Abstract
Vascular endothelial growth factor receptor 2 (VEGFR2) is an essential factor in tumor angiogenesis and in the growth of pancreatic cancer. Immunotherapy using epitope peptide for VEGFR2 (VEGFR2‐169) that we identified previously is expected to improve the clinical outcome. Therefore, a phase I clinical trial combining of VEGFR2‐169 with gemcitabine was conducted for patients with advanced pancreatic cancer. Patients with metastatic and unresectable pancreatic cancer were eligible for the trial. Gemcitabine was administered at a dose of 1000 mg/m2 on days 1, 8, and 15 in a 28‐day cycle. The VEGFR2‐169 peptide was subcutaneously injected weekly in a dose‐escalation manner (doses of 0.5, 1, and 2 mg/body, six patients/one cohort). Safety and immunological parameters were assessed. No severe adverse effect of grade 4 or higher was observed. Of the 18 patients who completed at least one course of the treatment, 15 (83%) developed immunological reactions at the injection sites. Specific cytotoxic T lymphocytes (CTL) reacting to the VEGFR2‐169 peptide were induced in 11 (61%) of the 18 patients. The disease control rate was 67%, and the median overall survival time was 8.7 months. This combination therapy for pancreatic cancer patients was tolerable at all doses. Peptide‐specific CTL could be induced by the VEGFR2‐169 peptide vaccine at a high rate, even in combination with gemcitabine. From an immunological point of view, the optimal dose for further clinical trials might be 2 mg/body or higher. This trial was registered with ClinicalTrial.gov (no. NCT 00622622). (Cancer Sci 2009)
Pancreatic cancer shows an extremely poor prognosis with an overall 5‐year survival of 5%.( 1 ) A curative surgical operation of pancreatic cancer should significantly improve the patient’s prognosis, but a great majority of patients with pancreatic cancer are diagnosed at an advanced stage, which makes curative resection very difficult.( 2 , 3 ) Single‐agent gemcitabine treatment is the standard chemotherapy for unresectable pancreatic cancer at present, although its effect is very limited.( 4 , 5 ) Although some phase III trials of combination chemotherapy of cytotoxic agents with gemcitabine have been attempted, they have failed to prove a statistically‐significant improvement in survival, compared with the treatment of gemcitabine alone.( 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ) The addition of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, to gemcitabine revealed survival benfits.( 15 ) However, the improvement of the survival period and the increased risk of toxicities indicate that its clinical benefit is also very limited.( 16 ) Thus, new treatment modalities to provide a prolonged survival benefit with a minimum risk of adverse reactions for patients with advanced pancreatic cancer are urgently required.
A number of specific immunotherapies, especially peptide vaccine therapy, has been attempted since the identification of epitope peptides.( 17 ) However, because their clinical responses have been minimal, several mechanisms of immune evasion of tumors have been implicated as issues to improve cancer immunotherapy.( 18 ) For example, the antitumor effect of cytotoxic T lymphocytes (CTL) induced by peptide vaccine was suspected to be inhibited due to tumor cell heterogeneity and also the downregulation or loss of human leukocyte antigen (HLA) or antigen proteins.( 19 , 20 ) These immune‐escape mechanisms should be overcome by the development of novel effective approaches.
We previously focused on immunotherapy targeting the vascular endothelial growth factor (VEGF) signaling pathway which plays critical roles in the development and progression of pancreatic cancer.( 21 , 22 , 23 , 24 , 25 , 26 , 27 ) In particular, VEGFR2, which is a functional molecule associated with neovascularization, is highly expressed in newly‐induced tumor blood vessels, but not in normal vessels. Therefore, an epitope peptide derived from VEGFR2 was expected to overcome one of the problems mentioned above. The VEGFR2‐169 peptide is an immunogenic peptide derived from VEGFR2 restricted with HLA‐A*2402, which is the most common HLA‐A allele in Japanese population.( 28 , 29 ) Even in cancer patients, specific CTL were strongly induced by this peptide.( 28 ) Since gemcitabine, the standard agent for the treatment of pancreatic cancer, was indicated to enhance immunological responses,( 30 , 31 ) we have attempted a clinical trial to evaluate the safety, tolerability, and immune response of the administration of the VEGFR2‐169 peptide in combination with gemcitabine.
