and small molecules. Hum Antibodies 2006; 15: 103–11. [PubMed] [Google Scholar]
- 5. MacKenzie MJ. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol 2004; 5: 541–9. [DOI] [PubMed] [Google Scholar]
- 6. Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors: a promising tool for gene therapy. Appl Biochem Biotechnol 2006; 133: 9–29. [DOI] [PubMed] [Google Scholar]
- 7. Gunzburg WH, Salmons B. Novel clinical strategies for the treatment of pancreatic carcinoma. Trends Mol Med 2001; 7: 30–7. [DOI] [PubMed] [Google Scholar]
- 8. Warshaw AL, Fernandez‐del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–65. [DOI] [PubMed] [Google Scholar]
- 9. Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13 560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg 1995; 82: 111–15. [DOI] [PubMed] [Google Scholar]
- 10. Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev 2002; 2: 897–909. [DOI] [PubMed] [Google Scholar]
- 11. Jaffee EM, Hruban RH, Canto M, Kern SE. Focus on pancreas cancer. Cancer Cell 2002; 2: 25–8. [DOI] [PubMed] [Google Scholar]
- 12. Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res 1995; 55: 1129–38. [PubMed] [Google Scholar]
- 13. Bloomston M, Zervos EE, Rosemurgy AS 2nd. Matrix metalloproteinases and their role in pancreatic cancer. a review of preclinical studies and clinical trials. Ann Surg Oncol 2002; 9: 668–74. [DOI] [PubMed] [Google Scholar]
- 14. Jimeno A, Hidalgo M. Molecular biomarkers: their increasing role in the diagnosis, characterization, and therapy guidance in pancreatic cancer. Mol Cancer Ther 2006; 5: 787–96. [DOI] [PubMed] [Google Scholar]
- 15. Sato N, Goggins M. The role of epigenetic alterations in pancreatic cancer. J Hepatobiliary Pancreat Surg 2006; 13: 286–95. [DOI] [PubMed] [Google Scholar]
- 16. Ogura Y, Mizumoto K, Nagai E et al . Peritumoral injection of adenovirus vector expressing NK4 combined with gemcitabine treatment suppresses growth and metastasis of human pancreatic cancer cells implanted orthotopically in nude mice and prolongs survival. Cancer Gene Ther 2006; 13: 520–9. [DOI] [PubMed] [Google Scholar]
- 17. Murakami M, Nagai E, Mizumoto K et al . Suppression of metastasis of human pancreatic cancer to the liver by transportal injection of recombinant adenoviral NK4 in nude mice. Int J Cancer 2005; 117: 160–5. [DOI] [PubMed] [Google Scholar]
- 18. Mulvihill S, Warren R, Venook A et al . Safety and feasibility of injection with an E1B‐55 kDa gene‐deleted, replication‐selective adenovirus (ONYX‐015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther 2001; 8: 308–15. [DOI] [PubMed] [Google Scholar]
- 19. Sangro B, Mazzolini G, Ruiz J et al . Phase I trial of intratumoral injection of an adenovirus encoding interleukin‐12 for advanced digestive tumors. J Clin Oncol 2004; 22: 1389–97. [DOI] [PubMed] [Google Scholar]
- 20. Topf N, Worgall S, Hackett NR, Crystal RG. Regional ‘pro‐drug’ gene therapy: intravenous administration of an adenoviral vector expressing the E. coli cytosine deaminase gene and systemic administration of 5‐fluorocytosine suppresses growth of hepatic metastasis of colon carcinoma. Gene Ther 1998; 5: 507–13. [DOI] [PubMed] [Google Scholar]
- 21. Shieh GS, Shiau AL, Yo YT et al . Low‐dose etoposide enhances telomerase‐dependent adenovirus‐mediated cytosine deaminase gene therapy through augmentation of adenoviral infection and transgene expression in a syngeneic bladder tumor model. Cancer Res 2006; 66: 9957–66. [DOI] [PubMed] [Google Scholar]
- 22. Lee CT, Seol JY, Lee SY et al . The effect of adenovirus‐IkappaBalpha transduction on the chemosensitivity of lung cancer cell line with resistance to cis‐diamminedichloroplatinum (II) (cisplatin) and doxorubicin (adriamycin). Lung Cancer 2003; 41: 199–206. [DOI] [PubMed] [Google Scholar]
- 23. Lee J, Park JO, Kim WS et al . Phase II study of gemcitabine combined with uracil‐tegafur in metastatic pancreatic cancer. Oncology 2004; 66: 32–7. [DOI] [PubMed] [Google Scholar]
