Abstract
Gene expression profiles of HCC and surrounding non‐cancerous tissues in rats fed a CDAA diet for 70 weeks, as well as normal liver tissues, were explored using an oligonucleotide microarray for 3757 genes. A total of 146 genes were identified as differentially expressed; the affected functions including metabolism, apoptosis, cell cycling, RNA splicing, Wnt signaling, reactive oxygen species‐induced stress, and fibro/cirrhogenesis. The genes were found to fit into four distinct expression patterns after classification by hierarchical and k‐means clustering procedures. Notably, genes within the same functional category tended to be found within the same cluster, thus gene functions appeared to be related to their expression patterns. For example, genes encoding receptors (Fisher's exact test, P < 0.01) and cytokines (Fisher's exact test, P < 0.05) were both enriched in a cluster characterized by low expression in HCC compared to their surrounding tissues. While some of the receptors in this cluster had cell‐proliferative potential, others are known to be growth‐suppressive. It was noted, however, that four of the 10 receptor genes encode G‐protein‐coupled receptors, for which growth‐suppressive potential has been reported. The seven growth factors in the same cluster included two fibroblast growth factors. The current findings suggest the possibility that genes differentially expressed in this multistep carcinogenic model may be classified into relatively few clusters according to their expression patterns, and that these clusters may be associated with gene functional categories. (Cancer Sci 2005; 96: 414 – 424)
Abbreviations:
- CDAA
choline‐deficient, l‐amino acid‐defined
- HCC
hepatocellular carcinoma
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- RT‐PCR
reverse transcription polymerase chain reaction.
Hepatocellular carcinomas are common in Asia and Africa, and the incidence is increasing in Europe and North America. The prognosis for HCC is extremely poor. In human HCC, both genetic and epigenetic alterations have been detected with regard to particular genes such as p53, cyclin D, p16 lnk4 , p21 Waf1/ Cip1 , Rb, β‐catenin, mannose‐6‐phosphate/insulin‐like growth factor II receptor (M6P/IGF2R), E‐cadherin, cyclo‐oxygenase (COX)‐2, and telomerase reverse transcriptase (hTERT). ( 1 , 2 , 3 ) In addition to studies on individual genes, microarray technology has allowed the exploration of comprehensive changes in expression during HCC development. To gain insight into the overall picture, suitable animal models are necessary so that samples reflecting various stages of carcinogenesis can be collected. It should be noted, however, that in animal models featuring the use of chemical carcinogens, unavoidable carcinogen‐specific molecular alterations may mask generic and essential changes. In addition, the major risk factor in human HCC has been established as being continuous chronic liver injury, which occurs in hepatitis virus infections.( 1 , 2 , 3 ) Thus we have chosen to use an animal model that employs the administration of a CDAA diet, which induces HCC on a background of continuous hepatic injury and cirrhosis. It has been confirmed that this model resembles human carcinogenesis caused by chronic viral hepatitis, hemochromatosis, and Wilson's disease in many respects.( 1 , 2 , 3 , 4 )
The CDAA diet is hepatocarcinogenic in male rats of Fischer 344 and Wistar strains.( 4 ) As with other diets deficient in choline and low in methionine (CMD diets), HCC are induced in the absence of chemical carcinogens. In the CDAA model, HCC occur at a high rate through the induction and growth of preneoplastic hepatocellular lesions, which are followed by progression to hepatocellular adenomas and subsequent conversion to malignancy.( 4 ) Chronic feeding of CMD diets in rats has also been accepted as an animal model for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH), a condition in which a substantial number of patients develop HCC.( 5 ) Here we have performed microarray analyses to obtain gene‐expression profiles for HCC and surrounding non‐cancerous tissues in CDAA treated rats, as well as normal liver samples, so as to gain insight into the mechanisms of multistep hepatic carcinogenesis.
Materials and Methods
Ethical considerations. The experimental protocols were approved by the Animal Experimentation Committee of the Sasaki Institute prior to their execution. The experiment was conducted under monitoring by the committee in accordance with the National Institute of Health's Guidelines for the Care and Use of Laboratory Animals, Japanese Government Animal Protection and Management Law Number 105, and Japanese Government Notification on Feeding and Safekeeping of Animals Number 6.
Animals, diets and animal treatment. A total of 10 male 5‐week‐old Fischer 344 rats were purchased from Charles River Japan (Atsugi, Kanagawa, Japan). They were divided into two groups of five animals, housed in plastic cages with white‐flake bedding in an airconditioned room (25 ± 3°C temperature, 55 ± 8% relative humidity, 10–12 times/h ventilation and 12‐h dark/light cycle). They were used after a 1‐week acclimation on basal diet (CRF‐1, Oriental Yeast Corporation, Itabashi, Tokyo, Japan) and allowed free access to food and tap water throughout the acclimation and experimental periods. Bodyweight, food consumption, and water intake were monitored weekly. The CDAA diet was obtained from Dyets (Bethlehem, PA, USA).
After acclimation, group 1 rats were administered the basal diet, while group 2 rats were placed on the CDAA diet for 70 weeks and then killed. The livers were then macroscopically examined. Portions of group 1 livers, and the macroscopic tumors and surrounding non‐cancerous tissues of group 2 livers were fixed in 10% neutrally buffered‐formalin for 24 h, embedded in paraffin, processed for the routine HE staining procedure, and histologically examined. Remaining portions of these three types of liver tissues were immediately frozen in liquid nitrogen and stored at −80°C. Group 1 liver tissues, group 2 liver tissues from non‐cancerous areas, and group 2 liver tissues from tumors (after being histologically diagnosed as HCC) were employed as normal liver (CON), surrounding non‐cancerous liver (NC), and HCC samples (CA). The microarray and RT‐PCR experiments and the subsequent data analysis were performed using five CON samples from individual group 1 rats and five matched pairs of NC and CA samples from individual group 2 animals.
RNA isolation and probe labeling. Total RNA from liver tissues was isolated using an RNeasy Midi kit (QIAGEN, Hilden, Germany) and its integrity checked by electrophoresis on 1% agarose‐formaldehyde gels. Five micrograms of total RNA from individual samples were then labeled with Cy3 (Amersham Biosciences, Uppsara, Sweden) using a BD Atlas PowerScript Fluorescent Labeling kit (BD Biosciences Clontech, Palo Alto, CA, USA) according to the manufacturer's protocols.
Hybridization, scanning and quantification. Cy3‐labeled probes were hybridized to Atlas Rat 3.8 I microarrays (BD Biosciences Clontech) containing 3757 genes for 16 h at 50°C. After hybridization, microarrays were washed, dried and scanned using a GMS 418 confocal laser scanner (Genetic MicroSystems, Woburn, MA, USA). Fluorescence intensities of the Cy3 channels were quantified using ImaGene 4.0 software (BioDiscovery, El Segundo, CA, USA).
Microarray analysis and annotation of gene function. Data analysis was performed using GeneSpring software, version 5.1 (Silicon Genetics, Redwood City, CA, USA), including appropriate statistics. Dividing by the median calculated from all of the signal intensities for the Cy3 in a given sample, the fluorescence signal for each gene was normalized and the expression ratio was then calculated by dividing the normalized signal by the median for each gene to offset differences in expression levels between genes. Values were displayed as average of five animal samples for the three groups and were then logged (base 10) for further analyses. Standard deviations of the normalized values were calculated for the three groups, and genes in which standard deviation was more than two for any of the three groups were excluded. Genes differentially expressed among the three tissue types were detected by one‐way anova (P = 0.02).
