Abstract
Burkitt's lymphoma and atypical Burkitt/Burkitt‐like lymphoma (BL/BLL) are considered highly aggressive B‐cell lymphomas with a rapid proliferative rate and high rate of apoptosis. The aim of the present study was to confirm whether apoptotic and cell proliferative factors affect BL/BLL clinical outcomes. We retrospectively analyzed the relationship between the clinical and immunophenotypic features of 43 BL/BLL patients by immunohistochemical staining for bcl‐2 and double staining for Ki‐67 plus caspase‐3. In double staining experiments, all patients were divided into high and low groups for the expression of caspase‐3, Ki‐67, and both Ki‐67 and caspase‐3, by using the medians of their percentages as limits. The 43 BL/BLL patients were divided into high caspase‐3 (n = 19) and low caspase‐3 (n = 24) groups. There was a significant difference in the overall survival between the high (77%) and low caspase‐3 (33%) groups; the survival rate of patients in the low caspase‐3 group who received aggressive short‐term chemotherapy (58%) was significantly better than that of patients who received cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) therapy (17%). All patients positive for bcl‐2 were in the low caspase‐3 group (high caspase‐3 group, 0%; low caspase‐3 group, 42%). The overall survival tended to be better in the high caspase‐3 and bcl‐2‐negative group (76%) than in the low caspase‐3 and bcl‐2‐negative (50%) group. In addition, the low caspase‐3 and bcl‐2‐positive group tended to show the worst prognosis (16%). We suggest that caspase‐3 may function as an indicator of the prognosis of BL/BLL. Furthermore, intensive short‐term chemotherapeutic regimens may improve the prognosis of the patients in the low caspase‐3 group. (Cancer Sci 2008; 99: 1564–1569)
Apoptosis is an important programmed cell suicide mechanism that regulates the cell cycle. Morphologically, it is characterized by the shrinkage of cell volume and the condensation of nuclear chromatin.( 1 , 2 , 3 ) The caspase family proteins play an essential role in apoptotic cell death.( 4 , 5 , 6 ) In particular, caspase‐3 is the main effector caspase in the apoptotic cascade in many apoptotic processes, including the dismantling of cells and the formation of apoptotic bodies within the cells.( 5 , 7 , 8 , 9 ) Therefore, the immunohistochemical expression of caspase‐3 has been used for the detection of apoptotic cells in some recent studies.( 5 , 7 ) Burkitt's lymphoma (BL) is considered a subtype of highly aggressive mature B‐cell lymphoma with a rapid proliferative rate and high rate of apoptosis. Histopathologically, BL is characterized by a diffuse monotonous pattern of medium‐sized cells with round nuclei and clumped chromatin, that is, a ‘starry sky’ appearance with numerous macrophages and a cohesive pattern called the ‘jigsaw puzzle‐like’ pattern. Atypical Burkitt/Burkitt‐like lymphoma (BLL) is defined as a variant of BL in the new World Health Organization (WHO) classification,( 10 ) which includes greater pleomorphism in nuclear size and shape with fewer and more prominent nucleoli. However, in both BL and BLL, a starry sky pattern is generally present. Morphologically, the starry sky pattern imparted by numerous benign macrophages that have ingested apoptotic tumor cells is compatible with an extremely high rate of both cell proliferation and apoptosis.( 11 , 12 , 13 )
According to the new WHO classification, the presence of c‐MYC translocations is required to conclusively diagnose BL and BLL. The c‐MYC oncogene plays a central role in the transcriptional regulation of a set of downstream genes that controls diverse cellular processes, including cell cycle progression and apoptosis.( 14 ) Therefore, c‐MYC translocation is considered to induce rapid cell proliferation, leading to a highly aggressive clinical course.( 15 )
In immunohistochemical analysis, the typical BL demonstrates a proliferation (MIB‐1) index of nearly 100%, and lack of expression of bcl‐2, which is an anti‐apoptotic marker. The lack of expression of bcl‐2 reflects enhanced apoptosis, while the overexpression of MIB‐1 (Ki‐67) signifies rapid cell proliferation and increased mitosis in BL; however, it was reported that a small part of BL and BLL expressed bcl‐2.( 12 , 16 , 17 )
To confirm whether apoptotic factors and cell proliferative factors affect the clinical outcomes of BL/BLL, we retrospectively analyzed clinical and immunophenotypic features and clinical outcomes of 43 patients with BL/BLL.
