Abstract
HAb18G/CD147, a transmembrane glycoprotein highly expressed in various types of malignant cells, mainly functions as an inducer of matrix metalloproteinases to promote tumor growth, invasion and metastasis. However, whether there are other mechanisms underlying the role of HAb18G/CD147 in tumor progression remains to be elucidated. In this study, we investigated the functional effects of HAb18G/CD147 on autophagy in hepatoma cell line SMMC7721 using immunofluoscence staining, Western blot and transmission electronmicroscopy. Our data showed that specific small interference RNA (siRNA) considerably down‐regulated the expression of HAb18G/CD147 in SMMC7721 cells at both messenger RNA (mRNA) and protein levels. The down‐regulation of HAb18G/CD147 significantly promoted starvation‐induced autophagy in a dose‐dependent manner. Using trypan blue exclusion assay, we found that HAb18G/CD147 notably enhanced the survival of SMMC7721 cells through inhibiting starvation‐induced autophagy. In addition, we demonstrated that HAb18G/CD147 down‐regulated the expression of autophagy‐regulating protein Beclin 1 in SMMC7721 cells. Furthermore, our data indicated that HAb18G siRNA‐transfected SMMC7721 cells had a significantly decreased level of phosphorylated serine/threonine protein kinase B (pAkt) and the expression of Beclin 1 was inversely associated with the level of pAkt, suggesting that the Class I phosphatidylinositol 3 kinase‐Akt pathway may be involved in the down‐regulation of Beclin 1 by HAb18G/CD147. Overall, we provide the first experimental evidence to show that HAb18G/CD147 may play an important role in the inhibitory regulation of autophagy. Therefore, our data suggest a new molecular mechanism for HAb18G‐mediated hepatoma progression. (Cancer Sci 2009; 100: 837–843)
We previously cloned HAb18G, a hepatoma‐associated antigen, using monoclonal antibody HAb18 through screening human hepatocellular carcinoma (HCC) complementary DNA (cDNA) library.( 1 ) Moreover, we confirmed that the amino acid sequence of HAb18G is identical to that of CD147, thus naming it HAb18G/CD147. Multiple independent laboratories have also discovered the HAb18G/CD147 protein in different origins of human cells and tissues, designating it extracellular matrix metalloproteinase inducer (EMMPRIN),( 2 ) basigin,( 3 ) or M6 antigen.( 4 )
A series of previous studies reported that CD147 molecule is highly expressed on the surface of various malignant tumor cells, including cancers of liver,( 5 ) skin,( 6 ) bladder,( 7 ) lung and breast.( 8 ) A variety of in vitro studies have suggested that CD147 mainly functions as a cellular adhesion molecule involved in the cell–cell and cell–extracellular matrix interaction to induce the secretion of matrix metalloproteinases (MMPs, mainly including MMP‐1, MMP‐2 and MMP‐9) in tumor local environment and thus promote tumor invasion and metastasis.( 9 , 10 , 11 ) In addition to being an MMP inducer, CD147 has also been reported to promote tumor angiogenesis.( 12 ) However, whether there are other mechanisms explaining the role of HAb18G/CD147 in tumor development and progression remains to be elucidated.
Autophagy is a highly conservative intracellular process that consists of several sequential steps: sequestration, transport to lysosomes, degradation, and utilization of degradation products. Many key molecules are involved in this biological process, especially Beclin 1. Beclin 1 is a mammalian homolog of yeast Atg6/Vps30 and an essential regulator that promotes autophagosome formation through mediating the localization of other autophagy proteins on the pre‐autophagosomal membrane.( 13 ) Class I phosphoinositide 3‐kinase (PI3K) is a heterodimeric enzyme consisting of a 110‐kDa catalytic subunit and an 85‐kDa regulatory subunit. Class I PI3K catalyzes the production of secondary messenger phosphatidylinositol‐3,4,5‐triphosphate, which in turn activates a wide range of downstream targets, mainly including the serine/threonine kinase B (Akt). The Class I PI3K‐Akt pathway regulates a wide spectrum of cellular processes, including cell proliferation, survival, growth, and motility.( 14 , 15 ) A previous study demonstrated that the CD147 molecule could activate the Class I PI3K‐Akt pathway in tumor cells.( 15 ) In addition, the stimulation of Class I PI3K activity has been indicated to inhibit autophagy at the sequestration step of autophagy.( 16 ) These findings lead us to hypothesize that Class I PI3K‐Akt pathway might be involved in the regulation of autophagy by HAb18G/CD147.
Previous studies have clearly demonstrated that autophagy has a wide variety of roles in physiological and pathological processes, such as starvation adaptation,( 17 ) embryonic development,( 18 ) cell survival and death,( 19 ) and tumor suppression.( 20 ) The role of autophagy in cancer has been a topic of intense discussion. Since the late 1970s, an inverse relationship between autophagic activity and malignant transformation has been established in cells and experimental animal models.( 21 , 22 ) Recent publications have reported two opposite functions of autophagy in tumor progression.( 23 ) On one hand, autophagy allows cancer cells to act in response to changing environmental conditions such as nutrient deprivation. On the other hand, autophagy also directly or indirectly induces cell death through excessive self‐digestion and the activation of apoptosis and inhibits tumor progression.( 20 ) The detailed mechanisms underlying the inter‐relationships between autophagy, cell survival and cell death are largely unknown. Pattingre et al.( 24 ) suggested a conceptual model suggesting that the absence of autophagy increases susceptibility to death when cells confront stressful stimuli. In contrast, if induced to a level beyond a physiological range, autophagy can also contribute to death execution.