The functions of the genes were assigned referring to a table attached to Atlas Rat 3.8 I microarrays (BD Biosciences Clontech), and classified into 21 categories: cell surface antigens, transcription factors, cell cycle‐related factors, cell adhesion receptors/proteins, extracellular transport/carriers, stress response proteins, membrane channels/transporters, extracellular matrix proteins, trafficking/targeting proteins, metabolism‐related factors, post‐translational modification/protein folding‐related factors, translation‐related factors, apoptosis‐related factors, RNA processing/turnover/transport‐related factors, DNA binding and chromatin proteins, cell receptors, cell signaling/extracellular communicating factors, intracellular transducers/effectors/modulators, protein turnover‐related factors, cytoskeleton/motility proteins, and others. Statistical significance for frequencies of genes of each functional category in each cluster was assessed as follows: values of the other categories in the relevant cluster, values of the other clusters in the relevant category, and values of the other clusters in the other categories were all combined, and the resultant combined values were compared with the relevant value by Fisher's exact test for the 2 × 2 table.
Semi‐quantitative RT‐PCR. Semi‐quantitative RT‐PCR was performed for seven genes, at least one gene from each cluster. They were endothelin receptor B (EDNRB), zinc finger protein 265 (ZNF265), neurofibromatosis type 1 (NF1), hepatic nuclear factor 1/transcription factor 1 (HNF1/TCF1), leukemia/lymphoma related factor (LRF), cyclin L (CCNL), and lamin A (LMNA). cDNA was synthesized from 3 µg of total RNA using a First‐Strand cDNA Synthesis kit (Amersham Biosciences) and oligo(dT)18 primer according to the manufacturer's instructions. RT‐PCR was carried out using the GeneAmp PCR system 9700 (Applied Biosystems, Foster City, CA, USA) and cycling conditions were 2 min at 94°C, followed by 30 cycles (25 cycles for β‐actin) of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. Forward and reverse primer sequences were: EDNRB, 5′‐TTGATGTGATTACGTCGGAC‐3′ and 5′‐GGACTGTTTTTCCTCAAACG‐3′; ZNF265, 5′‐AGAAGTACTACATCTGCTAG‐3′ and 5′‐TTCCCAGTTGTCAGCTTTGC‐3′; NF1, 5′‐GTACACCAAATACCATGAGC‐3′ and 5′‐ATGAAGAGGGTGTTGTTGGC‐3′; HNF1/TCF1, 5′‐TGCACTAGAAAGGCTGCTTC‐3′ and 5′‐GGTTTCTTGCAGTACCGAGG‐3′; LRF, 5′‐TGGGCCGCCTGAATGTAGCG‐3′ and 5′‐GTATGTCAGTGGTGGCCATG‐3′; CCNL, 5′‐TAATAGGCGAAGTCGATCTG‐3′ and 5′‐CATCGTCACACTGCATATGG‐3′; LMNA, 5′‐ATGAGAGCAGGTCTGAAGCC‐3′ and 5′‐AAGCATGGCAGATTTGCCTC‐3′; β‐actin (used as control), 5′‐TTGAACACGGCATTGTAACC‐3′ and 5′‐ATCTCTTGCTCGAAGTCTAG‐3′. PCR products were analyzed on 2% agarose gels with ethidium bromide and subsequently underwent densitometry. The obtained values were then normalized to those for β‐actin.
Western blotting. Western blotting was conducted using the liver samples. Liver tissues were homogenized in 5 volumes of extraction buffer (10 mM Tris‐HCl, pH 6.8, 1% SDS) using a polytron homogenizer at setting 7 for 90 s. The homogenates were heated on boiling water for 5 min and centrifuged at 13 000 × g for 30 min. The supernatants were used for western blotting. Protein concentrations of the lysates were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). Samples containing 5 µg of protein were separated on 7.5% SDS polyacrylamide gel electrophoresis and transferred to Hybond‐P PVDF membranes (Amersham Biosciences, Buckinghamshire, UK). The blots were blocked with 5% dried milk in PBS for 1 h, incubated with 1:200 dilution of rabbit anti‐EDNRB polyclonal antibody (Chemicon International, Temecula, CA, USA), washed in 0.1% Tween 20 in PBS, and incubated with 20 000‐fold diluted antirabbit donkey IgG conjugated with peroxidase (Amersham Biosciences). Both primary and secondary antibodies were diluted with 0.1% Tween 20 in PBS, and incubation was at room temperature for 1 h. The immunoreactive bands were detected using ECL Plus Western Blotting Detection Reagents (Amersham Biosciences).
Results
General findings. All rats survived until their scheduled killing in relatively healthy conditions, but the mean bodyweight of group 2 animals was lighter than that of group 1 animals (Table 1). Group 1 livers showed no particular pathological changes either macroscopically or histologically. All of group 2 livers were macroscopically yellowish‐white and appeared cirrhotic with 1 or 2 large tumoral nodules with a dark color. The mean relative liver weight was greater in group 2 compared to group 1 animals (Table 1). Tumors were histologically diagnosed as relatively well‐differentiated HCC, chiefly showing a trabecular pattern. The surrounding non‐cancerous areas were cirrhotic, featuring intrahepatocellular fat accumulation, frequent hepatocellular apoptosis, and nuclear divisions of hepatocytes. These findings are in accordance with our previous results.( 4 ) Representative histology of the three groups of liver samples is shown in Figure 1.
Table 1.
Final body and relative liver weights
| Group | Treatment | Animal | Final bodyweight (g) | Relative liver weight (g/100 g bodyweight) |
|---|---|---|---|---|
| 1 | Control | 1 | 456 | 3.26 |
| 2 | 448 | 3.04 | ||
| 3 | 462 | 2.78 | ||
| 4 | 460 | 2.88 | ||
| 5 | 450 | 3.12 | ||
| Mean | 455 | 3.02 | ||
| Standard deviation | 6 | 0.19 | ||
| 2 | CDAA | 6 | 416 | 5.12 |
| 7 | 422 | 4.88 | ||
| 8 | 418 | 4.66 | ||
| 9 | 408 | 5.38 | ||
| 10 | 404 | 4.72 | ||
| Mean | 414* | 4.95* | ||
| Standard deviation | 7 | 0.3 |
Significantly different from the group 1‐value (P = 0.0079 by the two‐tailed Mann–Whitney non‐parametric test).
Figure 1.
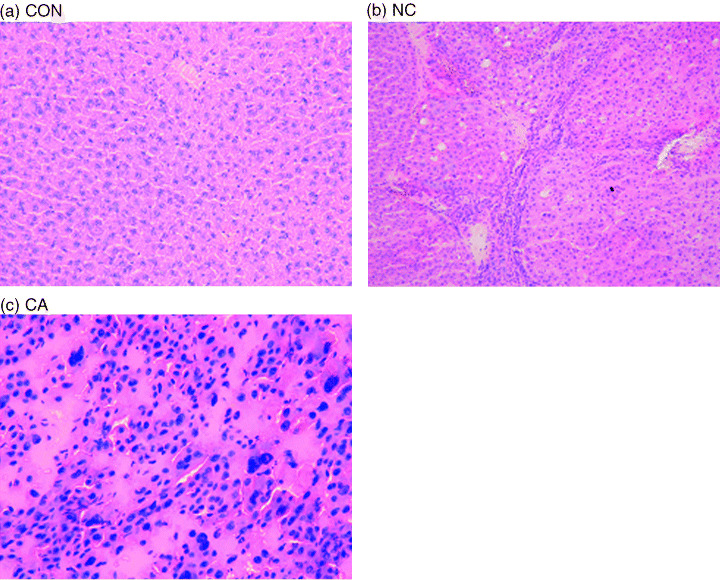
Representative histology of the liver groups: CON, normal liver (a); NC, surrounding non‐cancerous liver (b); CA, HCC sample (c). HE staining.