Materials and Methods
Patient selection and materials. We selected 43 BL/BLL patients diagnosed between October 1984 and May 2007 from a list of lymphoma files in the Departments of Pathology of Fukuoka and Kurume Universities. The ages of the patients ranged 3–85 years (median, 49 years), including 17 patients who were less than 16 years of age and 15 patients who were 60 years of age or over. Some patients had been previously reported by us.( 18 ) In the current study, the histological findings of all patients were reviewed by four experienced hematopathologists (T. Y., K. O., S. N. and M. K.) according to the criteria of the new WHO classification. Biopsy was performed after obtaining informed consent from all patients. None of the cases with BL/BLL that were included in this study had AIDS, post‐transplant lymphoproliferative disorder or other immunodeficiency. All clinical and laboratory data of the patients along with the follow‐up data were obtained from the medical records of the respective institutions.
Immunohistochemical staining. Paraffin sections from each specimen were immunostained using monoclonal antibodies against CD3, CD20, CD10, bcl‐2, Ki‐67 (MIB‐1) and TdT. All immunohistochemical reactions and analyses were performed in a single laboratory, as described previously.( 19 )
To understand the relationship between apoptosis and cell proliferation in BL/BLL, we performed immunohistochemical double staining for Ki‐67 plus caspase‐3 in this study. The paraffin sections from each specimen were immunostained with antibody cocktails of monoclonal antibodies against Ki‐67 and polyclonal antibodies against caspase‐3 (Ki‐67 + caspase‐3 pre‐diluted double stain antibody). In the antibody cocktails, cleaved caspase‐3 detects the endogenous levels of the large fragment (17/19 kDa) of activated caspase‐3. Activation of caspase‐3 requires proteolytic processing of its inactive zymogen into the activated p17 and p12 subunits. The cleavage of caspase‐3 requires aspartic acid at the P1 position. This antibody does not cross‐react with other cleaved caspases.( 7 ) All immunohistochemical double stainings were performed in a single laboratory, as described previously,( 20 ) by following the protocol manual provided by Biocare Medical (Concord, CA, USA). Briefly, the first reaction products, which were brown, were developed by incubation with diaminobenzidine (DAB), while the subsequent red reaction products were developed using new fuchsin after an alkaline phosphatase reaction (Fig. 1).
Figure 1.
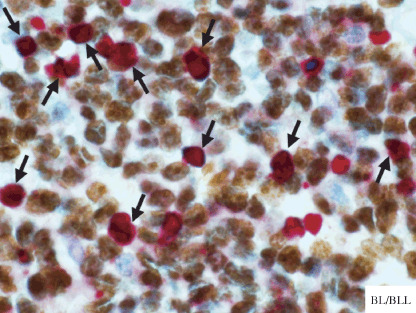
Double staining for Ki‐67 plus caspase‐3 on Burkitt's lymphoma or atypical Burkitt/Burkitt‐like lymphoma (BL/BLL). Caspase‐3‐positive cells (red) are intermingled in the proliferation of Ki‐67‐positive cells (brown). A proportion of proliferating cells, which are Ki‐67‐positive, are also simultaneously stained as apoptotic cells, which are caspase‐3‐positive (red + brown, arrows). In this case, the percentage of cells positive for caspase‐3 is approximately 25% (low caspase‐3 group).
Immunohistological scoring. For immunohistochemistry analysis, two experienced hematopathologists (K. O. and M. K.) counted 200 cells and calculated the number of cells that stained positively at three sites. The average of the percentages of cells that stained positive for Ki‐67 was calculated to yield the immunohistological score of 0–100% (MIB‐1 index). With regard to bcl‐2 expression, the categorization was performed as follows: negative (positively‐stained tumor cells, <40%) and positive (positively‐stained tumor cells, ≥40%).
In the double staining for caspase‐3 and Ki‐67, we calculated the average percentage of tumor cells that were positive for caspase‐3 and Ki‐67 from the total number of tumor cells. Furthermore, to evaluate the proportion of cells that underwent both apoptosis and cell proliferation, we analyzed the percentages of cells positive for both Ki‐67 and caspase‐3 to those that were positive only for caspase‐3.