Materials and Methods
Patient eligibility. Patients diagnosed with unresectable pancreatic cancer were enrolled in this trial from November 2006 to March 2008 at Wakayama Medical University Hospital (WMUH), Wakayama, Japan. The eligibility criteria included unresectable pancreatic cancer with metastatic, recurrent, and/or locally‐advanced disease based on diagnostic imaging using computed tomography (CT) or histological examinations. Other inclusion criteria included the HLA‐A*2402‐positive status, as determined by commercially‐available genomic DNA typing tests (SRL, Tokyo, Japan); an Eastern Cooperative Oncology Group performance status of 0–2; age between 20 and 80 years; life expectancy ≥3 months; and adequate hepatic, renal, and bone marrow function (white blood cell count ≥2000/μL, platelets ≥75 000/μL, aspartate aminotransferase ≤150 IU/L, alanine aminotransferase ≤150 IU/L, total bilirubin ≤3 g/dL, and serum creatinine ≤1.5 mg/dL). The exclusion criteria included pregnancy or lactation, active infection, other active malignancy, unhealed wound, intestinal obstruction or interstitial pneumonia, and concurrent treatment with steroids or immunosuppressive agents. Written, informed consent was obtained from all patients, and the study was approved by the institutional review board at WMUH.
Study design and end‐points. This study was a non‐randomized, open‐label, phase I clinical trial with dose‐escalation of the VEGFR2‐169 peptide combined with gemcitabine for patients with advanced unresectable pancreatic cancer. The primary end‐point of this trial was the safety of the peptide vaccination combined with gemcitabine. The secondary end‐points were immunological responses, clinical outcome, and the determination of the optimal dose of peptide for further clinical trials. Immunological responses were assessed by measuring γ‐interferon (IFN‐γ) production from specific CTL responding to the VEGFR2‐169 peptide, the proportion of regulatory T (Treg) cells, and immunological reactions at the injection sites (RAI). RAI was defined by erythema and/or induration at the injection site of the vaccine. Clinical outcomes include assessment using CT scanning in accordance with the Response Evaluation Criteria in Solid Tumors, time to progression (TTP), and overall survival (OS). CT scanning was performed after one and two cycles, and once every 3 months after three cycles. TTP was determined as the time from the date of the initial vaccination until the documentation of clear disease progression. OS was estimated from the date of the initial vaccination to the date of death. This trial was registered with ClinicalTrials.gov (no. NCT00622622).
Treatment protocol. The dose was escalated as 0.5, 1, and 2 mg/body of the vaccinated peptide. The VEGFR2‐169 peptide was administered emulsified with incomplete Freund’s adjuvant (Montanide ISA‐51VG; SEPPIC, Paris, France) and subcutaneously administered on days 1, 8, 15, and 22 in a 28‐day treatment cycle. Gemcitabine was intravenously administered at a dose of 1000 mg/m2 on days 1, 8, and 15. The administration of VEGFR2‐169 and gemcitabine was repeatedly performed until the patients had completed at least one course.
Toxicity assessment and dose‐limiting toxicity. Toxicity was assessed based on the common terminology criteria for adverse effects version 3.0. Dose‐limiting toxicity was defined as a hematological toxicity of grade 4 or greater and non‐hematologic toxicity of grade 3 or greater.
Peptides. The VEGFR2‐169 peptide (RFVPDGNRI) was synthesized by the American Peptide Company (Sunnyvale, CA, USA) according to a standard solid‐phase synthesis method and purified by reversed‐phase high‐performance liquid chromatography (HPLC). The purity (>90%) and the identity of the peptides were determined by analytical HPLC and mass spectrometry analysis, respectively. The VEGFR2‐169 peptide and the epitope peptide derived from the HIV–Envelope protein restricted with HLA‐A*2402 (RYLRDQQLL) were used for the measurement of CTL responses.
Cells. TISI cells, HLA‐A*2402‐positive B‐lymphoblastoid cell lines, were purchased from the IHWG Cell and Gene Bank (Seattle, WA, USA). Peripheral blood cells were periodically collected from the enrolled patients. Peripheral blood mononuclear cells (PBMC) were isolated using Ficoll‐Paque Plus (GE Healthcare, Uppsala, Sweden) density gradient centrifugation and frozen immediately after isolation. The PBMC of each patient were simultaneously thawed and used in the measurement of the CTL responses and flow cytometric analysis.
Measurement of CTL responses. An enzyme‐linked immunospot (ELISPOT) assay was performed to measure the specific CTL response against the peptide. PBMC were obtained from patients before the vaccination treatment and at the end of each course, and then frozen and stored in liquid nitrogen until their use. The frozen PBMC were thawed and used in the in vitro sensitization. In brief, PBMC were stimulated with 10 μg/mL VEGFR2‐169 peptide and 20 IU/mL interleukin‐2 at 37°C with 5% CO2 for 2 weeks. The VEGFR2‐169 peptide was added twice at days 1 and 8. After incubation, harvested cells were used as responder cells, and peptide‐pulsed TISI cells were used as stimulator cells (1×105 cells per well). The HLA‐A*2402‐restricted epitope peptide derived from the HIV–Env protein was used as the negative control peptide. An IFN‐γ ELISPOT kit and AEC substrate set (BD Biosciences Pharmingen, San Diego, CA, USA) were used to measure the CTL responses throughout this clinical trial. Spots were captured and analyzed using an automated ELISPOT reader, ImmunoSPOT 4S (CTL, Cleveland, OH, USA). The assessment of positive CTL responses was identified when the average spot‐forming cells per well in response to the VEGFR2‐169 peptide was ≥10 spot‐forming cells per well in response to the control peptide.