Changes in gene expression during carcinogenesis. We compared the gene expression profiles among CON, NC and CA using oligonucleotide microarrays containing 3757 genes. A total of 146 genes were detected as differentially expressed among the three tissue types. To verify the results of microarray analysis, expression of a total of seven genes (at least one gene from each cluster) was investigated by semiquantitative RT‐PCR. These genes are involved in cell functions important in tumor progression, such as transcriptional regulation (HNF1/TCF1 and LRF), RNA splicing (ZNF265 and CCNL), signal transduction (NF1 and EDNRB), and nuclear structure (LMNA) (Fig. 2). For comparison, the relative expression levels of CON were set to one. The expression patterns among the three sample groups were similar for these genes, although there are some discrepancies (Fig. 2). We considered that the results of microarray analysis were generally reproducible in RT‐PCR, but the causes of the discrepancies are yet to be investigated.
Figure 2.
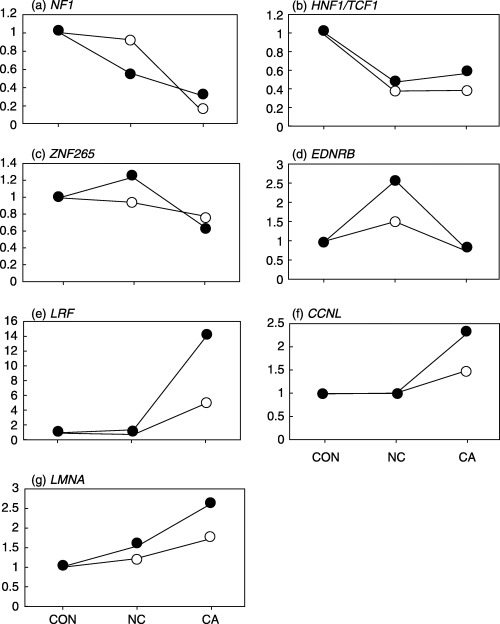
Comparison of results obtained with microarray and semiquantitative RT‐PCR for relative mRNA expression levels among normal liver (CON), surrounding non‐cancerous liver (NC), and HCC samples (CA). The average expression levels of CON samples were set at 1. (○), data from RT‐PCR; (•), data from microarray analysis. The indicated data for RT‐PCR are from a single representative experiment, which was reproduced at least three times. (a), neurofibromatosis 1 (NF1); (b), hepatic nuclear factor 1/transcription factor 1 (HNF1/TCF1); (c), zinc finger protein 265 (ZNF265); (d), endothelin receptor B (EDNRB); (e), leukemia/lymphoma related factor (LRF); (f), cyclin L (CCNL); (g), lamin A (LMNA).
Western blotting. In some cases, expression intensities at mRNA level and protein levels are not in parallel. So, we have investigated the expression levels of EDNRB by western blotting for comparison with data obtained by microarray and semiquantitative RT‐PCR. At protein levels, the tendency of EDNRB expression was as follows: CON < NC > CA (Fig. 3a,b). So, in the case of EDNRB, changes in mRNA levels appear to be accompanied by changes in protein levels. High expression levels of EDNRB in rat( 6 ) and human( 7 ) liver cirrhosis compared to normal liver tissues have already been reported. The activated endothelin system can increase tonality of the hepatic microvasculature, which may contribute to the formation of liver cirrhosis.( 6 , 7 )
Figure 3.
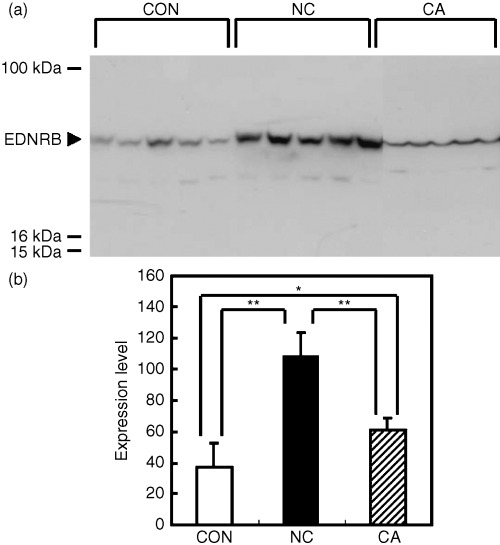
(a) Expression of endothelin receptor B (EDNRB) detected in rat‐liver samples by western blotting. (b) Protein levels of EDNRB compared between normal liver (CON), surrounding non‐cancerous liver (NC), and HCC sample (CA). Data are expressed as mean ± SD in arbitrary units. There was a significant difference in expression levels among the three groups by anova (P < 0.01). *P < 0.05 and **P < 0.01 by Student–Neuman–Keuls’ test. The indicated data for western blotting are from a single representative experiment that was reproduced three times.
Detection of multiple patterns of expression with CON, NC and CA. Only the 146 genes that showed differential expressions were subjected to further analysis to prevent invariant genes from negatively affecting the clustering results. We first used a hierarchical clustering procedure and a dendrogram for classification of genes based on expression patterns (Fig. 4a). The genes were thereby divided along two major branches and then subdivided into two branches (Fig. 4a). Accordingly, we chose four for the total number of clusters in the subsequent k‐means clustering analysis. The gene members contained in the four clusters obtained by k‐means clustering were identical to those obtained by hierarchical clustering. We named them clusters 1–4, containing 18, 54, 45, and 29 members shown in 2, 3, 4, 5, respectively. Each of the four clusters had its own different pattern of expression profiles, with tendencies as follows: Cluster 1, CON > NC ≈ CA; cluster 2, CON < NC > CA [≈ CON]; cluster 3, CON ≥ NC < CA [> CON]; cluster 4, CON < NC < CA (Fig. 4b).
Figure 4.
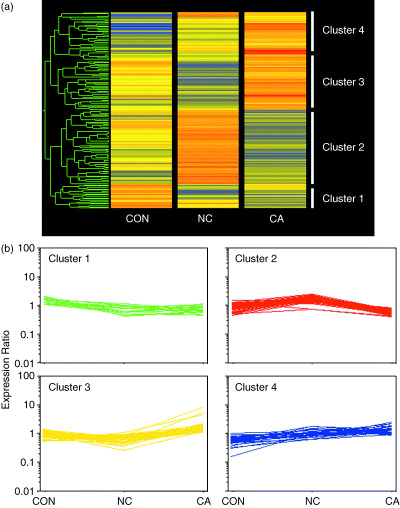
Gene expression profiles among normal liver (CON), surrounding non‐cancerous liver (NC), and HCC samples (CA). One hundred and forty‐six genes were identified as demonstrating differential expressions among the three tissue types and subjected to further analysis. (a) Hierarchical clustering. A dendrogram is shown on the left side of the diagram. Tissue types are represented by columns and genes in rows. Red, yellow and blue represent the higher, equal and lower mRNA levels, respectively, relative to that of the median of each gene. The dendrogram indicates that data are divided along two main branches, each divides further into two branches. (b) Expression patterns of the genes included in the four clusters. The 146 genes were classified by hierarchical and k‐means clustering procedures. Expression ratios of the genes are indicated.
Table 2.