Our previous studies demonstrated that HAb18G/CD147 plays an important role in invasion and metastasis of hepatocellular carcinoma, mainly by inducing MMP secretion.( 11 , 25 ) Although increasing evidence has linked autophagy to tumor progression, whether autophagy‐mediated cell survival and cell death are involved in the functional roles of HAb18G/CD147 in hepatocellular carcinoma (HCC) has not been evaluated. In the present study, we investigated the effects of HAb18G/CD147 expression on autophagy in hepatoma cell line SMMC7721 and the potential molecular mechanisms. To the best of our knowledge, this is the first study to explore the relationship between HAb18G/CD147 and autophagy.
Materials and Methods
Cell cultures. Human hepatoma cell line SMMC7721 provided by the Institute of Cell and Biochemistry, Chinese Academy of Sciences (Shanghai, China) was regularly grown in RPMI1640 medium supplemented with 10% fetal bovine serum at 37°C under a mixture of 95% air and 5% CO2. For studies of amino acid starvation‐induced autophagy, SMMC7721 cells were cultured in EBSS medium (Earle's Balanced Salt Solution) at 37°C under mixture of 95% air and 5% CO2 for 12 h to induce autophagy as previously described.( 26 , 27 )
Small interfering RNA (siRNA). SMMC7721 cells growing up to 70–80% confluency were transfected with siRNA that specifically targets the HAb18G/CD147 gene (hereafter referred to as HAb18G siRNA) or non‐silencing control siRNA using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, US) according to the manufacturer's protocol. Transfected cells were cultured for 24 h in normal medium and then used for the induction of autophagy and further functional analyses. When used for transfection, the final concentration of siRNA was 100 pM if not otherwise stated. To examine the dose effect of HAb18G/CD147 on autophagy, SMMC7721 cells were transfected with HAb18G siRNAs at a final concentration of 0, 20, 50 and 100 pM. All siRNA duplexes were synthesized by Ambion Inc. (Austin, TX, US). The sequences of siRNA duplex targeting HAb18G/CD147 are as follows: 5′‐GUUCUUCGUGAGU UCCUCdTdT‐3′ and 3′‐dTdTCAAGAAGCACUCAAGGAG‐5′.
Reverse transcription‐polymerase chain reaction (RT‐PCR). After transfection with HAb18G siRNA or control siRNA, SMMC7721 cells were cultured in normal medium for 24 h, and then cultured for another 12 h in EBSS medium. Finally, cells were harvested and the expression of HAb18G/CD147, Beclin 1 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was analyzed by RT‐PCR. In brief, total RNA was extracted using Trizol reagent (Invitrogen) and cDNA was synthesized using Superscript first strand synthesis kit (Invitrogen) according to the manufacturer's instructions. PCR was carried out under the following thermal cycling conditions: one cycle at 94°C for 5 min; 25 cycles at 94°C for 30 s, 52–55°C for 60 s when appropriate, and 72°C for 30 s; one cycle at 72°C for 10 min. The sequences of primers used for PCR of indicated genes were described in previous studies.( 28 ) All primers were synthesized by Shanghai Sangon Biological Engineering Technology and Services Co. Ltd (Shanghai, China).
Western blot analysis. SMMC7721 cell samples were lyzed with radioimmuno precipitation assay (RIPA) buffer (Beyotime, Inc., NanTong, China) containing 100 µM phenylmethylsulfonyl fluoride (PMSF) and protein concentrations were determined using the BCA kit (Pierce, Rockford, IL, US). Equal amounts of total protein were separated using 12% sodium dodecylsulfate – polyacrylamide gel electrophoresis (SDS‐PAGE), and then transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, US). The membranes were subsequently immunoblotted with the appropriate primary antibody diluted in total‐serum bilirubin (TBS) buffer containing 0.05% Tween‐20 and 5% non‐fat dry milk at room temperature for 2 h. The following primary antibodies were used in this study: anti‐HAb18G/CD147 mouse monoclonal antibody (1:3000) prepared in our laboratory, anti‐pAkt rabbit monoclonal antibody (1:100) and anti‐Beclin 1 rabbit polyclonal antibody (1:1000) from Cell Signaling Technology, Inc. (Beverly, MA, US), and anti‐GAPDH mouse monoclonal antibody (1:500) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, US). After extensive washings, the membranes were incubated with appropriate secondary horseradish peroxidase‐conjugated goat antimouse (1:5000) or goat antirabbit (1:5000) antibody (Pierce).
