Abstract
We previously identified three novel HLA‐A24‐restricted epitope peptides, which were derived from three cancer‐testis antigens, TTK protein kinase (TTK), lymphocyte antigen 6 complex locus K (LY6K), and insulin‐like growth factor (IGF)‐II mRNA binding protein 3 (IMP‐3), as targets for cancer vaccination against esophageal squamous cell carcinoma (ESCC). To examine the safety, immunogenicity, and antitumor effect of vaccine treatment using a combination of these three peptides, 10 HLA‐A2402‐positive advanced ESCC patients who failed to standard therapy were enrolled in a phase I clinical trial. Each of the three peptides (1 mg each) was intradermally administered with 1 mL of incomplete Freund's adjuvant to the neck in three separate regions weekly for 5 weeks. The cancer vaccination therapy was well tolerated without any treatment‐associated adverse events of grade 3 or 4. The TTK‐, LY6K‐, and/or IMP‐3‐specific T‐cell immune responses were observed by enzyme‐linked immunospot assay in peripheral blood lymphocytes obtained from nine of the 10 ESCC patients after their vaccination. The median survival time after the vaccination was 6.6 months. The vaccination could induce good clinical responses in 50% of the 10 patients. One patient experienced a complete response in hepatic metastasis lasting 7 months, one showed objective responses in all lung metastasis lesions, and three patients revealed a stable disease condition for at least 2.5 months. The cancer vaccine therapy using these three peptides demonstrated satisfactory safety and good immunogenicity as well as promising disease control rate, and therefore warrants further clinical studies. (Cancer Sci 2009)
Despite the aggressive treatment modalities such as surgical tumor resection with extensive lymphadenectomy and chemo‐radiotherapy, the long‐term disease control of advanced esophageal squamous cell carcinoma (ESCC) remains difficult.( 1 , 2 , 3 ) Therefore, development of novel treatment modalities such as immunotherapy is eagerly awaited. Several evidences obtained in clinical trials suggest that cancer vaccination with immunogenic epitopes derived from tumor‐associated antigens (TAA) could induce specific T‐cell responses in patients with cancers.( 4 , 5 , 6 , 7 , 8 , 9 , 10 ) Cancer‐testis antigens (CTA) such as MAGE, BAGE, GAGE, and NY‐ESO‐1 are considered to be attractive TAA due to their unique expressions in malignant tumors.( 4 , 6 ) In addition, tumor regression in some metastatic renal cell carcinomas by immunotherapy and reduction of recurrence rate in melanoma patients by adjuvant cancer vaccination after surgical treatment have been reported.( 7 , 8 ) Although recent progresses in cancer vaccine therapies have been very rapid, its efficacy is still limited to a very small subset of cancer patients.( 4 , 5 , 6 , 7 , 8 , 9 , 10 )
By using cDNA microarray technology coupled with laser microdissection, we recently identified three novel HLA‐A24‐restricted epitope peptides as targets for cancer vaccination for ESCC patients.( 11 ) These antigenic peptides were derived from three different CTAs, TTK protein kinase (TTK), lymphocyte antigen 6 complex locus K (LY6K), and insulin‐like growth factor (IGF)‐II mRNA binding protein 3 (IMP‐3).( 11 ) Our gene expression profile data revealed that TTK, LY6K, and IMP‐3 were highly expressed in a large proportion (>90%) of esophageal cancers, while these transcripts were hardly detectable in normal organs, except the testis and/or placenta.( 12 , 13 ) Immunohistochemical analyses revealed that LY6K and IMP‐3 proteins were mainly localized in cytoplasm, while TTK protein was detected in the cytoplasm and nucleus of esophageal cancer cells.( 14 ) These peptides could stimulate CTL that recognized and killed ESCC cells endogenously expressing these antigens in vitro. ( 11 ) Moreover, we recently found pre‐existence of specific T‐cells, which could respond to the peptides derived from TTK, LY6K, and IMP‐3, in tumor‐infiltrating lymphocytes (TIL), regional lymph node lymphocytes (RLNL), and Peripheral blood lymphocytes (PBLs) obtained from HLA‐A*2402‐positive patients with ESCC.( 14 ) The evidence strongly encouraged us to apply these CTA peptides to cancer vaccine therapy for ESCC patients.