Genes of cluster 1
| Category | Gene name | GenBank accession number | Expression ratio | ||
|---|---|---|---|---|---|
| CON | NC | CA | |||
| Cell surface antigens | CD48 antigen | X13016 | 1.59 | 1.13 | 0.74 |
| Transcription factors | D site albumin promoter binding protein | J03179 | 1.28 | 0.61 | 0.46 |
| Hepatic nuclear factor 1/TCF1 | J03170 | 1.66 | 0.62 | 0.66 | |
| Cell cycle‐related factors | Synaptonemal complex protein 3 | X75785 | 1.84 | 0.99 | 0.56 |
| Cell adhesion receptors/proteins | Milk fat glolbule membrane protein/O‐acetyltransferase | D84068 | 1.57 | 0.44 | 0.63 |
| Extracellular matrix proteins | Collagen type X α1 | AJ131848 | 1.41 | 0.91 | 0.99 |
| Metabolism‐related factors | α‐1,3‐Fucosyltransferase | U58860 | 1.82 | 0.99 | 0.71 |
| UDP‐GalNAc:polypeptide N‐acetylgalactosaminyltransferase T5 | AF049344 | 1.15 | 0.82 | 0.81 | |
| Glucose‐6‐phosphatase | D78592 | 1.77 | 0.50 | 0.46 | |
| Branched chain aminotransferase 1 | AF165887 | 1.29 | 0.76 | 0.90 | |
| Post‐translational modification/ protein folding‐related factorsTranslation‐related factors | FK506‐binding protein 1 | D86641 | 1.95 | 0.57 | 1.12 |
| Ubiquitin‐conjugating enzyme E2D 3 | AB006852 | 1.41 | 0.76 | 0.64 | |
| Ribosomal protein S15a | X77953 | 1.47 | 0.79 | 1.10 | |
| Cytoskeleton/motility proteins | Keratin complex 1, acidic, gene 18 | U67992 | 1.09 | 0.55 | 0.94 |
| Others | Ras homolog enriched in brain | U08227 | 1.05 | 1.00 | 0.65 |
| Peptidyl arginine deiminase, type 3 | D88034 | 2.05 | 0.42 | 0.59 | |
| Prostatic binding protein | X05034 | 1.69 | 1.01 | 0.48 | |
| Scrapie responsive gene 1 | AJ132434 | 1.17 | 0.63 | 0.83 | |
CON, normal liver; NC, surrounding non‐cancerous liver; CA, HCC sample.
Table 3.
Genes of cluster 2
| Category | Gene name | GenBank accession number | Expression ratio | ||
|---|---|---|---|---|---|
| CON | NC | CA | |||
| Cell surface antigens | CD36 antigen‐like 2 | M68965 | 0.73 | 2.10 | 0.67 |
| Membrane channels/transporters | Inositol 1,4,5‐triphosphate receptor 3 | L06096 | 0.87 | 1.56 | 0.63 |
| X transporter protein 2 | U12973 | 0.87 | 1.67 | 0.45 | |
| ATPase, H+K+ transporting, non‐gastric, α polypeptide | M90398 | 0.69 | 1.65 | 0.55 | |
| Dopa decarboxylase | M27716 | 0.87 | 1.41 | 0.73 | |
| Synaptotagmin 2 | M64488 | 1.19 | 1.63 | 0.55 | |
| Fatty acid binding protein 2 | M35992 | 0.74 | 1.78 | 0.56 | |
| Interleukin‐2 receptor, β chain | M55050 | 1.15 | 1.44 | 0.55 | |
| Metabolism‐related factors | Cytochrome P450 1B1 | U09540 | 1.32 | 2.48 | 0.70 |
| Sucrose isomaltase | X15546 | 1.03 | 2.22 | 0.69 | |
| Propionyl coenzyme A carboxylase, β polypeptide | M14634 | 1.30 | 1.56 | 0.62 | |
| Mevalonate kinase | M29472 | 0.45 | 1.36 | 0.64 | |
| Hexokinase 2 | M68971 | 0.56 | 1.51 | 0.61 | |
| Plasma glutathione peroxidase precursor | D00680 | 1.34 | 1.19 | 0.52 | |
| Testis lipid binding protein | U07870 | 1.03 | 1.86 | 0.68 | |
| Lactate dehydrogenase 3, C chain | U07177 | 1.01 | 1.41 | 0.71 | |
| Solute carrier family 18, member 2 | M97381 | 0.56 | 1.71 | 0.68 | |
| Glycerol kinase | D16102 | 1.05 | 1.97 | 0.64 | |
| Cytochrome P450 3A9 | U60085 | 1.11 | 1.25 | 0.56 | |
| Apoptosis‐related factors | Caspase 3 | U49930 | 1.01 | 1.33 | 0.69 |
| α‐Inhibin | M36453 | 1.18 | 1.50 | 0.83 | |
| RNA processing/turnover/ transport‐related factors | Zinc finger protein 265 | AF013967 | 1.18 | 1.47 | 0.78 |
| Cell receptors | Opioid receptor, κ1 | D16829 | 0.82 | 1.55 | 0.78 |
| Neural receptor protein‐tyrosine kinase TrkB | M55291 | 0.51 | 1.42 | 0.67 | |
| Somatostatin receptor subtype 1 | X62314 | 1.01 | 2.15 | 0.58 | |
| Platelet‐derived growth factor receptor α | M63837 | 0.63 | 1.88 | 0.75 | |
| Diphtheria toxin receptor | L05489 | 0.79 | 1.84 | 0.67 | |
| Glutamate receptor, ionotropic, δ1 | U08255 | 0.95 | 1.72 | 0.72 | |
| Interleukin‐1 receptor‐like 1 | U04317 | 0.72 | 1.70 | 0.45 | |
| Endothelin receptor type A | X57764 | 0.60 | 1.53 | 0.50 | |
| Interleukin‐1 receptor, type I | M95578 | 0.80 | 1.47 | 0.56 | |
| Endothelin receptor type B | M60786 | 0.93 | 1.56 | 0.54 | |
| Cell signaling/extracellular communicatimg factors | β‐Nerve growth factor | M36589 | 0.54 | 1.72 | 0.81 |
| Cytokine‐induced neutrophil chemoattractant‐2 | D21095 | 1.29 | 1.45 | 0.46 | |
| Placental growth factor | L40030 | 0.96 | 1.90 | 0.42 | |
| Neurotrophin‐3 | M34643 | 1.23 | 1.36 | 0.74 | |
| Fibroblast growth factor‐5 | D64085 | 1.18 | 2.43 | 0.57 | |
| c‐fos induced growth factor | AF014827 | 0.97 | 1.82 | 0.66 | |
| Fibroblast growth factor‐9 | D14839 | 0.76 | 1.92 | 0.65 | |
| Intracellular transducers/ effectors/modulators | Annexin 1/p35/lipocortin 1 | M19967 | 1.04 | 1.37 | 0.61 |
| Insulin receptor substrate 1 | X58375 | 1.03 | 1.67 | 0.77 | |
| Neurofibromatosis type 1 | D45201 | 1.32 | 0.75 | 0.40 | |
| Phosphatidylinositol 4,5‐bisphosphate 5‐phosphatase, A | AB032551 | 0.84 | 1.48 | 0.47 | |
| Endothelin converting enzyme‐like 1 | AB026293 | 0.85 | 1.79 | 0.79 | |
| Src related tyrosine kinase | U09583 | 0.77 | 1.65 | 0.61 | |
| S6 kinase | M58340 | 0.99 | 1.79 | 0.51 | |
| Guanine nucleotide binding protein, γ7 | L23219 | 1.11 | 1.44 | 0.78 | |
| Polo‐like kinase homolog | U10188 | 1.02 | 1.40 | 0.47 | |
| Protein tyrosine phosphatase, non‐receptor type 5 | S49400 | 1.04 | 1.46 | 0.61 | |
| Cytoskeleton/motility proteins | Troponin 1, type 2 | M73701 | 0.56 | 1.89 | 0.77 |
| Others | P‐glycoprotein/multidrug resistance 1 | M81855 | 1.04 | 1.53 | 0.57 |
| Secretory zymogen granule membrane glycoprotein GP2 | M58716 | 1.05 | 1.53 | 0.56 | |
| Probasin | M27156 | 0.80 | 1.54 | 0.42 | |
| Palmitoyl‐protein thioesterase | L34262 | 0.51 | 2.30 | 0.62 | |
CON, normal liver; NC, surrounding non‐cancerous liver; CA, HCC sample.