Immunofluorescence staining. Immunofluorescence staining was performed as previously described,( 29 ) to examine the autophagic level. In brief, after culture in EBSS‐medium for 12 h, transfected SMMC7721 cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X‐100, then washed three times with phosphate‐buffered saline (PBS) containing 0.01% Triton X‐100 and 5% goat serum, followed by incubation with anti‐LC3 mouse monoclonal antibody (1:1000) from MBL Inc. (Nagoya, Japan) for 1 h at room temperature. The cells were next incubated with fluorescein isothiocyanate (FITC)‐labeled goat‐anti mouse IgG (1 : 800) (Molecular Probes Inc., Eugene, OR, US) for 1 h at room temperature. Finally, cells were imaged using an Olympus BX60 fluorescence microscope (Olympus, Tokyo, Japan). The percentage of cells with LC3 (microtubule‐associated protein 1 light chain 3) punctate dots was determined as previously described.( 24 ) Briefly, a minimum of 100 cells was counted for each sample in three independent experiments. The percentage of cells with LC3 punctate dots was calculated by dividing the number of cells with punctate dots by total cells counted.
Transmission electron microscope (TEM) analysis. TEM analysis was performed as previously described,( 30 ) to examine the autophagic level. Briefly, after culturing in EBSS‐medium for 12 h, transfected SMMC7721 cells were fixed with 3% glutaraldehyde in 0.2 M phosphate buffer (pH 7.3) for 4 h at 4°C, then postfixed with 1% osmium tetroxide and 0.5% tannic acid for 1 h at 4°C and washed three times with 0.1 M phosphate buffer (pH 7.3). Next, cells were dehydrated and embedded in Epon812 (Electron Microscopy Sciences, Fort Washington, PA, US). Finally, sections were counterstaind with uranyl acetate and lead citrate, and then examined using a JEM‐2000EX transmission Electron Microscope (JEOL, Ltd, Tokyo, Japan). The number of autophagosomes in 50 cross‐sectioned cells was counted for each sample and the mean value in each cross‐sectioned cell was calculated.
Trypan blue exclusion assay. The starvation‐induced cell death was evaluated by trypan blue exclusion as described in a previous study.( 31 ) In brief, after the transfected SMMC7721 cells were cultured in EBSS‐medium for 12 h, both adherent and nonadherent cells were harvested, washed three times with PBS, and resuspended in 100 µL PBS. After mixing with 100 µL of 0.8% Trypan blue, the cells were counted using a hemocytometer. The number of dead cells with disrupted membranes (blue cells) in 200 cells was counted in three replicates. Cell death was represented by the mean percentage of blue cells/total cells.
Statistical analysis. All statistical analyses were performed using the SPSS 13.0 statistical software package (SPSS, Chicago, IL, US). Statistical significance of the difference was determined using Student's t‐test. All P‐values were based on two‐sided tests. A probability level of 0.05 was used as the criterion for statistical significance.
Results
HAb18G/CD147 inhibits starvation‐induced autophagy in SMMC7721 cells. After transfection with HAb18G siRNA or control siRNA, the expression of HAb18G/CD147 in SMMC7721 cells was evaluated by RT‐PCR and Western blot. We found that HAb18G siRNA significantly down‐regulated the expression of HAb18G/CD147 at both mRNA and protein levels, compared with control siRNA (Fig. 1A,B). Immunofluorescence staining was used to examine the level of starvation‐induced autophagy in transfected SMMC7721 cells. Our data showed that SMMC7721 cells transfected with HAb18G siRNA exhibited significantly higher levels of autophagy in response to starvation than those transfected with control siRNA (P < 0.01). The percentages of cells with LC3 punctate dots, which represent the level of autophagy, were 92%± 6% and 21% ± 4% in SMMC7721 cells transfected with HAb18G siRNA and control siRNA, respectively (Fig. 1C,D). To confirm the inhibitory effect of HAb18G/CD147 on starvation‐induced autophagy, we further evaluated the level of starvation‐induced autophagy in transfected SMMC7721 cells using quantitative electron microscopy (Fig. 1E,F). Our findings indicated that there were a significantly higher number of autophagosomes per cross‐sectioned cell in SMMC7721 cells transfected with HAb18G siRNA than those transfected with control siRNA (mean ± SD; 16.00 ± 2.503 vs. 9.667 ± 1.358, P < 0.01). Taken together, these results strongly suggest that HAb18G/CD147 inhibits starvation‐induced autophagy in SMMC7721 cells.
Figure 1.