We here report a phase I clinical cancer vaccination study with a combination of multiple peptides that were derived from TTK, LY6K, and IMP‐3 for the HLA‐A*2402 (+) patients with advanced ESCC who had been refractory to standard ESCC therapy, and evaluate the safety, feasibility, immunological response, and clinical effectiveness of the vaccination.
Materials and Methods
Study design. A phase I clinical tumor vaccination trial with single group assignment was performed. Vaccination with multiple peptides that were derived from TTK, LY6K, and IMP‐3 that was mixed with an incomplete Freund's adjuvant (IFA); Montanide ISA 51, SEPPIC was performed on ESCC patients (n = 10) with locally advanced, recurrent, or metastatic tumors who had been resistant to standard therapy. Primary endpoints were to evaluate the safety and feasibility of the therapy. Secondary endpoints were to investigate the immunological responses, clinical response, and overall survivals. Toxicities caused by the vaccination therapy were assessed by Common Terminology Criteria for Adverse Events version 3 (CTCAE). Immunological monitoring was performed by enzyme‐linked immunospot (ELISPOT) assay using in vitro culturing lymphocytes derived from PBLs at pre‐ and postvaccination periods, as described below. To assess the clinical response, computed tomography imaging was performed before and after the vaccination. Every measurable legion such as liver, lung, or lymph node metastasis was evaluated by RECIST (response evaluation criteria in solid tumors). In addition to RECIST, when the patients revealed the reduction in size at any measurable tumors, the patients were evaluated as objective response cases in the present study. Overall survival rates were analyzed by the Kaplan–Meier method, and survival was measured in days from the first vaccination to death. This study was approved by the ethical committee of University of Yamanashi (approval no. 270) and is registered with ClinicalTrials.gov (no. NCT00682227). Written informed consent was obtained from all individuals. The trial was carried out in accordance with the Helsinki declaration on experimentation on human subjects.
Patient eligibility. The eligibility criteria of patients participating in the clinical trial were as follows. (a) ESCC patients with locally advanced, recurrent, or metastatic tumors who had failed to respond to the standard therapy; (b) HLA‐A*2402‐positive patients evaluated by DNA typing of HLA‐A genetic variations with polymerase chain reaction and DNA sequencing; (c) adequate bone‐marrow, cardiac, pulmonary, hepatic, and renal functions including white blood cell count = 2000/mm3, platelet count = 75 000/mm3, total bilirubin = 2× the institutional normal upper limits, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase = 2.5× the institutional normal upper limits, creatinine = 1.5× the institutional normal upper limits; (d) no therapy 4 weeks prior to the initiation of the trial; (e) ECOG performance status of 0–2; and (f) age of ≥20 and ≤80 years old. The exclusion criteria of patients participating in the clinical trial were as follows. (a) Pregnancy (women of childbearing potential: refusal or inability to use effective means of contraception); (b) breastfeeding; (c) serious bleeding disorder; (d) serious infections requiring antibiotics; (e) concomitant treatment with steroids or immunosuppressing agent; and (f) decision of unsuitableness by principal investigator or physician‐in‐charge.
Treatment protocol. Ten eligible ESCC patients were enrolled. Each of the three peptides (1 mg each) was emulsified in 1‐mL IFA, and injected into bilateral sites of the neck at three separate regions. The vaccination was given intradermally once a week for 5 weeks.
Peptides. The peptides derived from TTK‐567 (SYRNEIAYL), LY6K‐177 (RYCNLEGPPI), and IMP‐3–508 (KTVNELQNL) that bound to HLA‐A24 molecule were synthesized as described elsewhere.( 11 ) The purity (>97%) and identity of the peptides were determined by analytical high‐performance liquid chromatography and mass spectrometry analysis, respectively. The endotoxin levels and bioburden of these peptides were tested and determined to be within acceptable levels as GMP grade for the vaccines (NeoMPS, San Diego, CA, USA). Peptides were dissolved in dimethylsulfoxide at the concentration of 20 mg/mL and stored at –80°C.