We divided all patients who stained positive for caspase‐3 and Ki‐67 into either the high or low group by using the medians of these percentages as the limits. The categorization was performed as follows. The low group comprised cases where tumor cells positive for caspase‐3 were 30% or less, those positive for Ki‐67 were 95% or less and those positive for both Ki‐67 and caspase‐3 were 55% or less; the high group comprised cases where tumor cells positive for caspase‐3 were more than 30%, those positive for Ki‐67 were more than 95% and those positive for both were more than 55%.
Cytogenetic and molecular genetic analysis. We used the LSI MYC Dual Color Break Apart Rearrangement probe (Vysis, Downers Grove, IL, USA) to detect the c‐MYC translocation by performing routine fluorescence in situ hybridization (FISH) analysis on frozen and paraffin sections, as described previously.( 18 , 21 , 22 )
In this study, we detected c‐MYC translocation in all the 43 cases of BL/BLL.
Statistical analysis. The survival curves were constructed by the Kaplan–Meier method, and the level of significance of the differences in the survival rates were tested using the generalized log–rank test. The correlations between the two groups were examined using the χ2‐test and Student's t‐test, and differences were considered significant at P < 0.05.
Clinical analysis. As our previous study,( 18 ) all relevant data were available, including the individual risk factors that constitute the International Prognostic Index (IPI; age, clinical stage, performance status, lactate dehydrogenase [LDH] level, and the number of extranodal disease sites). The IPI score ranged 0–5 and, based on it, the cases were classified as low risk (IPI score, 0–2) or high risk (IPI score, 3–5). Clinical staging was based on the Ann Arbor classification. With regard to chemotherapy, we could obtain the clinical information regarding the treatment modalities of 40 of the 43 BL/BLL patients. To eliminate disparity caused by the use of different treatment modalities, we divided the patients with BL/BLL into two groups, namely, groups A and B. Group A included patients treated with the cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) regimen, which is the standard chemotherapeutic regimen for diffuse large B‐cell lymphoma (DLBCL), or with the modified CHOP regimen. Group B included patients treated with intensive short‐term chemotherapeutic regimens used for children with BL; for example, cyclophosphamide, vincristine, doxorubicin and high‐dose methotrexate (CODOX‐M) and ifosfamide, etoposide and high‐dose cytarabine (IVAC).
Results
Table 1 shows the characteristics of 43 patients with BL/BLL according to the proportion of cells positive for caspase‐3; that is, characteristics of patients in the high caspase‐3 group (n = 19) and those of patients in the low caspase‐3 group (n = 24). The median ages of the patients in the high and low caspase‐3 groups were 13 and 64.5 years, respectively. The high caspase‐3 group comprised a significantly higher percentage of patients who were less than 16 years of age as compared to the low caspase‐3 group (high caspase‐3 group, 63%; low caspase‐3 group, 21%; P = 0.005). With regard to the chemotherapeutic regimen, significantly more patients of the high caspase‐3 group (84%) received the intensive short‐term regimen (Group B) than those of the low caspase‐3 group (38%) (P = 0.003). We could not detect significant differences between the high and low caspase‐3 groups with regard to clinical features, including sex, performance status, site, clinical stage and IPI.
Table 1.
Characteristics of patients with classified Burkitt and Burkitt‐like lymphoma (BL/BLL) according to the proportion of positive cells for caspase‐3
| BL/BLL | High caspase‐3 group † | Low caspase‐3 group ‡ | P‐value |
|---|---|---|---|
| Age | n = 19 | n = 24 | |
| Median (range) | 13 years (3–64) | 64.5 years (5–85) | |
| Child: ≤15 years | 12 (63%) | 5 (21%) | 0.005 |
| Senior: ≥60 years | 1 (5%) | 14 (58%) | <0.001 |
| Sex | n = 19 | n = 24 | 0.92 |
| Male | 9 (47%) | 11 (46%) | |
| Female | 10 (53%) | 13 (54%) | |
| PS § | n = 19 | n = 24 | 0.35 |
| 2–4 | 9 (47%) | 8 (33%) | |
| Site | n = 19 | n = 24 | 0.09 |
| Extranodal | 15 (79%) | 13 (54%) | |
| Stage | n = 19 | n = 24 | 0.977 |
| III/IV | 11 (58%) | 14 (58%) | |
| IPI score ¶ | n = 19 | n = 24 | 0.515 |
| High risk | 10 (53%) | 15 (63%) | |
| Treatment | n = 19 | n = 21 ††† | 0.003 |
| Group A †† | 3 (16%) | 13 (62%) | |
| Group B ‡‡ | 16 (84%) | 8 (38%) | |
| bcl‐2 | n = 19 | n = 24 | 0.001 |
| Negative | 19 (100%) | 14 (58%) | |
| Ki‐67 | n = 19 | n = 24 | 0.058 |
| Median percentage (range) | 97.5% (95–100) | 94% (82.5–100) | |
| High group §§ | 11 (58%) | 7 (29%) | |
| Both Ki‐67 and caspase‐3 | n = 19 | n = 24 | 0.001 |
| Median percentage (range) | 65% (50–80) | 50% (35–80) | |
| High group ¶¶ | 14 (74%) | 6 (25%) |
High caspase‐3 group: positively‐stained tumor cells for caspase‐3, >30%.