Flow cytometry. Analysis of the proportion of Treg cells in the peripheral blood cells was performed using a FACSCalibur (Becton Dickinson, San Jose, CA, USA). Frozen PBMC samples were periodically thawed and used to measure Treg cells directly. In this experiment, CD4+CD25highFoxp3+ cells were judged as Treg cells. The antibodies used to measure Treg cells were as follows: CD4–fluorescein isothiocyanate, Foxp3–phycoerythrin, and CD25–Allophycocyanin (e‐Bioscience, San Diego, CA, USA).
Statistical analysis. Statistical analyses were performed using either the unpaired Student’s t‐test or the Wilcoxon signed rank test. TTP and OS curves were estimated using Kaplan–Meier methodology. Any correlations between the clinical outcome and RAI were estimated using Fisher’s exact test.
Results
Demographics and clinical characteristics. A total of 21 patients were enrolled in this trial. Eighteen of the 21 patients who received four or more vaccinations (at least one course) were evaluated for further analysis; the remaining three patients, who dropped out from this trial because of their own clinical condition and decision, were excluded from further analysis. Six patients were enrolled in each dose condition. The demographics and clinical characteristics of the 18 patients are shown in Table 1.
Table 1.
Demographics and clinical characteristics of the eligible patients
| Characteristics | Peptide | ||
|---|---|---|---|
| 0.5 mg | 1.0 mg | 2.0 mg | |
| (n = 6) | (n = 6) | (n = 6) | |
| Sex | |||
| Male/Female | 4/2 | 5/1 | 5/1 |
| Age (years)† | 66 (38–75) | 66 (56–79) | 64 (58–78) |
| Performance status | |||
| 0 | 4 | 5 | 5 |
| 1 | 2 | 1 | 1 |
| Disease stage | |||
| Locally advanced | 1 | 4 | 2 |
| Metastatic | 5 | 2 | 4 |
| Prior therapy | |||
| Chemotherapy | 2 | 1 | 0 |
Number of patients is indicated for each cohort with regards to the performance status, disease stage, and prior therapy. †Median (range).
Toxicity. The overall toxicity of the 18 patients is summarized in Table 2. No patient showed any toxicities of grade 4 or greater. Fifteen of the 18 patients (83%) developed immunological reactions, erythema, and/or induration at the injection sites (RAI). A grade 3 hemorrhage from the duodenum was observed in one patient due to the progression of pancreatic cancer invasion to the duodenum, but we judged that there was no correlation between the hemorrhage and this treatment. In this study, no case revealed vascular adverse events, such as hypertension, bleeding, and thromboembolism. We observed two patients with grade 3 hepatic transaminase elevation and one patient with grade 2 hyperbilirubinemia, probably due to obstructive jaundice by the progression of the disease. Grade 3 leucopenia and neutropenia were observed in two (11%) and 10 (56%) patients, respectively. All patients recovered from the toxicity by postponing gemcitabine for 1 week, the administration of granulocyte colony‐stimulating factor, or the reduction of the doses of gemcitabine according to the dose reduction criteria. No dose‐limiting toxicity was observed in this trial.
Table 2.