Cell lines. Human B‐lymphoblastoid cell line, TISI cells, that express HLA‐A24 on their surface, were cultured in RPMI‐1640 medium (Sigma‐Aldrich, St. Louise, MO, USA) containing 5% heat inactivated fetal calf serum (FCS; Invitrogen., Grand Island, NY, USA) and 1% penicillin and streptomycin. COS‐7 cells were cotransfected with the vectors expressing TTK, LY6K, or IMP‐3 in the combination of HLA‐A*2402 or HLA‐A*0201 using FuGENE6 (Roche, Indianapolis, IN, USA) reagent, as described previously.( 14 ) Thereafter, 3 × 105 cells/well were incubated in DMEM (Sigma‐Aldrich) with 10% FCS, and 1% antimycobiotics (Sigma‐Aldrich) in six‐well flat bottom plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA). Two days after culture, the cells were used as target cells in ELISPOT assay.
Lymphocyte preparation and culture for immunologic monitoring. PBLs were obtained from patients at prevaccination periods as well as 14 days and 42 days after the first vaccination. PBLs were isolated with Ficoll‐Paque Plus (GE Healthcare, Piscataway, NJ, USA) density gradient solution and were stored at –80°C in cell stock media (IBL). For the in vitro culture, PBLs were thawed simultaneously and 5 × 104 cells per well were incubated in X‐VIVO15 medium (Cambrex, East Rutherford, NJ, USA) with 100 units/mL of recombinant interleukin‐2 (rIL‐2; Peprotech, Rocky Hill, NJ, USA) without any antigen stimulations. On day 14, the cultured lymphocytes were subjected to ELISPOT assay.
Enzyme‐linked immunospot (ELISPOT) assay. For detecting antigen‐specific immune response, ELISPOT assay was performed with the human γ‐interferon (IFN‐γ) ELISPOT kit (Mabtech, Nacka Strand, Sweden). 96‐well plates with nitrocellulose membranes (Millipore, Molshelm, France) were precoated with primary anti‐IFN‐γ antibody (1‐D1K) at 4°C overnight. The plates were then prereacted with X‐VIVO15 containing 1% human serum albumin (Sigma‐Aldrich). TISI cells (2 × 104/well) were incubated for 24 h in triplicate with responder cells (2 × 103/well) and CTA peptides (2, 20, or 50 µg/mL) or HIV‐peptides (ILKEPVHGV, 20 µg/mL) in a total of 200 µL/well of X‐VIVO15. On the other hand, COS‐7 cells (2 × 103/well) transfected with individual genes (please see above) and responder cells (2 × 103/well) were mixed and incubated in a final volume of 200 µL/well of X‐VIVO15 for 24 h in triplicate. These cell mixtures were treated with biotinylated secondary anti‐IFN‐γ antibody (7‐B6‐1) and incubated for 2 h. Then the plates were incubated with streptavidin‐alkaline phosphatase reagent and stained with NBT and BCIP (Sigma‐Aldrich). The spots were quantified with the auto‐analyzing system KS ELISPOT Compact (Zeiss, Göttingen, Germany). Positivity of antigen‐specific T‐cell response was defined as follows: the number of spots in the specific peptide (TTK, LY6K, or IMP‐3 at the final concentration of 20 µg/mL)‐pulsed TISI was ≥ twice as many as that in HIV peptide‐pulsed TISI at day 42 after the first vaccination. We defined the assessable lower limit of the spot per well as 40 spots.