Table 4.
Genes of cluster 3
| Category | Gene name | GenBank accession number | Expression ratio | ||
|---|---|---|---|---|---|
| CON | NC | CA | |||
| Cell surface antigens | MHC class II antigen RT1.B‐1 β‐chain | X56596 | 0.85 | 0.49 | 1.44 |
| Transcription factors | Hepatic nuclear factor 4 | D10554 | 1.09 | 0.60 | 1.56 |
| Mini chromosome maintenance deficient 6 | U17565 | 1.04 | 0.71 | 1.46 | |
| Leukemia/lymphoma related factor | D88450 | 0.62 | 0.83 | 8.57 | |
| Trafficking/targeting proteins | Clathrin, heavy polypeptide | J03583 | 1.25 | 0.60 | 1.58 |
| Metabolism‐related factors | Peroxiredoxin 6 | AF110732 | 1.23 | 0.68 | 1.79 |
| Superoxide dismutase 3 | Z24721 | 1.19 | 0.67 | 1.16 | |
| Glutamate dehydrogenase | X14044 | 0.95 | 0.82 | 1.51 | |
| Prostaglandin‐endoperoxide synthase 1/cyclo‐oxygenase 1 | NM017043 | 0.73 | 0.63 | 1.12 | |
| Dimethylglycine dehydrogenase precursor | X55995 | 1.01 | 0.49 | 1.72 | |
| Hydroxyacid oxidase 3/glycolate oxidase 3 | X67156 | 1.33 | 0.68 | 1.14 | |
| Glycerol‐3‐phosphate dehydrogenase 2 | X78593 | 1.27 | 0.45 | 1.55 | |
| β‐4N‐acetylgalactosaminyltransferase | D17809 | 0.57 | 0.53 | 1.44 | |
| Post‐translational modification/ protein folding‐related factors | Peptidylglycine α‐amidating monooxygenase | M25732 | 0.55 | 1.14 | 4.71 |
| Translation | Eukaryotic translation initiation factor 2B, subunit 2 | U31880 | 0.91 | 0.78 | 1.48 |
| RNA processing/turnover/ transport‐related factors | Cyclin L | AF030091 | 0.75 | 0.75 | 1.71 |
| DNA binding and chromatin proteins | Histone H10 | U49737 | 1.25 | 0.68 | 1.11 |
| Cell signaling/extracellular communicating factors | Endothelin‐2 | U64949 | 1.03 | 0.55 | 1.17 |
| Intracellular transducers/ effectors/modulators | Insulin receptor substrate 3 | U93880 | 0.95 | 0.91 | 1.35 |
| PCTAIRE3 | AB005541 | 0.81 | 0.37 | 2.07 | |
| Calbindin 1 | M31178 | 0.79 | 0.75 | 1.85 | |
| Arrestin, β1 | M91589 | 0.90 | 0.93 | 1.55 | |
| Adenylyl cyclase 2 | M80550 | 0.83 | 0.68 | 1.34 | |
| Homeodomain‐interacting protein kinase 3 | AF036959 | 0.93 | 0.66 | 1.65 | |
| Tuberous sclerosis 2 | U24150 | 1.04 | 0.75 | 1.66 | |
| AMP‐activated protein kinase | Z29486 | 1.35 | 0.74 | 1.49 | |
| Sialyltransferase 5 | X76988 | 1.02 | 0.62 | 1.46 | |
| Thyroid hormone receptor interactor 10 | AB006914 | 0.58 | 0.67 | 1.81 | |
| Peroxiredoxin 5 | Y17295 | 1.02 | 0.87 | 1.47 | |
| Sialyltransferase 8 | U55938 | 1.10 | 0.68 | 2.16 | |
| Cytoskeleton/motility proteins | Kinesin heavy chain member 2 | AF155824 | 0.91 | 0.76 | 1.21 |
| Others | Cell growth regulatory with EF‐hand domain | U66470 | 1.36 | 0.61 | 1.33 |
| Homeobox protein R3 | M37567 | 1.01 | 0.55 | 2.28 | |
| ADAMTS‐1/METH‐1 | AF149118 | 0.94 | 0.67 | 1.29 | |
| Outer mitochondrial membrane receptor rTOM20 | U21871 | 1.01 | 0.94 | 1.92 | |
| Coronin, actin‐binding protein, 1B | AJ006064 | 1.41 | 0.53 | 1.40 | |
| Unconventional myosin Myr2 I heavy chain | X74800 | 0.98 | 0.86 | 1.41 | |
| Replication factor C 2 | AF208499 | 0.81 | 0.76 | 1.26 | |
| Solute carrier family 29, member 1/ENT1 | AF015304 | 0.94 | 0.55 | 1.47 | |
| Aspartyl‐tRNA synthetase/DRS1 | U30812 | 1.16 | 0.50 | 1.16 | |
| Quinoid dihydropteridine reductase | J03481 | 1.18 | 0.40 | 1.44 | |
| Fibrinogen, γ‐polypeptide | J00734 | 0.63 | 0.48 | 5.35 | |
| Myosin light chain kinase 2 | J03886 | 0.98 | 0.46 | 1.51 | |
| Hyperpolarization‐activated cyclin nucleotide‐gated cation channel 1 | AF247450 | 1.00 | 0.27 | 1.20 | |
| ECL | X56190 | 0.79 | 0.50 | 1.57 | |
CON, normal liver; NC, surrounding non‐cancerous liver; CA, HCC sample.
Table 5.
Genes of cluster 4
| Category | Gene name | GenBank accession number | Expression ratio | ||
|---|---|---|---|---|---|
| CON | NC | CA | |||
| Extracellular transportors/carriers | Apolipoprotein A‐V | AF202887 | 0.45 | 1.50 | 1.30 |
| Stress response proteins | Solute carrier family 22, member 2/OCT2 | D83044 | 0.52 | 0.64 | 0.95 |
| Membrane channels/transporters | Solute carrier family 6, member 13/GAT2 | M95762 | 0.73 | 1.38 | 1.14 |
| Chloride channel 1, skeletal muscle | X62894 | 0.60 | 1.77 | 1.39 | |
| Potassium voltage‐gated channel, subfamily H, member 2 | Z96106 | 0.31 | 0.78 | 2.54 | |
| Metabolism‐related factors | Protease 28 subunit, β/prosome/macropain | NM017257 | 0.33 | 0.66 | 2.28 |
| Protease, cysteine, 1/legumain | AF154349 | 0.51 | 1.04 | 1.32 | |
| Malate dehydrogenase‐like enzyme | AF093773 | 0.65 | 0.89 | 1.67 | |
| Translation‐related factors | Ribosomal protein S2 | U92698 | 0.78 | 1.10 | 1.13 |
| Apoptosis‐related factors | Lifeguard/neural membrane protein 35 | AF044201 | 0.16 | 1.05 | 2.19 |
| Cell receptors | Galanin receptor 3 | AF073798 | 0.84 | 1.05 | 1.90 |
| Fc receptor, IgG, low affinity III | M32062 | 0.44 | 1.41 | 0.89 | |
| Polymeric immunoglobulin receptor | X15741 | 0.40 | 0.74 | 1.33 | |
| Neuromedin B receptor | U37058 | 0.36 | 1.76 | 1.17 | |
| Cell signaling/extracellular communicating factors | Glucose‐dependent insulinotropic peptide | L08831 | 0.65 | 0.93 | 1.34 |
| Intracellular transducers/ effectors/modulatorsProtein turnover‐related factors | Phospholipase C, β3 | M99567 | 0.67 | 0.86 | 1.32 |
| Thiol‐specific antioxidant‐like protein | AF053093 | 0.51 | 0.82 | 1.69 | |
| Serine protease inhibitor | X16359 | 0.57 | 1.13 | 1.45 | |
| Calpain, small subunit 1 | U53859 | 0.55 | 0.94 | 1.65 | |
| Cytoskeleton/motility proteins | Actinin α1 | AF115386 | 0.69 | 0.73 | 1.13 |
| Lamin A | X66870 | 0.56 | 0.87 | 1.47 | |
| β‐Spectrin 3 | AB001347 | 0.64 | 0.85 | 1.15 | |
| Others | Thymosin, β10 | M58404 | 0.55 | 1.42 | 1.07 |
| Solute carrier family 19, member 1/RFC1 | AF099010 | 0.70 | 0.87 | 1.37 | |
| Vitronectin | U44845 | 1.04 | 1.22 | 1.78 | |
| Dendrin | X96589 | 0.91 | 0.96 | 1.05 | |
| HLA‐B‐associated transcript 3 | AB018791 | 0.75 | 0.97 | 1.08 | |
| Rhodopsin/retinitis pigmentosa 4, autosomal dominant | Z46957 | 0.48 | 0.64 | 1.14 | |
| Myosin heavy chain Myr 8 | AF209114 | 0.59 | 0.95 | 0.98 | |
CON, normal liver; NC, surrounding non‐cancerous liver; CA, HCC sample.