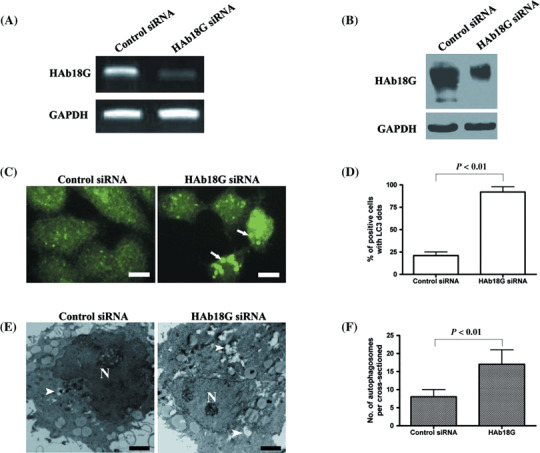
Inhibition of starvation‐induced autophagy by HAb18G/CD147 in SMMC7721 cells. SMMC7721 cells were transfected with control small interfering RNA (siRNA) or HAb18G siRNA, and cultured for 24 h in normal medium. Then, cells were subject to 12 h of starvation in Earle's Balanced Salt Solution (EBSS) medium for the induction of autophagy. (A) and (B) HAb18G/CD147 expression was analyzed by using reverse transcription – polymerase chain reaction and Western blot, respectively. (C and D) Immunofluorescence staining with anti‐LC3 antibody was used to examine the autophagic level that was indicated as the percentage of cells with LC3 punctate dots representing autophagosomes in a minimum of 100 cells for each sample. Representative photomicrographs of cellular images exhibiting LC3 punctate dots are shown in (C). Arrows denote representative LC3 punctate dots. Scale bars, 10 µm. The mean percentage of cells with LC3 punctate dots from triplicate samples is displayed in (D) for two different groups (control siRNA or HAb18G siRNA). (E and F) Autophagic level was evaluated by transmission electron microscopy. The number of autophagosomes in 50 cross‐sectioned cells was counted for each sample and mean values in each cross‐sectioned cell was calculated. Representative electron micrographs exhibiting autophagosomes are shown in (E). Scale bars, 2 µm. Arrowheads indicate autophagosomes. The number of autophagosomes per cross‐sectioned cells is displayed in (F) for two different groups. In (D) and (F), error bars represent standard deviation (SD). Statistical significance was determined by Student's t‐test.
Inhibitory effect of HAb18G/CD147 on starvation‐induced autophagy is dose‐dependent. To examine the dose effect of HAb18G/CD147 on autophagy, we measured the level of starvation‐induced autophagy in SMMC7721 cells transfected with different concentrations of HAb18G siRNA. Western blot analysis showed that HAb18G siRNAs inhibited the expression of HAb18G/CD147 protein in a dose‐dependent manner and 100 pM of HAb18G siRNAs exhibited the greatest inhibitory effect (Fig. 2A). Immunostaining assay indicated that the inhibition of starvation‐induced autophagy by HAb18G/CD147 was dose‐dependent and the percentages of cell with LC3 dots were 19%, 34%, 65% and 92% in SMMC7721 cells transfected with HAb18G siRNAs at a final concentration of 0, 20, 50 and 100 pM, respectively (Fig. 2B,C). These findings demonstrated an inverse dose–response relationship between the expression of HAb18G/CD147 and starvation‐induced autophagy.
Figure 2.
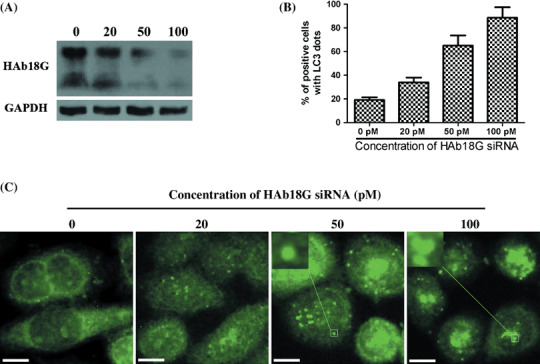
Dose–response effect of HAb18G small interfering RNA (siRNA) on autophagy in SMMC7721 cells. SMMC7721 cells were transfected with HAb18G siRNAs at a final concentration of 0, 20, 50 and 100 pM, respectively, and cultured for 24 h in normal medium. Then, cells were subject to 12 h of starvation in Earle's Balanced Salt Solution (EBSS) medium for the induction of autophagy. (A) HAb18G/CD147 expression was evaluated by using Western blot. (B and C) Immunofluorescence staining with anti‐LC3 antibody was used to observe the autophagic level as described above. The mean percentage of cells with LC3 punctate dots from triplicate samples was displayed in (B) for different treatment groups. Error bars represent ±SD. Representative photomicrographs were shown in (C). Indication: inserted boxes in (C) indicate the magnification of pane‐labeled areas. Scale bars, 10 µm.
HAb18G/CD147 enhances survival of SMMC7721 cells by inhibiting starvation‐induced autophagy. We investigated the starvation‐induced cell death in transfected SMMC7721 cells by using trypan blue exclusion assay. Starvation‐induced cell death includes traditional apoptosis and autophagic cell death. The 3‐methyladenine (3‐MA) is a common autophagy inhibitor. In our study, 3‐MA was used to inhibit autophagy, and thus decrease the autophagic cell death, but not other cell deaths. Our data shown in Fig. 3 indicated a weak inhibition on total cell death. However, we undoubtedly found that the starvation‐induced cell death was evidently decreased by 3‐MA in cells transfected with HAb18G siRNA (45%vs. 35%, P < 0.05). A similar trend was also observed in control siRNA‐transfected cells (23%vs. 18%, P = 0.12), although the inhibitory effect of 3‐MA on the starvation‐induced cell death seems to be weak. The difference of inhibitory effect might be explained by the different expression levels of CD147 in both cells. When the expression of CD147 was knocked down by specific siRNA, autophagic cell death might be significantly increased, the therefore inhibitory effect of 3‐MA would be significant. These findings strongly suggest that HAb18G/CD147 might enhance survival of SMMC7721 cells by inhibiting starvation‐induced autophagy. In addition, our results indicated that SMMC7721 cells transfected with HAb18G siRNA had a significantly higher percentage of cell deaths under starvation conditions than those transfected with control siRNA in both the absence (45%vs. 23%, P < 0.01) and presence (35%vs. 18%; P < 0.05) of 10 mM 3‐MA (Fig. 3).