Immunohistochemical analysis. Four‐µm thick sections of archival, formalin‐fixed, paraffin‐embedded tissue block (ESCC and adjacent normal esophagus) were used for immunohistochemical analysis. To investigate the expression of TTK, LY6K, and IMP‐3 protein, we stained tissue sections using ENVISION + Kit/HRP (Dako Cytomation, Glostrup, Denmark) as previously described.( 14 ) A rabbit polyclonal antihuman TTK antibody (NB 100–463, 70 mg/mL; Nobus Biologicals, San Diego, CA, USA), a rabbit polyclonal antihuman LY6K antibody (TM38, originally generated to recombinant LY6K,( 14 ) 4 mg/mL), or a goat polyclonal antihuman IMP‐3 antibody (sc47892, 2.5 mg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was added to deparaffinized sections after blocking of endogenous peroxidase. Then, the sections were incubated with HRP‐labeled antirabbit or antigoat IgG as the secondary antibody. Substrate‐chromogen was added and the specimens were counterstained with hematoxylin. HLA class I staining was performed as described previously.( 15 ) In brief, the sections were dewaxed, followed by antigen retrieval with Epitope Retrieval Solution (10 mmol citrate buffer [pH 6.0]; Dako Cytomation) in an autoclave at 121°C for 20 min. Endogenous peroxidase was blocked by Chemmate Peroxidase Blocking Solution (Dako Cytomation). The primary antibody EMR8‐5 (anti‐HLA class I heavy chain, diluted by PBS, 1:100; Cosmo Bio Co., Tokyo, Japan) was applied to the sections at 4°C overnight. Thereafter, the sections were incubated with streptavidin‐biotin complex (Simple Stain MAX‐PO kit; Nichirei, Tokyo, Japan) for 30 min. The sections were then treated with 3, 3′‐diaminobenzidine (Dako Cytomation) for 5 min, and counterstained with hematoxylin. Normal epithelium or lymphocytes served as positive controls. Negative control staining was performed with isotype IgGs (Dako Cytomation). Three independent investigators assessed the staining positivity semiquantitatively without prior knowledge of clinicopathological or immunological data. The intensity of TTK, LY6K, IMP‐3, or HLA class I staining was evaluated using following criteria: strong positive (2+), dark brown staining in more than 50% of tumor cells completely obscuring cytoplasm; weak positive (1+), any lesser degree of brown staining appreciable in tumor cells; negative, no appreciable staining in tumor cells.
Statistical analysis. Overall survival rates were analyzed by the Kaplan–Meier method, and survival was calculated in days from the first vaccination to death. All statistical analyses were performed with SPSS statistics 17.0 (SPSS, Chicago, IL, USA).
Results
Feasibility and adverse reactions. Ten patients satisfying the eligibility criteria were enrolled in this study (the patients’ characteristics are shown in Table 1). Nine of 10 patients developed grade 1 or 2 local skin reactions with redness and swelling in the injection sites. High‐grade fever, fatigue, diarrhea, headache, rash, and itching were not observed in any patients. No hematologic, cardiovascular, hepatic, or renal toxicity was observed during or after the vaccination. Vaccination protocol was well tolerated in all patients enrolled.
Table 1.
Characteristics of the patients with esophageal squamous cell carcinoma
| Patient | Age/Gender (M/F) | Stage* | Prior Therapy | Target organ | Performance status (ECOG) |
|---|---|---|---|---|---|
| 1 | 51/M | IVB | Operation, chemo (5FU+CDDP) | Lymph node | 1 |
| 2 | 71/M | III | Operation, chemo (5FU+CDDP) | Lung | 1 |
| 3 | 78/M | IVB | Operation, chemo (5FU+CDDP) | Lung, lymph node | 1 |
| 4 | 54/M | III | Operation, chemo (5FU+CDDP) | Lung | 1 |
| 5 | 66/M | IVB | Operation, chemo (5FU+CDDP+Docetaxel) | Lung, liver | 1 |
| 6 | 61/M | III | Operation, chemo (5FU+CDDP) | Liver | 1 |
| 7 | 63/M | IVB | Operation, chemo (5FU+CDDP+Docetaxel) | Liver | 0 |
| 8 | 73/M | IVB | Chemo (5FU+CDDP) | Primary tumor, Liver | 1 |
| 9 | 51/M | III | Operation, chemo (5FU+CDDP+Docetaxel) | Lymph node, lung | 1 |
| 10 | 66/M | IVB | Chemo (5FU+CDDP) | Primary tumor, Liver | 1 |
Stage according to the TNM classification for esophageal cancer (UICC) at primary diagnosis.
CDDP, cisplatin; Chemo, chemotherapy; ECOG, European Cooperative Oncology Group; 5FU, 5‐fluorouracil; M/F, Male/Female.