Gene function category of differentially expressed genes. In cluster 2, genes categorized as ‘cell receptors’ (P < 0.01) and ‘cell signaling/extracellular communications’ (P < 0.05) were significantly enriched. Genes in the categories of ‘intracellular transducers’ (P < 0.05) and ‘cell receptors’ (P < 0.01) were significantly low in clusters 1 and 3, respectively (Fig. 5).
Figure 5.
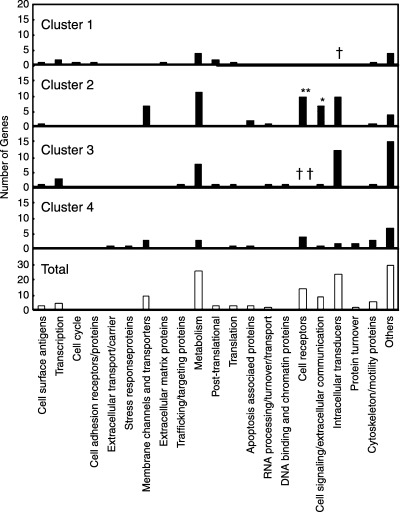
Distribution of functionally categorized genes in each cluster. Numbers of genes within each functional category are shown. Frequency of each category in each cluster was evaluated by Fisher's exact test. Significantly elevated (*P < 0.05, **P < 0.01) and lowered († P < 0.05, †† P < 0.01) frequencies for genes in each cluster are indicated.
Discussion
Significance of the four clusters in hepatocarcinogenesis. The present results revealed that the gene expression profiles were differentially altered in HCC and their adjacent non‐cancerous, cirrhotic liver tissues in rats. The differentially expressing genes could be divided into four clusters according to their expression patterns. Although we need to be cautious with the interpretation of these clusters, we speculate on the possible meanings of the expression patterns observed in the current study as follows. Genes belonging to cluster 1 were down‐regulated in NC and also stayed low in CA, suggesting that the aberrant function of these genes may be involved in continuous liver injury, including fibro/cirrhogenesis, and early stage hepatocarcinogenesis. We have explored the expression of the cluster 1 gene HNF1/TCF1 also by RT‐PCR, and the roles of this gene in hepatocarcinogenesis are discussed further. Genes belonging to cluster 2 were upregulated in NC and then downregulated to the control level in CA, suggesting that the aberrant function of these genes is involved both in liver injury and hepatocarcinogenesis but with opposite influences. Alternatively, it is possible that these genes are necessary to be upregulated for liver injury but no longer have to be aberrantly expressed for carcinogenic processes. Genes belonging to cluster 3 were normally expressed in NC but then upregulated in CA, suggesting that the function of these genes may not be involved in liver injury but mainly in hepatocarcinogenesis. Genes belonging to cluster 4 were upregulated in NC and further upregulated in CA, suggesting that the function of these genes is positively involved both in liver injury and in hepatocarcinogenesis. However, to fully understand the roles of differentially expressed genes, exploration of the sequential expression of these genes at more time points may be needed.
Genes in the category of ‘cell receptors’ enriched in cluster 2. The present study showed that genes differentially expressed among CON, NC and CA fit into relatively few clusters, each showing distinct expression patterns. Further analyses revealed that certain functionally categorized genes are enriched in some clusters (Fig. 5). Notably, genes categorized as ‘cell receptors’ and ‘cell signaling/extracellular communication proteins’ were both enriched in cluster 2 (Table 3), characterized by high gene expression levels in NC compared with CON or CA (Fig. 4b). The precise significance of this expression pattern is not clear at present, but at least four of the 10 receptors in this cluster, namely, opioid receptor κ1 (OPRK1), somatostatin receptor 1 (SSTR1), endothelin receptor A (EDNRA), and endothelin receptor B (EDNRB), are known for their inhibitory effects on cell growth.( 8 , 9 , 10 , 11 ) This is in theoretical accordance with the downregulation of these genes observed between NC and CA (Fig. 4b, Table 3), as this would give cells a growth advantage. These four genes also belong to the same family, namely, G‐protein‐coupled receptors. In fact, the somatostatin and opioid systems are the main inhibitory systems in mammals, and relevant molecules including OPRK1 and SSTR1 have been under intense investigation in terms of their roles in tumorigenesis.( 8 , 9 , 10 , 11 ) Moreover, downregulation of EDNRB has been studied in human prostate and nasopharyngeal cancer in relation to its abnormal methylation in CpG islands.( 12 , 13 ) Another receptor gene, platelet derived growth factor receptor α (PDGFRA), however, is known for its growth‐stimulating activity in certain types of cells. TrkB, a neurotrophic tyrosine kinase receptor, also found in this cluster, has recently been identified as a potent suppressor of anoikis and inducer of metastasis in epithelial cells.( 14 ) TrkB and its ligand brain‐derived neurotrophic factor are frequently coexpressed in human cancers, especially those with aggressive phenotypes,( 15 ) suggesting the formation of an autocrine signaling pathway. So, while the expression patterns of the receptor genes in cluster 2 appear similar to each other, the cell signals related to each gene are diverse and should be individually explored.
Genes in the category of ‘cell signaling/extracellular communications’ enriched in cluster 2. Genes in the category of ‘cell signaling/extracellular communications’ were also enriched in cluster 2 (Fig. 5, Table 3). The seven genes included in this cluster encode polypeptide cytokines with various functions and related signal transduction systems after binding to their cognate receptors. It should be noted that fibroblast growth factor 5 (FGF5) and FGF9, both members of the FGF family, were found in this cluster. For FGF members stimulate growth and differentiation in a variety of cell types, and their roles in carcinogenesis have been investigated.( 16 ) Hu et al. reported high expression levels of FGF1 in rat HCC induced by the Solt‐Farber protocol.( 17 ) In their results, FGF1 levels were elevated at early stages, decreased after one month, and remained low until the development of tumors. The reason why FGF5 and FGF9 were higher in NC rather than CA in the current study is not clear at present. However, there have been at least 23 FGF discovered thus far, and the roles of individual FGF in carcinogenesis have not been exactly elucidated.( 16 ) Sequential expression analysis of these molecules during carcinogenesis may also help fully understand the roles of individual FGF in hepatocarcinogenesis.