Low caspase‐3 group: positively‐stained tumor cells for caspase‐3, ≤30%.
§ PS: performance status.
IPI: International Prognostic Index (age, clinical stage, performance status, lactate dehydrogenase level, and the number of extranodal disease sites): high risk (score, 3–5).
†† Group A: patients with BL/BLL treated with cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) or the modified CHOP regimen.
‡‡ Group B: patients with BL/BLL treated with intensive short‐term chemotherapeutic regimen.
High group of Ki‐67: stained tumor cells, >95%.
High group both Ki‐67 and caspase‐3: stained cells for both Ki‐67 and caspase‐3, >55%.
††† We were able to obtain clinical information of treatment from 21 of 24 low caspase‐3 group in BL/BLL.
To confirm the relation between the proportion of cells positive for caspase‐3 and the clinical outcome, we analyzed the overall survival rates of patients in the high and low caspase‐3 groups and found a significant difference in the overall survival (high caspase‐3 group, 77%; low caspase‐3 group, 33%; P = 0.029) (Fig. 2a).
Figure 2.
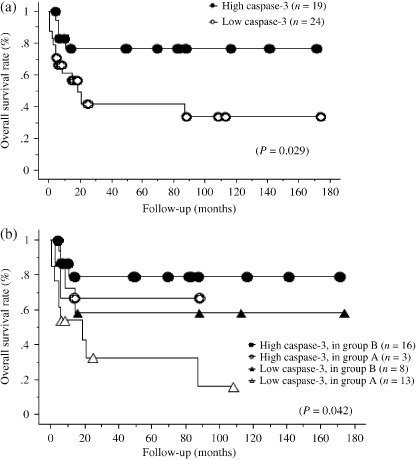
Comparison of the overall survival rates in Burkitt/Burkitt‐like lymphoma (BL/BLL) with regard to the proportion of cells that are positive for caspase‐3 and response to chemotherapy. (a) Overall survival rate of the high caspase‐3 group (positively‐stained tumor cells, >30%) and the low caspase‐3 group (positively‐stained tumor cells, ≤30) in BL/BLL. (b) Overall survival rate of the patients in the high and low caspase‐3 groups when treated with cyclophosphamide, doxorubicin, vincristine and prednisolone (CHOP) or modified CHOP (group A) and with highly intensive short‐term chemotherapeutic regimens (group B).
Furthermore, to eliminate disparity caused by the use of different treatment modalities, we compared the overall survival rates of patients in the high and low caspase‐3 groups by dividing them into treatment groups A and B (Fig. 2b) and found a significant difference (high caspase‐3: group A, 67% and group B, 79%; low caspase‐3: group A, 17% and group B, 58%; P = 0.042).
With regard to the correlation of caspase‐3 and bcl‐2, which inhibit apoptosis, we compared the percentage of patients who were negative for bcl‐2 in the high and low caspase‐3 groups (Table 2). In this study, the high caspase‐3 group had a significantly larger proportion of patients who were negative for bcl‐2 than the low caspase‐3 group (high caspase‐3 group, 100%; low caspase‐3 group, 58%; P < 0.005).
Table 2.