Immunological monitoring. PBLs obtained before, during, and after the vaccination periods were cultured in rIL‐2 without any antigen stimulation for 14 days and subjected to the ELISPOT assay to detect the antigen‐specific T‐cell response induced by the vaccination. Figure 1a shows representative data from ELISPOT assays against LY6K antigens in case 5. LY6K‐specific T‐cell responses were seen in PBLs derived from days 14 and 42 after the first vaccination, while no specific T‐cell response was observed in those obtained before the vaccination, indicating that antigen‐specific T‐cell responses were induced by the vaccination. In the condition of the immunological monitoring in the present study, any T‐cell responses specific for the cognate antigens were detected in none of the patients prior to the vaccination (Table 2). As summarized in 2, 3, all patients except case 3 showed antigen‐specific T‐cell responses to at least one of the three antigens after the vaccination. Antigen‐specific T‐cell responses were seen in 80% of the patients for LY6K, 70% for TTK, and 40% for IMP‐3.
Figure 1.
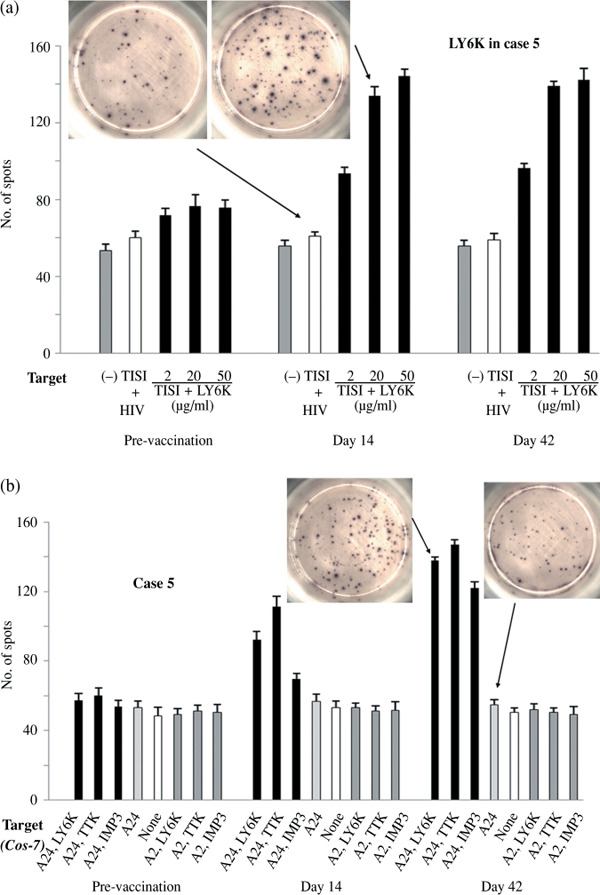
Enzyme‐linked immunospot (ELISPOT) assays detecting antigen‐specific T‐cell activity. (a) Peripheral blood lymphocyte (PBLs) obtained from case 5 at the time‐points prevaccination (day 0), day 14, and post‐vaccination periods (day 42) were cultured in recombinant interleukin‐2 (rIL‐2) without any antigen stimulation for 14 days and subjected to the ELISPOT assay to detect the antigen‐specific T‐cell response induced by the vaccination. TISI cells were incubated with responder cells in the presence of lymphocyte antigen 6 complex locus K (LY6K) peptides (2, 20, or 50 µg/mL) or HIV‐peptide (20 µg/mL). (b) ELISPOT assay using COS‐7 cells transfected with individual genes (LY6K, TTK, IMP3, HLA‐A*2401(A24), or HLA‐A*0201(A2)) and responder cells from case 5.
Table 2.