Other deregulated genes associated with carcinogenesis. Metabolism‐related factors found to be differentially expressed are mainly enzymes with a variety of functions and their genes did not show any significant enrichment in any of the four clusters (Fig. 5). Glucose‐6‐phosphatase is a negative phenotype marker of putatively preneoplastic foci of cellular alteration in the liver of rats,( 18 ) and its activity is frequently lost also in human HCC.( 19 ) CYP1B1 and CYP3A9 are both related to estrogen metabolism, and CYP enzymes are known as target genes of hepatic transcription factor 4 (HNF4). In human HCC, CYP enzymes are also generally down‐regulated compared to surrounding tissues.( 20 ) Aberrant expression of β‐4N‐acetylgalactosaminyl‐transferase, observed here, is frequently detected in human HCC and other cancers.( 21 ) Potassium voltage‐gated channels play an important role in the proliferation and metastasis of HCC cells.( 22 )
In the CDAA diet model, repeated apoptotic death and proliferation of hepatocytes and fibro/cirrhogenesis have been shown to be important in the processes underlying hepatocarcinogenesis.( 4 , 23 ) Genes with possible relation to fibro/cirrhogenesis included PDGFRA, diphtheria toxin receptor/heparin‐binding epidermal growth factor receptor, FGF5, FGF9, interleukin (IL)‐1 receptor‐like 1, IL‐1 receptor type B, IL‐2 receptor β‐chain, EDNRA, EDNRB, endothelin converting enzyme‐like 1, annexin 1/p35/lipocortin 1, insulin receptor substrate 1, Src related tyrosine kinase, and S6 kinase. ( 24 , 25 , 26 , 27 , 28 ) Differentially expressed genes in the current study included both pro‐apoptotic factors such as caspase 3 and α‐inhibin,( 29 , 30 ) and antiapoptotic factors such as calbindin 1, arrestin β1, homeodomain‐interacting protein kinase 3, lifeguard, and lamin A. ( 31 , 32 , 33 , 34 , 35 ) Cyclin L( 36 ) and ZNF265( 37 ) are regulatory factors for the RNA splicing machinery so that their aberrant expressions may result in abnormal RNA splicing.
Reactive oxygen species‐induced stress has been shown to be involved in human hepatocarcinogenesis,( 1 , 2 , 3 ) NAFLD/NASH( 5 ) and the rat CDAA diet model.( 4 , 22 ) The present study detected the altered expression of several genes related to both anti‐ and pro‐oxidative machinery, such as plasma glutathione peroxidase precursor, peroxiredoxins 5 and 6, superoxide dismutase 3, prostaglandin endoperoxide synthase 1/COX‐1, phospholipase C β3, and thiol‐specific antioxidant‐like protein.
Our results have also shown aberrant expression of HNF1/TCF1 and kinesin heavy chain member 2, two molecules involved in the Wnt signaling pathway, a major system involved in human hepatocarcinogenesis.( 38 ) Biallelic inactivation of HNF1/TCF1, detected in human hepatic adenoma and HCC, is suspected to be important in early stages of liver tumor development.( 39 )
Regarding tumor‐suppressor genes, the expression of both tuberous sclerosis 2 (TSC2) and its molecular target ras homolog enriched in brain (Rheb) was found to be altered in the current experiment. Neurofibromatosis type 1 (NF1) also exhibited variation between tissue types, and this may be the first report to suggest a potential involvement of NF1 in HCC development. Other altered genes, fibrinogen γ‐polypeptide, actinin α1 and vitronectin, have previously been reported to demonstrate corresponding changes in human hepatocarcinogenesis.( 40 , 41 )
Conclusion. Among the differentially expressed genes found in the present study, many have already been reported to have involvement in human and animal hepatocarcinogenesis. Statistical methods have proved useful for classifying these genes into relatively few clusters according to their expression patterns. Based on the functional classification of the differentially expressed genes, it was further indicated that these clusters may be associated with gene functional categories. For example, genes encoding the two major components of signal‐transducing systems, cell receptors and cytokines, were significantly enriched in a particular cluster. Data have recently been accumulated regarding behaviors of individual tumor‐related genes,( 1 , 2 , 3 ) but mechanisms for global regulation of particular groups of genes are not fully understood. Several factors are supposed to affect overall behaviors of multiple genes. These include concordant methylation of CpG islands, which can result in suppression of multiple genes as described in human cancers including HCC.( 42 ) Although the causation of the association between expression patterns of genes and gene functional categories suggested in the current study is yet to be elucidated, these findings may give insight into underlying mechanisms for the evolution of HCC.
Acknowledgments
The authors thank Dr Yoshiyuki Hashimoto (Kyoritsu University of Pharmacy) for constant advice; Drs Tsuneyuki Oikawa (Department of Cell Genetics, Sasaki Institute), Tetsuya Muroya, and Masaru Sakamoto (Department of Gynecology, Kyoundo Hospital, Sasaki Foundation) for generous scientific supports; Drs Akihiko Maekawa (Sasaki Institute), Yashige Kotake (Free Radical Biology and Aging Research Program, Oklahoma Medical Research Foundation), and Yoichi Konishi (Nara Medical University) for helpful comments; Ms Hiromi Ichihara, Ms Hiromi Asako, and Ms Chinami Kajiwara (Department of Pathology, Sasaki Institute) for technical assistance. This work was supported by a Research Grant of the Princess Takamatsu Cancer Research Fund (01–23308), Grant of the Foundation for Promotion of Cancer Research, and Grant R01 CA82506 from the National Institute of Health of the USA.