Summary of toxicity
| Toxicity | Peptide | Total patients (n = 18) (%) | |||||
|---|---|---|---|---|---|---|---|
| 0.5 mg (n = 6) | 1.0 mg (n = 6) | 2.0 mg (n = 6) | |||||
| Grade | Grade | Grade | |||||
| 1–2 | 3 | 1–2 | 3 | 1–2 | 3 | ||
| Blood/bone marrow | |||||||
| Anemia | 5 | 0 | 4 | 1 | 2 | 2 | 14 (78) |
| Leukopenia | 5 | 0 | 5 | 0 | 4 | 2 | 16 (89) |
| Neutropenia | 2 | 2 | 2 | 3 | 1 | 5 | 15 (83) |
| Thrombocytopenia | 4 | 0 | 4 | 0 | 3 | 2 | 13 (72) |
| Neutropenic fever | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Hepatic | |||||||
| Elevated AST | 3 | 1 | 3 | 1 | 1 | 1 | 10 (56) |
| Elevated ALT | 2 | 1 | 0 | 1 | 2 | 1 | 7 (39) |
| Elevated bilirubin | 1 | 0 | 0 | 0 | 1 | 0 | 2 (11) |
| Constitutional symptoms | |||||||
| Fatigue | 1 | 0 | 1 | 0 | 1 | 0 | 3 (17) |
| Fever | 1 | 0 | 2 | 0 | 1 | 0 | 4 (22) |
| Gastrointestinal | |||||||
| Nausea/vomiting | 0 | 0 | 2 | 0 | 3 | 0 | 5 (28) |
| Anorexia | 1 | 0 | 2 | 0 | 0 | 0 | 3 (17) |
| Constipation | 2 | 0 | 1 | 0 | 0 | 0 | 3 (17) |
| Diarrhea | 1 | 0 | 2 | 0 | 1 | 0 | 4 (22) |
| Dermatology/skin | |||||||
| Rash | 0 | 0 | 1 | 0 | 0 | 0 | 1 (6) |
| Pruritus | 1 | 0 | 0 | 0 | 0 | 0 | 1 (6) |
| Reaction at the injection site | 5 | 0 | 4 | 0 | 6 | 0 | 15 (83) |
| Alopecia | 0 | 0 | 0 | 0 | 1 | 0 | 1 (6) |
| Other | |||||||
| Headache | 0 | 0 | 0 | 0 | 1 | 0 | 1 (6) |
| Hemorrhage | 0 | 0 | 0 | 0 | 0 | 1 | 1 (6) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
RAI. RAI, which may have been caused by the immunological reaction, were observed in all patients that were judged as having stable disease (SD) or partial response (PR) (Table 3). The proportion of patients showing RAI was significantly higher in the group with SD or PR than that showing progressive disease (PD) (P < 0.05).
Table 3.
Correlation of antitumor effect with RAI
| RAI | ||
|---|---|---|
| + | − | |
| Antitumor response | ||
| SD or PR | 12 | 0 |
| PD | 3 | 3 |
Reaction at the injection sites (RAI) was observed in all patients who achieved stable disease (SD) or partial response (PR), whereas three patients without RAI had progressive disease (PD). Statistical analysis was performed by Fisher’s exact test (P < 0.05).
CTL response. An IFN‐γ ELISPOT assay was conducted using PBMC periodically obtained from patients to assess the cellular immune responses to VEGFR2‐169. Positive CTL responses specific to the vaccinated peptide were determined as described in the Materials and Methods section. The positive CTL responses were seen in three of the six patients (50%) receiving 0.5 mg vaccination, four of the six patients (67%) receiving 1 mg, and four of the six patients (67%) receiving 2 mg, respectively (Table 4). The positive CTL responses were observed in 11 (61%) of the 18 patients who received at least one course of the vaccination. The representative data from the IFN‐γ ELISPOT assay and CTL responses in patient no. 12 before and after one course of the treatment are shown in Figure 1. The positive CTL response against the VEGFR2‐169 peptide was observed after one course of the treatment, while no CTL response against the VEGFR2‐169 peptide was observed before the vaccination.
Table 4.
CTL response in patients
| No. patients | Peptide | CTL response | Frequency of positive CTL response |
|---|---|---|---|
| 1 | 0.5 mg | − | 3/6 |
| 3 | + | ||
| 4 | + | ||
| 15 | − | ||
| 17 | + | ||
| 18 | − | ||
| 5 | 1.0 mg | + | 4/6 |
| 6 | + | ||
| 7 | − | ||
| 11 | − | ||
| 12 | + | ||
| 14 | + | ||
| 8 | 2.0 mg | + | 4/6 |
| 10 | + | ||
| 13 | + | ||
| 19 | + | ||
| 20 | − | ||
| 21 | − |
CTL, cytotoxic T lymphocytes.
Figure 1.
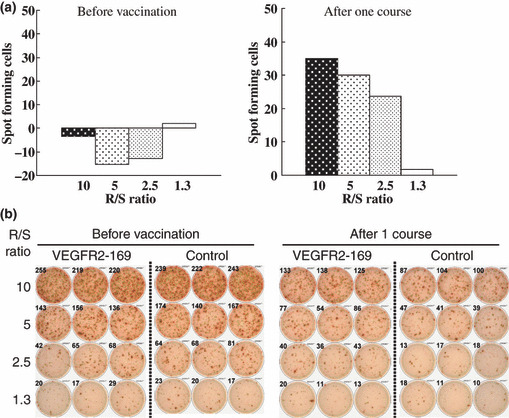
Cytotoxic T‐lymphocyte (CTL) responses in patient no. 12 before the vaccination and after one course of the treatment. A γ‐interferon enzyme‐linked immunospot T (ELISPOT) assay was performed against the vascular endothelial growth factor receptor 2‐169 (VEGFR2‐169) peptide or HIV peptide (control). Positive CTL responses were observed in 11 (61%) of the 18 patients. (a) Number of VEGFR2‐169‐specific spots is indicated as spot‐forming cells in these graphs. (b) representative ELISPOT assay. R/S, responder/stimulator ratio.