Figure 3.
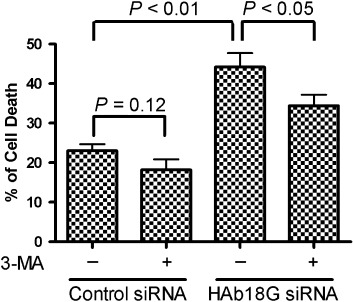
Inhibition of autophagic cell death by HAb18G/CD147 in SMMC7721 cells. SMMC7721 cells transfected with HAb18G small interfering RNA (siRNA) or control siRNA were first cultured in normal medium for 24 h, and then cultured for another 12 h in Earle's Balanced Salt Solution (EBSS) medium at the absence or presence of 10 mM 3‐MA (autophagy inhibitor). Cell death was detected using trypan blue exclusion assay. The percentage (%) of cell death was calculated as follows: (number of dead cells/number of total cells) × 100%. All data represent mean ± SD for eight replicates. Statistical significance was determined by Student's t‐test.
HAb18G/CD147 down‐regulates the expression of Beclin 1. Beclin 1 is one of the most critical molecules to positively regulate starvation‐induced autophagy.( 32 ) Therefore, to understand the potential molecular mechanisms underlying the above‐mentioned observations, we measured the expression level of Beclin 1 in transfected SMMC7721 cells (Fig. 4). We demonstrated that the expression of Beclin 1 was considerably up‐regulated at the protein level in SMMC7721 cells transfected with HAb18G siRNA compared with those transfected with control siRNA, suggesting that Beclin 1 might be an important mediator in the inhibition of starvation‐induced autophagy by HAb18G/CD147.
Figure 4.
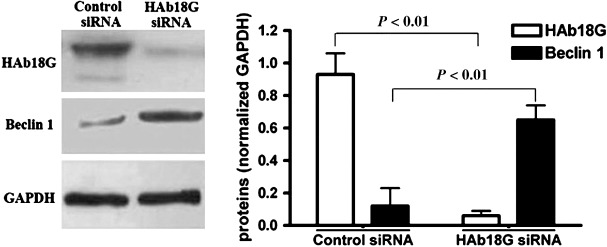
Expression change of Beclin 1 in small interfering RNA (siRNA) treated SMMC7721 cells. After transfection with HAb18G siRNA or control siRNA, SMMC7721 cells were cultured in normal medium for 24 h, and then cultured for another 12 h in Earle's Balanced Salt Solution (EBSS) medium. Finally, the expression of HAb18G/CD147 and Beclin 1 were analyzed by Western blot. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as the internal control for expression comparison.
Class I PI3K‐Akt pathway is involved in the down‐regulation of Beclin 1 by HAb18G/CD147. To verify if Class I PI3K‐Akt signaling pathway is involved in the down‐regulation of Beclin 1 by HAb18G/CD147, we examined the expression levels of pAkt and Beclin 1 in transfected SMMC7721 cells that were cultured with or without 40 µM LY294002, a specific inhibitor of Class I PI3K. Our data showed that SMMC7721 cells cultured without LY294002 had significantly higher levels of pAkt and lower expression of Beclin 1 than those cultured with LY294002 after transfection with control siRNA or HAb18G siRNA. We also found that, compared with control siRNA‐transfected SMMC7721 cells, HAb18G siRNA‐transfected SMMC7721 cells had a significantly decreased level of pAkt under culturing both with and without LY294002. In addition, we found that the expression level of Beclin 1 was notably increased in control cells treated with LY294002, compared with untreated cells. Similar trends were also noted in cells transfected with HAb18G/CD147 siRNA, although the increased amount of Beclin 1 seemed not to be as much as in control cells, which is possibly due to decreased levels of pAkt resulting from the transfection of HAb18G/CD147 siRNA. More importantly, we observed that the expression of Beclin 1 was significantly inversely associated with level of pAkt from a bar graph in Fig. 5(A). These results suggest that Class I PI3K‐Akt pathway might be involved in the down‐regulation of Beclin 1 by HAb18G/CD147. To further confirm the effect of Class I PI3K inhibition on autophagy, we also examined the autophagic level of transfected SMMC7721 cells under culturing with and without LY294002 using TEM assay. Our data indicated that the inhibition of Class I PI3K activity by LY294002 significantly promoted the autophagic level in SMMC7721 cells transfected with control siRNA or HAb18G siRNA (Fig. 5B,C).
Figure 5.