Correlation between caspase‐3 and bcl‐2 in 43 Burkitt and Burkitt‐like lymphoma (BL/BLL)
| High caspase‐3 group † | Low caspase‐3 group ‡ | ||
|---|---|---|---|
| n = 19 | n = 24 | ||
| bcl‐2‐negative | 19 (100%) | 14 (58%) | P = 0.00132 |
High caspase‐3 group: positively‐stained tumor cells for caspase‐3, >30%.
Low caspase‐3 group: positively‐stained tumor cells for caspase‐3, ≤30%.
Figure 3(a) shows the correlation between caspase‐3 and bcl‐2 in BL/BLL. Because all patients in the high caspase‐3 group were negative for bcl‐2, we divided the 43 BL/BLL patients into three groups according to the percentage of cells that were positive for caspase‐3 and the presence of bcl‐2: (i) the HN group, high caspase‐3 and bcl‐2‐negative (n = 19; caspase‐3‐positive cells, 40.1 ± 5.6%); (ii) the LN group, low caspase‐3 and bcl‐2‐negative (n = 14; caspase‐3‐positive cells 20.2 ± 8.3%); and (iii) the LP group, low caspase‐3 and bcl‐2‐positive (n = 10; caspase‐3‐positive cells, 15.3 ± 10.8%).
Figure 3.
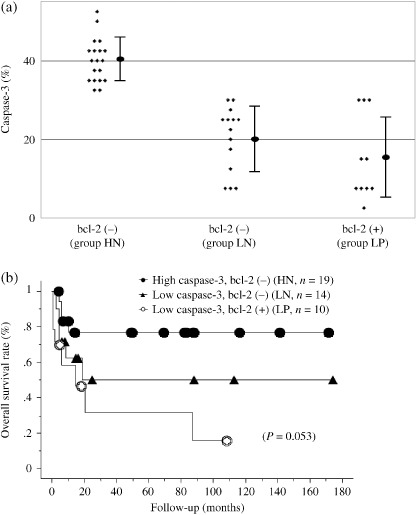
Correlation of the proportion of cells positive for caspase‐3 and bcl‐2 in Burkitt/Burkitt‐like lymphoma (BL/BLL). (a) BL/BLL patients are divided into three groups according to the proportion of cells positive for caspase‐3 (high caspase‐3 group, positively‐stained tumor cells, >30%; low caspase‐3 group, positively‐stained tumor cells, ≤30) and bcl‐2; high caspase‐3 and bcl‐2‐negative (HN), low caspase‐3 and bcl‐2‐negative (LN), low caspase‐3 and bcl‐2‐positive (LP). No patients in the high caspase‐3 group were bcl‐2‐positive. (b) Overall survival rate of BL/BLL patients with regard to the proportion of cells positive for caspase‐3 (high caspase‐3 group, positively‐stained tumor cells, >30%; low caspase‐3 group, positively‐stained tumor cells, ≤30) and bcl‐2; groups HN, LN and LP.
To confirm whether caspase‐3 and bcl‐2 – that is, the apoptosis‐associated factors – influenced the clinical outcome of BL/BLL, we analyzed the overall survival in HN, LN and LP groups (Fig. 3b). The overall survival tended to be the best in the HN group (76%), intermediate in the LN group (50%) and the worst in the LP group (16%); however, we could not detect a significant difference due to a small study population (P = 0.0528).
Discussion
The starry sky appearance, which is a characteristic of BL/BLL in histopathological findings, indicates that the tumor cells of BL/BLL have enhanced cell proliferation and apoptosis. Though, according to the new WHO classification, BLL is defined as a variant of BL, we did not distinguish between BL and BLL in this study for the following reasons: (i) it is known that the histopathological distinction of high‐grade mature B‐cell lymphomas with Burkitt‐like morphology has less reproduc‐ibility and concordance rate between diagnosticians; (ii) the number of patients in this study is small; and (iii) both BL and BLL exhibit a starry sky appearance.
To investigate these processes, we performed an immunohistochemical analysis for Ki‐67, bcl‐2 and caspase‐3. Ki‐67 is a known cell proliferation marker,( 23 ) and BL/BLL patients typically have a very high Ki‐67 proliferation index. In this study, Ki‐67 expression did not have a significant effect on the survival of BL/BLL patients (data not shown). Furthermore, though we analyzed the relation between Ki‐67 expression and the overall survival, including the factor of chemotherapy regimen, we could not detect a significant difference in this study (data not shown).