Treg cells. The proportion of Treg cells was measured using PBMC samples of the enrolled patients. The average ratios of Treg cells in the CD4+ cells of healthy donors and patients before the vaccination and those after one course and two courses of the treatment were 3.9%, 5.3%, 4.4%, and 4.2%, respectively. The proportion of Treg cells before the vaccination in pancreatic patients in this study (shown as pancreatic cancer with stage IV) was significantly higher than that of healthy donors (P < 0.05, Fig. 2a). The proportion of Treg cells after the vaccination was significantly reduced in comparison with that before the vaccination in the patients showing positive CTL responses (P < 0.05, Fig. 2b). The representative fluorescence‐activated cell sorter profile of Treg is shown in Figure 2(c).
Figure 2.
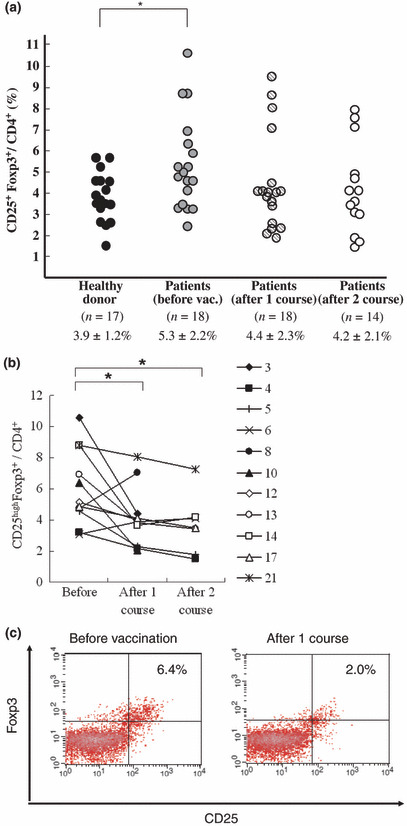
Proportion of CD25highFoxp3+ cells (Treg) in peripheral blood cells in pancreatic cancer patients by flow cytometry. (a) Proportion of Treg cells in CD4+ cells was examined in healthy donors, as well as patients with stage IV and unresectable pancreatic cancer in this trial. Number of samples, the average of the proportion, and standard deviation are indicated under the graph. Proportion of Treg cells in patients before the vaccination was significantly higher than that in the healthy donors. Statistical analysis was performed using unpaired Student’s t‐test (*P < 0.05). (b) Proportion of Treg cells in 11 patients who showed positive cytotoxic T‐lymphocyte responses. Numbers indicate the patients in this trial, respectively. After the vaccination, the proportion of Treg cells was significantly reduced compared with that before the vaccination. Statistical analysis was performed using the Wilcoxon signed rank test. (*P < 0.05) (c) Treg profile of patient no. 10 by flow cytometry is shown as representative data.
Clinical outcomes. One patient achieved PR, 11 patients (61%) achieved SD and six patients (33%) revealed PD (Table 5). Thus, the disease control rate (complete response, PR, and SD) in this study was calculated to be 67%. CT scan images of the patient with PR are shown in Figure 3. Seven months later, the tumor in the pancreatic body was reduced to 45% in comparison to that before the vaccination. This patient started to receive this treatment in May 2007 and is still alive. This patient revealed the longest progression‐free survival time of 19.4 months, although liver metastasis was found at the beginning of the treatment. CT scan images of one representative patient with SD are shown in Figure 4. The tumor in the pancreatic tail stayed at almost the same size, and one of the liver metastatic legions almost disappeared.
Table 5.
Clinical outcomes
| Peptide | |||
|---|---|---|---|
| 0.5 mg | 1.0 mg | 2.0 mg | |
| (n = 6) | (n = 6) | (n = 6) | |
| Injection times of peptide† | 8 (4–12) | 8 (5–11) | 8 (4–34) |
| Clinical outcome | |||
| Antitumor effect | |||
| PR/SD/PD | 0/4/2 | 0/4/2 | 1/3/2 |
| Progression‐free survival (days)† | 102 (28–148) | 135 (60–675) | 131 (31–589) |
| Overall survival (days)† | 233 (102–334) | 207 (135–675) | 344 (109–614) |
†Median (range). PD, progressive disease; PR, partial response; SD, stable disease.