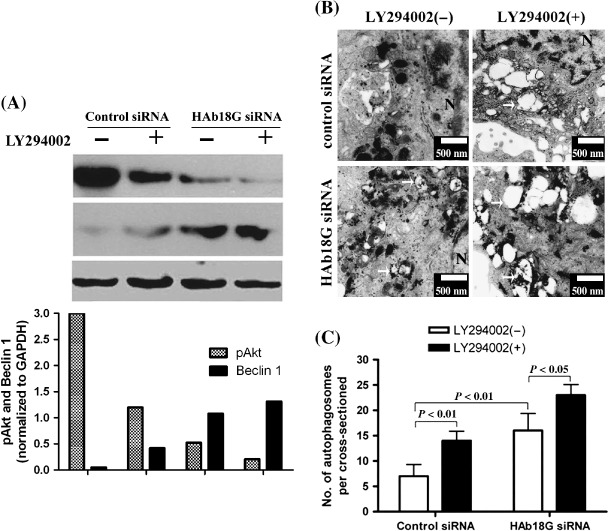
Analysis of signaling pathway involved in the down‐regulation of Beclin 1 by HAb18G/CD147 in SMMC7721 cells. SMMC7721 cells transfected with HAb18G small interfering RNA (siRNA) or control siRNA were first cultured in normal medium for 24 h, and then cultured for another 12 h in Earle's Balanced Salt Solution (EBSS) medium at the absence or presence of 40 µM LY294002. (A) Western blot analysis was performed to observe the expression level of phosphorylated kinase B (Akt) and Beclin 1. The expression of glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as internal control. (B and C) Autophagic level was examined by transmission electron microscopy as described previously. Representative electron micrographs exhibiting autophagosomes were indicated in (B). Scale bars, 500 nm. The number of autophagosomes per cross‐sectioned cells is shown in (C) for four groups. In (C), Error bars represent SD. Statistical significance was determined by Student's t‐test.
Discussion
In our study, we evaluated the effects of HAb18G/CD147 on starvation‐induced autophagy in SMMC7721 cells. We demonstrated that HAb18G/CD147 significantly inhibited starvation‐induced autophagy in a dose‐dependent manner. The inhibition of autophagy by HAb18G/CD147 notably enhanced the survival of SMMC7721 cells in starvation conditions and this process might be mediated by the down‐regulation of Beclin 1 via the Class I PI3K‐Akt pathway.
Our data indicated that HAb18G/CD147 targets the autophagy pathway and thus may be implicated in a wide variety of physiological and pathological processes. Therefore, the inhibition of autophagy by HAb18G/CD147 may represent a novel mechanism by which HAb18G/CD147 functions as an oncogenic and anticell death molecule and modulates tumor progression. In this study, we focused on the role of HAb18G/CD147 in starvation‐induced autophagy, since starvation is an important and commonly used physiologic inducer of autophagy across a broad range of eukaryotic species.( 33 ) Further studies using different stimuli of autophagy and other hepatoma cell lines are necessary to investigate if HAb18G/CD147 plays a more wide‐ranging role in the negative regulation of autophagy.
The role of autophagy in determining mammalian cell fate remains controversial. Autophagy functions in the degradation of cytoplasmic components in response to various cellular stresses, especially nutrition and energy deficiency.( 34 ) However, recent studies showed that excessive autophagy resulted in cell death.( 35 , 36 ) This may explain many of the controversial issues related to the toxicity of autophagy. However, the effects of autophagy seem to be strictly regulated in cells maintained in starvation conditions.( 37 ) Our results indicated that down‐regulation of HAb18G/CD147 led to autophagy induction in response to nutrient deprivation. The induction of autophagy was associated with cell death that can be prevented by 3‐MA, an autophagy inhibitor. These observations lead to the hypothesis that inhibition of autophagy by HAb18G/CD147 may prevent elevated cell death. Previous studies have indirectly indicated a role for HAb18G/CD147 in the positive regulation of survival in cancer cells.( 38 , 39 ) Baba et al.( 40 ) also reported that the inhibition of CD147 induces cancer cell death. However, direct evidence supporting a role of HAb18G/CD147 in promoting cell viability via inhibiting the autophagy pathway has been lacking. Our study is the first to provide direct evidence that HAb18G/CD147 may enhance cell survival by inhibiting starvation‐induced autophagy. In addition, our results showed that the percentage of cell death in HAb18G siRNA‐transfected cells is significantly higher than that in control siRNA‐transfected cells under culturing both with and without 3‐MA. These data are in agreement with a previous report, indicating an important role of CD147 in inhibiting apoptosis of oral cancer cell line KB/V.( 41 )
We also explored the potential molecular mechanism underlying the inhibition of starvation‐induced autophagy by HAb18G/CD147. Our results suggest the effect may be mediated by Beclin 1 gene. Previous studies have reported that Beclin 1 is frequently mono‐allelically deleted in human prostate,( 42 ) sporadic breast and ovarian cancers,( 43 ) leading to the classification of Beclin 1 as a candidate tumor suppressor gene. In the present study, we found that HAb18G/CD147‐mediated inhibition of starvation‐induced autophagy were reversed by Beclin 1 up‐regulation after transfection of HAb18G siRNA. Therefore, it is possible that HAb18G/CD147 down‐regulates the expression of Beclin 1 through direct inhibition of starvation‐induced autophagy.