It is possible that the results of the statistical analysis associated with Ki‐67 were influenced by the fact that all the patients in this study had a relatively high index of Ki‐67.
Bcl‐2 has anti‐apoptotic functions in that it inhibits the pathway for programmed cell death,( 23 ) and is usually absent in BL/BLL. Caspase‐3 is a marker of the early phase of apoptosis,( 24 ) and is essential for certain processes associated with the formation of apoptotic bodies.( 8 ) Hakimelahi et al. reported that one mechanism underlying the anti‐apoptotic function of bcl‐2 had been shown to operate via its inhibitory effect on the activation of the caspase cascade.( 25 ) They also suggested that bcl‐2 might function by directly binding to the adaptor proteins, thus inhibiting the activation of the caspase cascade.
Many investigators have described that frequent apoptosis in BL may stem from the rearrangement of the c‐MYC oncogene, which plays a critical role in cell cycle regulation, including proliferation, differentiation and apoptosis.( 12 , 13 , 14 , 26 , 27 ) The overexpression of c‐MYC in primary cells results in cell cycle arrest or apoptosis induced by p53‐dependent and p‐53‐independent pathways.( 14 , 27 ) Hecht et al. also suggested that bcl‐2 protects cells against both p53‐dependent and p‐53‐independent apoptotic pathways induced by c‐MYC.( 14 ) In this study, there were no significant differences in the correlation between caspase‐3 and Ki‐67 (data not shown). The reason for the absence of significant differences might be that all the patients in this study had c‐MYC rearrangement.
The cells that express both Ki‐67 and caspase‐3 involve a contradiction in the method of cell proliferation, because these cells undergo both proliferation and apoptosis simultaneously. However, accidental cell death often leads to compensatory proliferation because organ growth is highly dependent on the balance between cell proliferation and apoptotic cell death.( 28 , 29 , 30 ) For instance, a cyclic remodeling process characterized by high rates of proliferation and atresia is observed in ovarian follicular development.( 31 ) In their study on Drosophila, Hyung et al. reported that apoptotic cells activate the signaling cascades for compensatory proliferation.( 32 ) We did not observe this mechanism in this study; however, the ability of these cells to proliferate might be induced by the signaling cascades of the apoptotic cells surrounding them. In addition, although this study could not show a significant correlation between the percentage of cells positive for both Ki‐67 and caspase‐3 and the survival of patients in BL/BLL, the percentage of cells positive for both Ki‐67 and caspase‐3 could be used as a means to distinguish between BL/BLL and DLBCL because the percentage of these cells was significantly higher in BL/BLL than in DLBCL (data not shown).
Hickman described how the cytotoxicity of many chemotherapeutic agents is determined by their ability to induce apoptosis in tumor cells.( 33 ) In this study, we found a significant difference in the overall survival between the high and low caspase‐3 groups. Except bcl‐2‐positive patients, which has an atypical immunohistochemical reaction in BL/BLL, the patients in the high caspase‐3 group tended to better survive than those in the low caspase‐3 group, although we could not detect a significant difference in this study. This result suggests that an increase in caspase‐3 may indicate an increase in cell apoptosis and effective response to chemotherapy, resulting in a favorable outcome in patients with ordinary BL/BLL. In addition, Daniels et al. in their study using human BL cell lines, suggested that rituximab could kill human B cells via a caspase‐independent form of programmed cell death, and that this pathway might be relevant to the clinical efficacy of rituximab in BL patients.( 34 )
Recently, the survival rates of patients with BL/BLL have improved following the use of aggressive short‐term chemotherapy, particularly in children and adolescents,( 35 ) and several studies have reported high cure rates following the use of similar chemotherapeutic protocols in adults with BL or Burkitt's leukemia.( 36 , 37 ) We previously reported that BL/BLL patients who received aggressive short‐term chemotherapy showed better overall survival than those who received CHOP or CHOP‐like chemotherapy.( 18 ) In this study, while the patients in the low caspase‐3 group showed significantly worse overall survival than those in the high caspase‐3 group, intensive short‐term chemotherapeutic regimens improved the overall survival in the patients in the low caspase‐3 group. Therefore, it is possible that the use of aggressive short‐term chemotherapy may improve the prognosis of the patients in the low caspase‐3 group who demonstrated less apoptosis than those in the high caspase‐3 group. Moreover, in this study, the median of age distribution among the high caspase‐3 group and the low caspase‐3 group is apparently different (13 and 64.5 years, respectively). Therefore, we have hypothesized that the better response to chemotherapy in children with BL/BLL is because a large number of BL/BLL patients in the high caspase‐3 group were considerably younger than those in the low caspase‐3. However, this study was performed retrospectively and the number of patients was small. A large prospective study is required in the future to verify our results.