- 24. Endlicher E, Troppmann M, Kullmann A et al . Irinotecan plus gemcitabine and 5‐fluorouracil in advanced pancreatic cancer: a phase II study. Oncology 2007; 72: 279–84. [DOI] [PubMed] [Google Scholar]
- 25. Morizane C, Okusaka T, Furuse J et al . A phase II study of S‐1 in gemcitabine‐refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol 2009; 63: 313–19. [DOI] [PubMed] [Google Scholar]
- 26. Reni M, Cereda S, Mazza E et al . PEFG (cisplatin, epirubicin, 5‐fluorouracil, gemcitabine) regimen as second‐line therapy in patients with progressive or recurrent pancreatic cancer after gemcitabine‐containing chemotherapy. Am J Clin Oncol 2008; 31: 145–50. [DOI] [PubMed] [Google Scholar]
- 27. Macdonald JS, Jacobson JL, Modiano M et al . A phase II trial of etoposide, leucovorin, 5‐FU, and interferon alpha 2b (ELFI) + G‐CSF for patients with pancreatic adenocarcinoma: a Southwest Oncology Group study (SWOG 9413). Invest New Drugs 2000; 18: 269–73. [DOI] [PubMed] [Google Scholar]
- 28. Ebert M, Yokoyama M, Friess H et al . Coexpression of the c‐met proto‐oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res 1994; 54: 5775–8. [PubMed] [Google Scholar]
- 29. Paciucci R, Vila MR, Adell T et al . Activation of the urokinase plasminogen activator/urokinase plasminogen activator receptor system and redistribution of E‐cadherin are associated with hepatocyte growth factor‐induced motility of pancreas tumor cells overexpressing Met. Am J Pathol 1998; 153: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maehara N, Matsumoto K, Kuba K, Mizumoto K, Tanaka M, Nakamura T. NK4, a four‐kringle antagonist of HGF, inhibits spreading and invasion of human pancreatic cancer cells. Br J Cancer 2001; 84: 864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maehara N, Nagai E, Mizumoto K et al . Gene transduction of NK4, HGF antagonist, inhibits in vitro invasion and in vivo growth of human pancreatic cancer. Clin Exp Metastasis 2002; 19: 417–26. [DOI] [PubMed] [Google Scholar]
- 32. Saimura M, Nagai E, Mizumoto K et al . Intraperitoneal injection of adenovirus‐mediated NK4 gene suppresses peritoneal dissemination of pancreatic cancer cell line AsPC‐1 in nude mice. Cancer Gene Ther 2002; 9: 799–806. [DOI] [PubMed] [Google Scholar]
- 33. Tachimori A, Yamada N, Amano R et al . Combination therapy of S‐1 with selective cyclooxygenase‐2 inhibitor for liver metastasis of colorectal carcinoma. Anticancer Res 2008; 28: 629–38. [PubMed] [Google Scholar]
- 34. Okabe T, Okamoto I, Tsukioka S et al . Synergistic antitumor effect of S‐1 and the epidermal growth factor receptor inhibitor gefitinib in non‐small cell lung cancer cell lines: role of gefitinib‐induced down‐regulation of thymidylate synthase. Mol Cancer Ther 2008; 7: 599–606. [DOI] [PubMed] [Google Scholar]
- 35. Nakamura T, Nishizawa T, Hagiya M et al . Molecular cloning and expression of human hepatocyte growth factor. Nature 1989; 342: 440–3. [DOI] [PubMed] [Google Scholar]
- 36. Seki T, Ihara I, Sugimura A et al . Isolation and expression of cDNA for different forms of hepatocyte growth factor from human leukocyte. Biochem Biophys Res Commun 1990; 172: 321–7. [DOI] [PubMed] [Google Scholar]
- 37. Maemondo M, Narumi K, Saijo Y et al . Targeting angiogenesis and HGF function using an adenoviral vector expressing the HGF antagonist NK4 for cancer therapy. Mol Ther 2002; 5: 177–85. [DOI] [PubMed] [Google Scholar]
- 38. McGrory WJ, Bautista DS, Graham FL. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology 1988; 163: 614–17. [DOI] [PubMed] [Google Scholar]
- 39. Korst RJ, Bewig B, Crystal RG. In vitro and in vivo transfer and expression of human surfactant SP‐A‐ and SP‐B‐associated protein cDNAs mediated by replication‐deficient, recombinant adenoviral vectors. Hum Gene Ther 1995; 6: 277–87. [DOI] [PubMed] [Google Scholar]
- 40. Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett 1997; 420: 1–6. [DOI] [PubMed] [Google Scholar]