Figure 3.
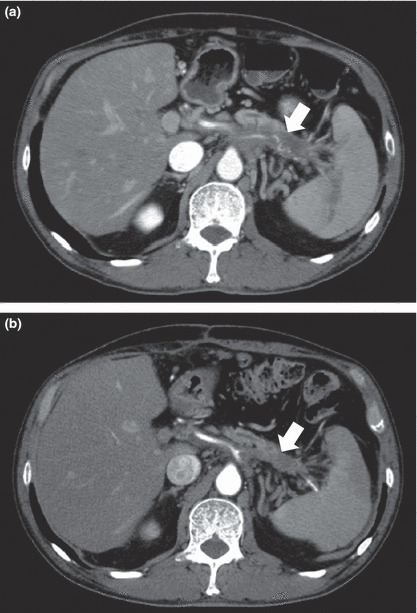
Axial contrast‐enhanced computed tomography (CT) images of a patient who showed a partial response. (a) Axial contrast‐enhanced CT image shows the locally‐advanced tumor of the pancreatic body (arrow). (b) Axial contrast‐enhanced CT image after 7 months shows the partial response of the pancreatic body mass (arrow). Tumor was reduced to 45% according to the Response Evaluation Criteria in Solid Tumors.
Figure 4.
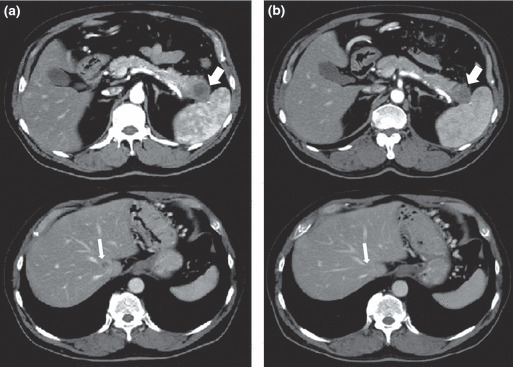
Axial contrast‐enhanced computed tomography (CT) images of a patient showing stable disease. (a) Axial contrast‐enhanced CT image shows a tumor of the pancreatic tail (thick arrow) and one of the multiple liver metastases (thin arrow). (b) Axial contrast‐enhanced CT image after 3 months shows a stable tumor of the pancreatic tail (thick arrow) and the disappearance of one of the liver metastasis (thin arrow).
The Kaplan–Meier curve for overall survival is shown in Figure 5. The median overall survival time (MST) was 7.7 months when we analyzed all 18 patients. However, the MST was a little bit longer (8.7 months) for the 15 patients who had no pretreatment of chemotherapy before enrolling in this clinical trial. The median TTP was 3.9 months for all 18 patients and 4 months for the 15 patients without pretreatment (data not shown).
Figure 5.
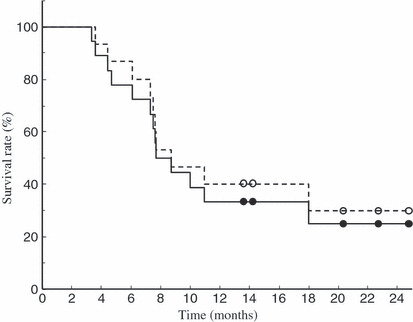
Overall survival measured by the Kaplan–Meier method. Mean overall survival time (MST) was 7.7 months for all 18 patients (––) and 8.7 months for 15 patients who had no chemotherapy before the enrollment in this clinical trial (‐ ‐ ‐).
Discussion
The prognosis of pancreatic cancer is one of the worst among the malignant tumors with a 5‐year survival rate of only approximately 5%. Gemcitabine is at present the only effective agent for pancreatic cancer. Combination therapies of cytotoxic agents with gemcitabine have been investigated, but have failed to prove their clinical benefit. Immunotherapies, including the granulocyte‐macrophage colony‐stimulating factor gene transduced tumor vaccines (GVAX) trial, mutated KRAS peptide vaccine, and gastrin peptide vaccine for pancreatic cancer, have also been tested and are expected to improve the prognosis of patients with the disease.( 32 , 33 , 34 ) The quantity of myeloid‐derived suppressor cells was reported to be reduced by gemcitabine treatment in vivo.( 30 ) Furthermore, gemcitabine was shown to enhance the sensitivity of tumor cells to killing‐mediated CTL and their induction.( 35 , 36 ) Considering this evidence, we believe that the combination of gemcitabine with immunotherapy might provide some clinical benefit to patients with pancreatic cancer.