Our results suggest that caspase‐3 may play the role of an indicator for the prognosis of BL/BLL. We conclude that caspase‐3 expression should be confirmed in patients with BL/BLL.
Acknowledgments
We thank Kyowa Medex for supporting this study.
References
- 1. Wyllie AH, Kerr JF, Currie AR. Cell death. the significance of apoptosis. Int Rev Cytol 1980; 68: 251–306. [DOI] [PubMed] [Google Scholar]
- 2. Arends MJ, Wyllie AH. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol 1991; 32: 223–54. [DOI] [PubMed] [Google Scholar]
- 3. Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol 1992; 10: 267–93. [DOI] [PubMed] [Google Scholar]
- 4. Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 1999; 6: 1028–42. [DOI] [PubMed] [Google Scholar]
- 5. Konstantinidou AE, Givalos N, Gakiopoulou H et al . Caspase‐3 immunohistochemical expression is a marker of apoptosis, increased grade and early recurrence in intracranial meningiomas. Apoptosis 2007; 12: 695–705. [DOI] [PubMed] [Google Scholar]
- 6. Reed JC. Mechanisms of apoptosis. Am J Pathol 2000; 157: 1415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gown AM, Willingham MC. Improved detection of apoptotic cells in archival paraffin sections: immunohistochemistry using antibodies to cleaved caspase 3. J Histochem Cytochem 2002; 50: 449–54. [DOI] [PubMed] [Google Scholar]
- 8. Porter AG, Janicke RU. Emerging roles of caspase‐3 in apoptosis. Cell Death Differ 1999; 6: 99–104. [DOI] [PubMed] [Google Scholar]
- 9. Woo M, Hakem R, Soengas MS et al . Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev 1998; 12: 806–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diebold JJE, Raphael M, Warnke RA. Burkitt lymphoma. In: Jaffe ES HN, Stein H, Vardiman JW, eds. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press, 2001. [Google Scholar]
- 11. Harris NL, Jaffe ES, Stein H et al . A revised European‐American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994; 84: 1361–92. [PubMed] [Google Scholar]
- 12. Chuang SS, Ye H, Du MQ et al . Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B‐cell lymphoma with very high proliferation index and with or without a starry‐sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol 2007; 128: 558–64. [DOI] [PubMed] [Google Scholar]
- 13. Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004; 104: 3009–20. [DOI] [PubMed] [Google Scholar]
- 14. Hecht JL, Aster JC. Molecular biology of Burkitt's lymphoma. J Clin Oncol 2000; 18: 3707–21. [DOI] [PubMed] [Google Scholar]
- 15. Pelengaris S, Khan M, Evan G. c‐MYC: more than just a matter of life and death. Nat Rev Cancer 2002; 2: 764–76. [DOI] [PubMed] [Google Scholar]
- 16. Braziel RM, Arber DA, Slovak ML et al . The Burkitt‐like lymphomas: a Southwest Oncology Group study delineating phenotypic, genotypic, and clinical features. Blood 2001; 97: 3713–20. [DOI] [PubMed] [Google Scholar]
- 17. Nakamura N, Nakamine H, Tamaru J et al . The distinction between Burkitt lymphoma and diffuse large B‐cell lymphoma with c‐myc rearrangement. Mod Pathol 2002; 15: 771–6. [DOI] [PubMed] [Google Scholar]
- 18. Nomura Y, Karube K, Suzuki R et al . High‐grade mature B‐cell lymphoma with Burkitt‐like morphology: results of a clinicopathologic study of 72 Japanese patients. Cancer Sci 2008; 99: 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohshima K, Kawasaki C, Muta H et al . CD10 and Bcl10 expression in diffuse large B‐cell lymphoma: CD10 is a marker of improved prognosis. Histopathology 2001; 39: 156–62. [DOI] [PubMed] [Google Scholar]