References
- 1. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 2002; 31: 339–46. [DOI] [PubMed] [Google Scholar]
- 2. Nita ME, Alves VAF, Carrilho FJ, Ono‐Nita SK, Mello ES, Gama‐Rodorigues JJ. Molecular aspects of hepatic carcinogenesis. Rev Inst Med Trop Sao Paulo 2002; 44: 39–48. [DOI] [PubMed] [Google Scholar]
- 3. Wang XW, Hussain SP, Huo TI et al. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology 2002; 181–182: 43–7. [DOI] [PubMed] [Google Scholar]
- 4. Nakae D. Endogenous liver carcinogenesis in the rat. Pathol Int 1999; 49: 1028–42. [DOI] [PubMed] [Google Scholar]
- 5. Rinella ME, Green RM. The methionine‐choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 2004; 40: 47–51. [DOI] [PubMed] [Google Scholar]
- 6. Yokomori H, Oda M, Ogi M et al. Enhanced expression of endothelin receptor subtypes in cirrhotic rat liver. Liver 2001; 21: 114–22. [DOI] [PubMed] [Google Scholar]
- 7. Yokomori H, Oda M, Yasogawa Y et al. Enhanced expression of endothelin B receptor at protein and gene levels in human cirrhotic liver. Am J Pathol 2001; 159: 1353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatzoglou A, Bakogeorgou E, Kampa M et al. Somatostatin and opioid receptors in mammary tissue. Role in cancer cell growth. Adv Exp Med Biol 2000; 480: 55–63. [DOI] [PubMed] [Google Scholar]
- 9. Lamberts SW, De Herder WW, Hofland LJ. Somatostatin analogs in the diagnosis and treatment of cancer. Trends Endocrinol Metab 2002; 13: 451–7. [DOI] [PubMed] [Google Scholar]
- 10. Ferjoux G, Bousquet C, Cordelier P et al. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J Physiol Paris 2000; 94: 205–10. [DOI] [PubMed] [Google Scholar]
- 11. Panagiotou S, Bakogeorgou E, Papakonstanti E et al. Opioid agonists modify breast cancer cell proliferation by blocking cells to the G2/M phase of the cycle: involvement of cytoskeletal elements. J Cell Biochem 1999; 73: 204–11. [DOI] [PubMed] [Google Scholar]
- 12. Nelson JB, Lee WH, Nguyen SH, Jarrard DF, Brooks JD, Magnuson SR. Opgenorth TJ, Nelson WG, Bova GS. Methylation of the 5′‐CpG island of the endothelin B receptor gene is common in human prostate cancer. Cancer Res 1997; 57: 35–7. [PubMed] [Google Scholar]
- 13. Lo KW, Tsang YS, Kwong J, To KF, Teo PM, Huang DP. Promoter hypermethylation of the EDNRB gene in nasopharyngeal carcinoma. Int J Cancer 2002; 98: 651–5. [DOI] [PubMed] [Google Scholar]
- 14. Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004; 430: 1034–40. [DOI] [PubMed] [Google Scholar]
- 15. Aoyama M, Asai K, Shishikura T et al. Human neuroblastomas with unfavorable biologies express high levels of brain‐derived neurotrophic factor mRNA and a variety of its variants. Cancer Lett 2001; 164: 51–60. [DOI] [PubMed] [Google Scholar]
- 16. McKeehan WL, Wang F, Kan M. The heparan sulfate‐fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol 1998; 59: 135–76. [DOI] [PubMed] [Google Scholar]
- 17. Hu Z, Evarts RP, Fujio K et al. Expression of transforming growth factor alpha/epidermal growth factor receptor, hepatocyte growth factor/c‐met and acidic fibroblast growth factor/fibroblast growth factor receptors during hepatocarcinogenesis. Carcinogenesis 1996; 17: 931–8. [DOI] [PubMed] [Google Scholar]
- 18. Williams GM. The significance of chemically‐induced hepatocellular altered foci in rat liver and application to carcinogen detection. Toxicol Pathol 1989; 17: 663–74. [DOI] [PubMed] [Google Scholar]
- 19. Gerber MA, Thung SN. Enzyme patterns in human hepatocellular carcinoma. Am J Pathol 1980; 98: 395–400. [PMC free article] [PubMed] [Google Scholar]
- 20. Xu XR, Huang J, Xu XG et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci USA 2001; 98: 15089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugita Y, Fujiwara Y, Hoon DS et al. Overexpresion of beta 1,4N‐acetylgalactosaminyl‐transferase mRNA as a molecular marker for various types of cancers. Oncology 2002; 62: 149–56. [DOI] [PubMed] [Google Scholar]
- 22. Zhou Q, Kwan HY, Chan HC, Jiang JL, Tam SC, Yao X. Blockage of voltage‐gated K+ channels inhibits adhesion and proliferation of hepatocarcinoma cells. Int J Mol Med 2003; 11: 261–6. [PubMed] [Google Scholar]
- 23. Nakae D, Uematsu F, Kishida H et al. Inhibition of development of hepatocellular carcinomas by phenyl N‐tert‐butyl nitrone in rats fed with a choline‐deficient, 1‐amino acid‐defined diet. Cancer Lett 2004; 206: 1–13. [DOI] [PubMed] [Google Scholar]
- 24. Bonner JC. Regulation of PDGF and its receptors in fibrotic disease. Cytokine Growth Factor Rev 2004; 15: 255–73. [DOI] [PubMed] [Google Scholar]
- 25. De Coupade C, Gillet R, Bennoun M, Briand P, Russo‐Marie F, Solito E. Annexin I expression and phophorylation are upregulated during liver regeneration and transformation in antithrombin III SV40 T large antigen transgenic mice. Hepatology 2000; 31: 371–80. [DOI] [PubMed] [Google Scholar]
- 26. Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis 2001; 21: 397–416. [DOI] [PubMed] [Google Scholar]
- 27. Svegliati‐Baloni G, Ridolfi F, Di Sario A et al. Insulin and insulin‐like growth factor‐1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: Differential effects on signal transduction pathways. Hepatology 1999; 29: 1743–51. [DOI] [PubMed] [Google Scholar]
- 28. Vishwanath BS, Frey FJ, Escher G, Reichen J, Frey BM. Liver cirrhosis induces renal and liver phopholipase A2 activity in rats. J Clin Invest 1996; 98: 365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bredesen DE, Mehlen P, Rabizadeh S. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol Rev 2004; 84: 411–30. [DOI] [PubMed] [Google Scholar]
- 30. Chen YG, Lui HM, Lin SL, Lee JM, Ying SY. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med 2002; 227: 75–87. [DOI] [PubMed] [Google Scholar]
- 31. Castro CY, Stephenson M, Gondo MM, Medeiros LJ, Cagle PT. Prognostic implications of calbindin‐D28k expression in lung cancer: Analysis of 452 cases. Mod Pathol 2000; 13: 808–13. [DOI] [PubMed] [Google Scholar]
- 32. Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein‐coupled receptor‐mediated apoptosis. J Biol Chem 2004; 279: 24578–84. [DOI] [PubMed] [Google Scholar]
- 33. Kondo S, Lu Y, Debbas M et al. Characterization of cells and gene‐targeted mice deficient for the p53‐binding kinase homeodomain‐interacting protein kinase I (HIP1). Proc Natl Acad Sci USA 2003; 100: 5431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Somia NV, Schmitt MJ, Vetter DE, Van Antwerp D, Heinemann SF, Verma IM. LFG: An anti‐apoptotic gene that provides protection from Fas‐mediated cell death. Proc Natl Acad Sci USA 1999; 96: 12667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho YS, Tsai PW, Yu CF, Liu HL, Chen RJ, Lin JK. Ketoconazole‐induced apoptosis through P53‐dependent pathway in human colorectal and hepatocellular carcinoma cell lines. Toxicol Appl Phrmacol 1998; 153: 39–47. [DOI] [PubMed] [Google Scholar]
- 36. Dickinson LA, Edgar AJ, Ehley J, Gottesfeld JM. Cyclin L is an RS domain protein involved in pre‐mRNA splicing. J Biol Chem 2002; 277: 25465–73. [DOI] [PubMed] [Google Scholar]
- 37. Adams DJ, Van Der Weyden L, Mayeda A, Stamm S, Morris BJ, Rasko JE. ZNF265: a novel splicesomal protein able to induce alternative splicing. J Cell Biol 2001; 154: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edamoto Y, Hara A, Biernat W et al. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer 2003; 106: 334–41. [DOI] [PubMed] [Google Scholar]
- 39. Bluteau O, Jeannot E, Bioulac‐Sage P et al. Bi‐allelic inactivation of TCF1 in hepatic adenomas. Nature Genet 2002; 32: 312–5. [DOI] [PubMed] [Google Scholar]
- 40. Kondoh N, Wakatsuki T, Ryo A et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res 1999; 59: 4990–6. [PubMed] [Google Scholar]
- 41. Nishiyama M, Ozturk M, Frohlich M, Mafune K, Steele G Jr, Wands JR. Expression of human alpha‐actinin in human hepatocellular carcinoma. Cancer Res 1990; 50: 6291–4. [PubMed] [Google Scholar]
- 42. Shen L, Ahuja N, Shen Y et al. DNA methylation and environmental exposures in human hepatocellular carcinoma. J Natl Cancer Inst 2002; 94: 755–61. [DOI] [PubMed] [Google Scholar]