For cancer immunotherapy, the loss or downregulation of HLA molecules on the tumor cells is considered to be one of the major reasons of limited clinical efficacy.( 19 , 20 ) Under these conditions, the development of vaccines against vascular endothelial cells induced in tumor tissues is one of the approaches to overcome such problems. Vascular endothelial cells play crucial roles in the growth and progression of tumors and stably express HLA molecules. Moreover, pancreatic cancer cells were reported to express VEGFR2 themselves and expected to be the target of CTL.( 37 , 38 )
The current study investigated a novel cancer vaccine therapy for pancreatic cancer using the VEGFR2‐169 peptide in combination with gemcitabine. We observed no severe adverse reaction related to the treatment in this trial, as shown in Table 2. Specific adverse events caused by this vaccine treatment were the RAI, but no event greater than grade 3 was observed. Therefore, this protocol is considered to be very safe and well tolerable. The immunological responses in this trial were measured by the RAI, CTL responses against the vaccinated peptide, and the proportion of Treg cells. The incidence of RAI was significantly correlated with the clinical outcome, as shown in Table 3. This result suggests that RAI may be one of the surrogate markers to predict clinical responses for this protocol, although the biological significance and specificity for RAI are not clear. The specific CTL responses against the vaccinated peptide were observed in 11 (61%) of the 18 patients, clearly demonstrating that CTL against VEGFR2 could be induced by a vaccination of the VEGFR2‐169 peptide with gemcitabine treatment in the patients. No obvious correlations of CTL response and clinical outcome were shown in this study. Further studies are required to confirm this issue because of the small number of patients in this phase I study. It is important to address the killing to antigen‐expressing cells endogenously. We did not monitor the CTL response against the endogenous target in this phase I study, because significant blood volumes are required to assess the endogenous killing assay. However, Wada et al. clearly demonstrated that the CTL induced with theVEGFR2‐169 peptide showed strong cytotoxicity against the human umbilical vein endothelial cells, which are endothelial cells naturally and endogenously expressing VEGFR2.( 28 ) Although the number of the patients was small, the proportion of Treg cells in patients with advanced pancreatic cancer was significantly higher than that in the healthy donors. The proportion of Treg cells in peripheral blood cells was significantly decreased in the patients who showed positive CTL responses by the vaccination. The Treg ratio increased in some patients without a CTL response; however, there was no statistical significance (data not shown). These data indicated that there might be some correlation between the decrease of Treg cells and the induction of positive CTL responses by treatment with the peptide vaccine and gemcitabine, possibly due to the enhancement of the antitumor immune response by gemcitabine. No dose‐limiting toxicity was observed in each dose cohort. However, the RAI was observed in all six patients with the 2 mg vaccination, as shown in Table 2. Furthermore, the RAI was observed in all patients with PR or SD. Therefore, we assume the optimal dose of the peptide for further clinical trials to be 2 mg/body.
This protocol was well tolerated, and peptide‐specific CTL were found to be induced by a VEGFR2‐169 peptide vaccine at a high rate, even in combination with the anticancer agent, gemcitabine. In future, a randomized, controlled clinical trial will be essential to demonstrate the clinical benefits of the VEGFR2‐169 peptide for advanced pancreatic cancer patients treated with gemcitabine.
References
- 1. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- 2. Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995 using the National Cancer Database. J Am Coll Surg 1999; 189: 1–7. [DOI] [PubMed] [Google Scholar]
- 3. Bramhall SR, Allum WH, Jones AG, Allwood A, Cummins C, Neoptolemos JP. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg 1995; 82: 111–5. [DOI] [PubMed] [Google Scholar]
- 4. Rothenberg ML, Moore MJ, Cripps MC et al. A phase II trial of gemcitabine in patients with 5‐FU‐refractory pancreas cancer. Ann Oncol 1996; 7: 347–53. [DOI] [PubMed] [Google Scholar]
- 5. Burris HA 3rd, Moore MJ, Andersen J et al. Improvement in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–13. [DOI] [PubMed] [Google Scholar]
- 6. Berlin JD, Catalano P, Thomas JP et al. Phase III study of gemcitabine combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002; 20: 3270–5. [DOI] [PubMed] [Google Scholar]
- 7. Rocha Lima CM, Green MR, Rotche R et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004; 22: 3776–83. [DOI] [PubMed] [Google Scholar]
- 8. Louvet C, Labianca R, Hammel P et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005; 23: 3509–16. [DOI] [PubMed] [Google Scholar]