- 20. Nomura Y, Sugita Y, Yoshida S et al . Estimation of apoptosis and cell proliferation in histiocytic necrotizing lymphadenitis using immunohistochemical double staining. Pathol Int 2008; 58: 98–103. [DOI] [PubMed] [Google Scholar]
- 21. Li JY, Gaillard F, Moreau A et al . Detection of translocation t(11;14) (q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization. Am J Pathol 1999; 154: 1449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kodama T, Ohshima K, Nomura K et al . Lymphomatous polyposis of the gastrointestinal tract, including mantle cell lymphoma, follicular lymphoma and mucosa‐associated lymphoid tissue lymphoma. Histopathology 2005; 47: 467–78. [DOI] [PubMed] [Google Scholar]
- 23. McCormick D, Chong H, Hobbs C, Datta C, Hall PA. Detection of the Ki‐67 antigen in fixed and wax‐embedded sections with the monoclonal antibody MIB1. Histopathology 1993; 22: 355–60. [DOI] [PubMed] [Google Scholar]
- 24. Chrysomali E, Nikitakis NG, Tosios K, Sauk JJ, Papanicolaou SI. Immunohistochemical evaluation of cell proliferation antigen Ki‐67 and apoptosis‐related proteins Bcl‐2 and caspase‐3 in oral granular cell tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96: 566–72. [DOI] [PubMed] [Google Scholar]
- 25. Hakimelahi S, Parker HR, Gilchrist AJ et al . Plakoglobin regulates the expression of the anti‐apoptotic protein BCL‐2. J Biol Chem 2000; 275: 10 905–11. [DOI] [PubMed] [Google Scholar]
- 26. Truffinet V, Pinaud E, Cogne N et al . The 3′ IgH locus control region is sufficient to deregulate a c‐myc transgene and promote mature B cell malignancies with a predominant Burkitt‐like phenotype. J Immunol 2007; 179: 6033–42. [DOI] [PubMed] [Google Scholar]
- 27. Sanchez‐Beato M, Sanchez‐Aguilera A, Piris MA. Cell cycle deregulation in B‐cell lymphomas. Blood 2003; 101: 1220–35. [DOI] [PubMed] [Google Scholar]
- 28. Zhang X, Zhang Q, Zhang Z, Na Y, Guo Y. Apoptosis profiles in benign prostatic hyperplasia: close associations of cell kinetics with percent area density of histologic composition. Urology 2006; 68: 905–10. [DOI] [PubMed] [Google Scholar]
- 29. Kondo S, Senoo‐Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Mol Cell Biol 2006; 26: 7258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol 2004; 14: 1262–6. [DOI] [PubMed] [Google Scholar]
- 31. Vital‐Reyes V, Rodriguez‐Burford C, Chhieng DC, Alvarado‐Cabrero I, Reyes‐Fuentes A, Grizzle WE. Ovarian expression of markers associated with proliferation or apoptosis in women with diminished ovarian reserve. Fertil Steril 2006; 86: 176–85. [DOI] [PubMed] [Google Scholar]
- 32. Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 2004; 7: 491–501. [DOI] [PubMed] [Google Scholar]
- 33. Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev 1992; 11: 121–39. [DOI] [PubMed] [Google Scholar]
- 34. Daniels I, Abulayha AM, Thomson BJ, Haynes AP. Caspase‐independent killing of Burkitt lymphoma cell lines by rituximab. Apoptosis 2006; 11: 1013–23. [DOI] [PubMed] [Google Scholar]
- 35. Cairo MS, Sposto R, Perkins SL et al . Burkitt's and Burkitt‐like lymphoma in children and adolescents: a review of the Children's Cancer Group experience. Br J Haematol 2003; 120: 660–70. [DOI] [PubMed] [Google Scholar]
- 36. Todeschini G, Tecchio C, Degani D et al . Eighty‐one percent event‐free survival in advanced Burkitt's lymphoma/leukemia: no differences in outcome between pediatric and adult patients treated with the same intensive pediatric protocol. Ann Oncol 1997; 8 (Suppl 1): 77–81. [PubMed] [Google Scholar]
- 37. Adde M, Shad A, Venzon D et al . Additional chemotherapy agents improve treatment outcome for children and adults with advanced B‐cell lymphomas. Semin Oncol 1998; 25: 33–9; discussion 45–8. [PubMed] [Google Scholar]