Furthermore, we investigated the potential signaling molecules involved in the down‐regulation of Beclin 1 by HAb18G/CD147. Our results indicated that the down‐regulation of HAb18G/CD147 significantly decreased the level of pAkt and the expression of Beclin 1 was negatively associated with levels of pAkt, suggesting that Class I PI3K‐Akt pathway may be involved in the down‐regulation of Beclin 1 by HAb18G/CD147. Our study for the first time linked this signaling pathway to the Beclin 1 regulation in autophagy. Previous studies have shown that this pathway is one of most important signaling pathways in the negative regulation of autophagy.( 16 , 44 ) Arico et al.( 45 ) also showed that a high rate of autophagy was observed in cells expressing a dominant negative form of PI3K/PKB. In addition, it also has been indicated that tumor‐derived HAb18G/CD147 could lead to the activation of Class I PI3K‐Akt pathway in tumor cells.( 15 ) These reports provide a strong support for our findings. However, based on our preliminary data, we cannot rule out the possible involvement of other signaling molecules and a thorough evaluation of a panel of related signaling pathways would shed further light on the mechanisms of HAb18G/CD147‐mediated autophagy regulation.
In conclusion, our study showed that HAb18G/CD147 significantly inhibits starvation‐induced autophagy in SMMC7721 cells, potentially through down‐regulating the expression of Beclin 1. Our findings highlight the importance of HAb18G/CD147 in the progression of hepatoma and give rise to the promise of therapeutic effects of this molecule in HCC treatments.
Acknowledgments
This work was supported by grants 2006CB708504 and 2009CB521704 from the National Basic Research Program of China and 30530720 National Natural Science Foundation of China.
Xingchun Gou, Qiang Ru and Hongxin Zhang equally contribute to this work.
References
- 1. Chen ZN, Yang Z, Mi L et al . Analysis on the structure and funtion of hepatoma transfer‐asociated factor HAb18G. J Cell Mol 1999; 15: 34. [Google Scholar]
- 2. DeCastro R, Zhang Y, Guo H et al . Human keratinocytes express EMMPRIN, an extracellular matrix metalloproteinase inducer. J Invest Dermatol 1996; 106: 1260–5. [DOI] [PubMed] [Google Scholar]
- 3. Miyauchi T, Kanekura T, Yamaoka A et al . Basigin, a new, broadly distributed member of the immunoglobulin superfamily, has strong homology with both the immunoglobulin V domain and the beta‐chain of major histocompatibility complex class II antigen. J Biochem 1990; 107: 316–23. [DOI] [PubMed] [Google Scholar]
- 4. Kasinrerk W, Fiebiger E, Stefanova I et al . Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX‐47, mouse basigin, and chicken HT7 molecule. J Immunol 1992; 149: 847–54. [PubMed] [Google Scholar]
- 5. Tsai WC, Chao YC, Lee WH et al . Increasing EMMPRIN and matriptase expression in hepatocellular carcinoma: tissue microarray analysis of immunohistochemical scores with clinicopathological parameters. Histopathology 2006; 49: 388–95. [DOI] [PubMed] [Google Scholar]
- 6. van den Oord JJ, Paemen L, Opdenakker G et al . Expression of gelatinase B and the extracellular matrix metalloproteinase inducer EMMPRIN in benign and malignant pigment cell lesions of the skin. Am J Pathol 1997; 151: 665–70. [PMC free article] [PubMed] [Google Scholar]
- 7. Muraoka K, Nabeshima K, Murayama T et al . Enhanced expression of a tumor‐cell‐derived collagenase‐stimulatory factor in urothelial carcinoma: its usefulness as a tumor marker for bladder cancers. Int J Cancer 1993; 55: 19–26. [DOI] [PubMed] [Google Scholar]
- 8. Polette M, Gilles C, Marchand V et al . Tumor collagenase stimulatory factor (TCSF) expression and localization in human lung and breast cancers. J Histochem Cytochem 1997; 45: 703–9. [DOI] [PubMed] [Google Scholar]
- 9. Sun J, Hemler ME. Regulation of MMP‐1 and MMP‐2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res 2001; 61: 2276–81. [PubMed] [Google Scholar]
- 10. Guo H, Zucker S, Gordon MK et al . Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem 1997; 272: 24–7. [PubMed] [Google Scholar]
- 11. Xu J, Xu HY, Zhang Q et al . HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res 2007; 5: 605–14. [DOI] [PubMed] [Google Scholar]
- 12. Tang Y, Nakada MT, Kesavan P et al . Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 2005; 65: 3193–9. [DOI] [PubMed] [Google Scholar]
- 13. Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy‐related protein. Cell Res 2007; 17: 839–49. [DOI] [PubMed] [Google Scholar]
- 14. Cantley LC. The phosphoinositide 3‐kinase pathway. Science 2002; 296: 1655–7. [DOI] [PubMed] [Google Scholar]
- 15. Tang Y, Nakada MT, Rafferty P et al . Regulation of vascular endothelial growth factor expression by EMMPRIN via the PI3K‐Akt signaling pathway. Mol Cancer Res 2006; 4: 371–7. [DOI] [PubMed] [Google Scholar]
- 16. Petiot A, Ogier‐Denis E, Blommaart EF et al . Distinct classes of phosphatidylinositol 3′‐kinases are involved in signaling pathways that control macroautophagy in HT‐29 cells. J Biol Chem J Biol Chem 2000; 275: 992–8. [DOI] [PubMed] [Google Scholar]