- 9. Abou‐Alfa GK, Letourneau R, Harker G et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. J Clin Oncol 2006; 24: 4441–7. [DOI] [PubMed] [Google Scholar]
- 10. Van Cutsem E, Van De Velde H, Karasek P et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004; 22: 1430–8. [DOI] [PubMed] [Google Scholar]
- 11. Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double‐blind placebo‐controlled, randomized study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 2002; 87: 161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinemann V, Quietzsch D, Gieseler F et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006; 24: 3946–52. [DOI] [PubMed] [Google Scholar]
- 13. Heinemann V, Quietzsch D, Gieseler F et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007; 25: 2212–7. [DOI] [PubMed] [Google Scholar]
- 14. Boeck S, Hoehler T, Seipelt G et al. Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (mGemOx): final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Ann Oncol 2008; 19: 340–7. [DOI] [PubMed] [Google Scholar]
- 15. Moore MJ, Goldstein D, Hamm J et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–6. [DOI] [PubMed] [Google Scholar]
- 16. Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol 2007; 25: 4506–7. [DOI] [PubMed] [Google Scholar]
- 17. Boon T, De Plaen E, Lurquin C et al. Identification of tumour rejection antigens recognized by T lymphocytes. Cancer Surv 1992; 13: 23–37. [PubMed] [Google Scholar]
- 18. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘‘tumor escape’’ phenotypes. Nat Immunol 2002; 3: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryschich E, Nötzel T, Hinz U et al. Control of T‐cell‐mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 2005; 11 (2 Pt 1): 498–504. [PubMed] [Google Scholar]
- 21. Itakura J, Ishiwata T, Friess H et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res 1997; 3: 1309–16. [PubMed] [Google Scholar]
- 22. Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 2000; 88: 2239–45. [DOI] [PubMed] [Google Scholar]
- 23. Niedergethmann M, Hildenbrand R, Wostbrock B et al. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas 2002; 25: 122–9. [DOI] [PubMed] [Google Scholar]
- 24. Fujimoto K, Hosotani R, Wada M et al. Expression of two angiogenic factors, vascular endothelial growth factor and platelet‐derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer 1998; 34: 1439–47. [DOI] [PubMed] [Google Scholar]
- 25. Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005; 109: 227–41. [DOI] [PubMed] [Google Scholar]
- 26. Kerbel RS. Tumor angiogenesis. N Engl J Med 2008; 358: 2039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers 2006; 10: 515–27. [DOI] [PubMed] [Google Scholar]
- 28. Wada S, Tsunoda T, Baba T et al. Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res 2005; 65: 4939–46. [DOI] [PubMed] [Google Scholar]
- 29. Date Y, Kimura A, Kato H, Sasazuki T. DNA typing of the HLA‐A gene: population study and identification of four new alleles in Japanese. Tissue Antigens 1996; 47: 93–101. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr‐1+/CD11b+ myeloid suppressor cells in tumor‐bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11: 6713–21. [DOI] [PubMed] [Google Scholar]
- 31. Plate JM, Plate AE, Shott S, Bograd S, Harris JE. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother 2005; 54: 915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jaffee EM, Hruban RH, Biedrzycki B et al. Novel allogeneic granulocyte‐macrophage colony‐stimulating factor‐secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001; 19: 145–56. [DOI] [PubMed] [Google Scholar]
- 33. Achtar M, Behrens RJ, Herrin V et al. Mutant ras vaccine in advanced cancers. Proc Am Soc Clin Oncol 2003; 22: A677. [Google Scholar]
- 34. Gilliam AD, Topuzov EG, Garin AM et al. Randomised double blind placebo controlled multi‐centre group sequential trial of G17DT for patients with advanced pancreatic cancer unsuitable or unwilling to take chemotherapy. Proc Am Soc Clin Oncol 2004; 23: A2511. [Google Scholar]
- 35. Dauer M, Herten J, Bauer C et al. Chemosensitization of pancreatic carcinoma cells to enhance T cell‐mediated cytotoxicity induced by tumor lysate‐pulsed dendritic cells. J Immunother 2005; 28: 332–42. [DOI] [PubMed] [Google Scholar]
- 36. Correale P, Cusi MG, Del Vecchio MT et al. Dendritic cell‐mediated cross‐presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5‐fluorouracil, and leucovorin, elicits a powerful human antigen‐specific CTL response with antitumor activity in vitro. J Immunol 2005; 175: 820–8. [DOI] [PubMed] [Google Scholar]
- 37. Itakura J, Ishiwata T, Shen B, Kornmann M, Korc M. Concomitant over‐expression of vascular endothelial growth factor and its receptors in pancreatic cancer. Int J Cancer 2000; 85: 27–34. [DOI] [PubMed] [Google Scholar]
- 38. Von Marschall Z, Cramer T, Höcker M et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology 2000; 5: 1358–72. [DOI] [PubMed] [Google Scholar]