- 41. Sato N, Maehara N, Mizumoto K et al . Telomerase activity of cultured human pancreatic carcinoma cell lines correlates with their potential for migration and invasion. Cancer 2001; 91: 496–504. [DOI] [PubMed] [Google Scholar]
- 42. Zhang L, Mizumoto K, Sato N et al . Quantitative determination of apoptotic death in cultured human pancreatic cancer cells by propidium iodide and digitonin. Cancer Lett 1999; 142: 129–37. [DOI] [PubMed] [Google Scholar]
- 43. Zhang M, Li S, Li J, Ensminger WD, Lawrence TS. Ionizing radiation increases adenovirus uptake and improves transgene expression in intrahepatic colon cancer xenografts. Mol Ther 2003; 8: 21–8. [DOI] [PubMed] [Google Scholar]
- 44. Qian J, Yang J, Dragovic AF, Abu‐Isa E, Lawrence TS, Zhang M. Ionizing radiation‐induced adenovirus infection is mediated by Dynamin 2. Cancer Res 2005; 65: 5493–7. [DOI] [PubMed] [Google Scholar]
- 45. Ohuchida K, Mizumoto K, Ohhashi S et al . S100A11, a putative tumor suppressor gene, is overexpressed in pancreatic carcinogenesis. Clin Cancer Res 2006; 12: 5417–22. [DOI] [PubMed] [Google Scholar]
- 46. Date K, Matsumoto K, Kuba K et al . Inhibition of tumor growth and invasion by a four‐kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene 1998; 17: 3045–54. [DOI] [PubMed] [Google Scholar]
- 47. Medina‐Kauwe LK. Endocytosis of adenovirus and adenovirus capsid proteins. Adv Drug Deliv Rev 2003; 55: 1485–96. [DOI] [PubMed] [Google Scholar]
- 48. Patterson S, Russell WC. Ultrastructural and immunofluorescence studies of early events in adenovirus‐HeLa cell interactions. J General Virol 1983; 64: 1091–9. [DOI] [PubMed] [Google Scholar]
- 49. Varga MJ, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol 1991; 65: 6061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP‐gamma S in nerve terminals. Nature 1995; 374: 186–90. [DOI] [PubMed] [Google Scholar]
- 51. Egami T, Ohuchida K, Mizumoto K et al . Radiation enhances adenoviral gene therapy in pancreatic cancer via activation of cytomegalovirus promoter and increased adenovirus uptake. Clin Cancer Res 2008; 14: 1859–67. [DOI] [PubMed] [Google Scholar]
- 52. Hayashi N, Asano K, Suzuki H et al . Adenoviral infection of survivin antisense sensitizes prostate cancer cells to etoposide in vivo . Prostate 2005; 65: 10–9. [DOI] [PubMed] [Google Scholar]
- 53. Lee WP, Tai DI, Tsai SL et al . Adenovirus type 5 E1A sensitizes hepatocellular carcinoma cells to gemcitabine. Cancer Res 2003; 63: 6229–36. [PubMed] [Google Scholar]
- 54. Reddy RM, Tsai WS, Ziauddin MF et al . Cisplatin enhances apoptosis induced by a tumor‐selective adenovirus expressing tumor necrosis factor‐related apoptosis‐inducing ligand. J Thorac Cardiovasc Surg 2004; 128: 883–91. [DOI] [PubMed] [Google Scholar]
- 55. Hecht JR, Bedford R, Abbruzzese JL et al . A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX‐015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res 2003; 9: 555–61. [PubMed] [Google Scholar]
- 56. Kikuchi K, Kanno H. Comparison for blood levels and clinical effects between tablet and other dossge forms of 5‐fluorouracil. Gan Kagaku Ryoho 1979; 6: 559–65. [Google Scholar]
- 57. Sawada M, Okudaira Y, Matsui Y et al . Pharmacokinetics of cisplatin. Sanfujinka No Jissai 1983; 32: 2117–22. [Google Scholar]
- 58. Park K. Clinical trial of NK 171s (etoposide miniaturized hard capsule) on malignant lymphoma. Kiso Rinshou 1992; 26: 1136–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cell proliferation assays with new SUIT‐2 cells cultured with precollected conditioned media derived from Ad‐NK4‐infected cells without chemotherapeutic agents (control), Ad‐NK4‐infected cells pretreated with 5‐fluorouracil (5FU) (10 µM), Ad‐NK4‐infected cells pretreated with cisplatin (5 µM) or Ad‐NK4‐infected cells pretreated with etoposide (5 µM). Each value represents the mean ± SD of proliferation ratio of triplicate wells to control at postseeding day 3.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