- 17. Krustev LP. Cell autophagy of the liver in starvation and undernutrition. Bibl Nutr Dieta 1976; 23: 145–54. [DOI] [PubMed] [Google Scholar]
- 18. Qu X, Zou Z, Sun Q et al . Autophagy gene‐dependent clearance of apoptotic cells during embryonic development. Cell 2007; 128: 931–46. [DOI] [PubMed] [Google Scholar]
- 19. Takagi H, Matsui Y, Sadoshima J. The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal 2007; 9: 1373–81. [DOI] [PubMed] [Google Scholar]
- 20. Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004; 23: 2891–906. [DOI] [PubMed] [Google Scholar]
- 21. Schwarze PE, Seglen PO. Reduced autophagic activity, improved protein balance and enhanced in vitro survival of hepatocytes isolated from carcinogen‐treated rats. Exp Cell Res 1985; 157: 15–28. [DOI] [PubMed] [Google Scholar]
- 22. Kisen GO, Tessitore L, Costelli P et al . Reduced autophagic activity in primary rat hepatocellular carcinoma and ascites hepatoma cells. Carcinogenesis 1993; 14: 2501–5. [DOI] [PubMed] [Google Scholar]
- 23. Mathew R, Karantza‐Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer 2007; 7: 961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pattingre S, Tassa A, Qu X et al . Bcl‐2 antiapoptotic proteins inhibit Beclin 1‐dependent autophagy. Cell 2005; 122: 927–39. [DOI] [PubMed] [Google Scholar]
- 25. Xu HY, Qian AR, Shang P et al . siRNA targeted against HAb18G/CD147 inhibits MMP‐2 secretion, actin and FAK expression in hepatocellular carcinoma cell line via ERK1/2 pathway. Cancer Lett 2007; 247: 336–44. [DOI] [PubMed] [Google Scholar]
- 26. Talloczy Z, Jiang W. Virgin HWt et al . Regulation of starvation‐ and virus‐induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A 2002; 99: 190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinet W, De Meyer GR, Herman AG et al . Amino acid deprivation induces both apoptosis and autophagy in murine C2C12 muscle cells. Biotechnol Lett 2005; 27: 1157–63. [DOI] [PubMed] [Google Scholar]
- 28. Yang W, Monroe J, Zhang Y et al . Proteasome inhibition induces both pro‐ and anti‐cell death pathways in prostate cancer cells. Cancer Lett 2006; 243: 217–27. [DOI] [PubMed] [Google Scholar]
- 29. Lum JJ. Bauer DE, Kong M et al . Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120: 237–48. [DOI] [PubMed] [Google Scholar]
- 30. Espert L, Denizot M, Grimaldi M et al . Autophagy is involved in T cell death after binding of HIV‐1 envelope proteins to CXCR4. J Clin Invest 2006; 116: 2161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valentim L, Laurence KM, Townsend PA et al . Urocortin inhibits Beclin1‐mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol 2006; 40: 846–52. [DOI] [PubMed] [Google Scholar]
- 32. Yue Z, Jin S, Yang C et al . Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. PNAS Proc Natl Acad Sci USA 2003; 100: 15 077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol 2004; 36: 2445–62. [DOI] [PubMed] [Google Scholar]
- 34. Levine B, Klionsky DJ. Development by self‐digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6: 463–77. [DOI] [PubMed] [Google Scholar]
- 35. Comes F, Matrone A, Lastella P et al . A novel cell type‐specific role of p38alpha in the control of autophagy and cell death in colorectal cancer cells. Cell Death Differ 2007; 14: 693–702. [DOI] [PubMed] [Google Scholar]
- 36. Qian W, Liu J, Jin J et al . Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up‐regulation of Beclin‐1. Leuk Res 2007; 31: 329–39. [DOI] [PubMed] [Google Scholar]
- 37. Kang C, You YJ, Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev 2007; 21: 2161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marieb EA, Zoltan‐Jones A, Li R et al . Emmprin promotes anchorage‐independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res 2004; 64: 1229–32. [DOI] [PubMed] [Google Scholar]
- 39. Zheng HC, Takahashi H, Murai Y et al . Upregulated EMMPRIN/CD147 might contribute to growth and angiogenesis of gastric carcinoma: a good marker for local invasion and prognosis. Br J Cancer 2006; 95: 1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baba M, Inoue M, Itoh K et al . Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem Biophys Res Commun 2008; 374: 111–16. [DOI] [PubMed] [Google Scholar]
- 41. Kuang YH, Chen X, Su J et al . RNA interference targeting the CD147 induces apoptosis of multi‐drug resistant cancer cells related to XIAP depletion. Cancer Lett 2008. [DOI] [PubMed]
- 42. Qu X, Yu J, Bhagat G et al . Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 2003; 112: 1809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liang XH, Jackson S, Seaman M et al . Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402: 672–6. [DOI] [PubMed] [Google Scholar]
- 44. Takeuchi H, Kondo Y, Fujiwara K et al . Synergistic augmentation of rapamycin‐induced autophagy in malignant glioma cells by phosphatidylinositol 3‐kinase/protein kinase B inhibitors. Cancer Res 2005; 65: 3336–46. [DOI] [PubMed] [Google Scholar]
- 45. Arico S, Petiot A, Bauvy C et al . The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3‐kinase/protein kinase B pathway. J Biol Chem 2001; 276: 35243–6. [DOI] [PubMed] [Google Scholar]