Antigen‐specific T‐cell responses in PBLs evaluated by ELISPOT assay during pre‐ and post‐vaccination periods
| Patient | Pre‐vaccination | Day 14 | Day 42 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTK | LY6K | IMP‐3 | Control | TTK | LY6K | IMP‐3 | Control | TTK | LY6K | IMP‐3 | Control | |
| 1 | 57.3 ± 8. | 58.7 ± 2.1 | 55.7 ± 4.2 | 53.3 ± 5.5 | 66.3 ± 12.5 | 61.7 ± 5.5 | 64.3 ± 3.5 | 55.7 ± 4.6 | 106.7 ± 7.5 | 105.3 ± 3.8 | 64.7 ± 7.0 | 50.3 ± 4.0 |
| 2 | 62.7 ± 7.6 | 75.3 ± 8.6 | 64.7 ± 5.0 | 61.7 ± 8.1 | 80.3 ± 12.4 | 112.7 ± 3.7 | 78.3 ± 6.5 | 65.3 ± 9.5 | 135.7 ± 6.7 | 144.3 ± 8.7 | 91.3 ± 4.7 | 63.3 ± 6.4 |
| 3 | 59.7 ± 5.5 | 58.7 ± 2.1 | 56.0 ± 2.6 | 53.7 ± 3.1 | 62.3 ± 5.1 | 65.3 ± 9.3 | 61.7 ± 5.5 | 60.3 ± 7.0 | 61.3 ± 3.5 | 59.3 ± 6.5 | 62.0 ± 6.1 | 59.0 ± 7.2 |
| 4 | 72.3 ± 5.5 | 73.7 ± 5.1 | 68.3 ± 2.1 | 66.7 ± 6.0 | 97.7 ± 4.2 | 94.3 ± 5.5 | 87.3 ± 7.8 | 66.7 ± 6.0 | 135.7 ± 6.1 | 129.7 ± 6.4 | 99.7 ± 16.6 | 59.7 ± 5.7 |
| 5 | 69.3 ± 4.2 | 77.3 ± 4.2 | 63.7 ± 3.8 | 60.3 ± 4.8 | 111.3 ± 6.0 | 132.3 ± 5.5 | 79.7 ± 3.1 | 60.7 ± 4.2 | 147.0 ± 2.6 | 139.0 ± 2.0 | 122.0 ± 3.6 | 58.7 ± 3.2 |
| 6 | 60.7 ± 3.2 | 57.3 ± 7.5 | 56.7 ± 8.1 | 55.7 ± 3.1 | 87.3 ± 10.6 | 64.7 ± 9.1 | 63.7 ± 8.1 | 62.7 ± 4.0 | 135.0 ± 11.0 | 65.3 ± 7.0 | 61.3 ± 2.5 | 60.7 ± 6.1 |
| 7 | 71.7 ± 6.5 | 69.7 ± 6.1 | 64.7 ± 7.0 | 62.3 ± 5.1 | 115.3 ± 7.2 | 94.0 ± 7.0 | 87.7 ± 6.1 | 54.3 ± 6.8 | 148.7 ± 5.3 | 138.3 ± 7.8 | 129.7 ± 4.5 | 60.3 ± 7.0 |
| 8 | 54.7 ± 4.7 | 58.7 ± 5.0 | 53.3 ± 4.6 | 52.7 ± 7.2 | 63.3 ± 6.4 | 70.3 ± 8.3 | 71.0 ± 6.0 | 62.3 ± 4.2 | 55.3 ± 2.1 | 106.7 ± 7.5 | 112.7 ± 3.7 | 52.7 ± 6.5 |
| 9 | 70.0 ± 8.5 | 71.3 ± 8.6 | 72.3 ± 5.1 | 69.3 ± 5.9 | 56.0 ± 6.0 | 74.7 ± 2.5 | 64.7 ± 4.7 | 55.7 ± 3.1 | 87.0 ± 9.5 | 126.3 ± 11.1 | 92.0 ± 7.8 | 61.7 ± 5.9 |
| 10 | 66.3 ± 9.5 | 59.7 ± 7.0 | 58.7 ± 5.0 | 59.3 ± 5.8 | 126.7 ± 10.2 | 107.3 ± 7.5 | 114.7 ± 11.0 | 58.3 ± 9.7 | 146.0 ± 7.0 | 123.0 ± 9.6 | 136.3 ± 6.5 | 60.3 ± 8.1 |
The number of spots on ELISPOT assay was demonstrated as mean ± SD for TISI pulsed with TTK, LY6K, IMP3, or HIV (control) peptide (20 mg/mL).
Positivity of antigen‐specific T‐cell response was defined as follows: the number of spots in specific peptide pulsed TISI was > twice as many as that in HIV peptide‐pulsed TISI (underlined).
ELISPOT, enzyme‐linked immunospot; IMP3, insulin‐like growth factor (IGF)‐II mRNA binding protein 3; LY6K, lymphocyte antigen 6 complex locus K; TTK, TTK protein kinase.
Table 3.
Clinical responses related to T‐cell response and antigen expression
| Patient | RECIST | Objective response | Survival (day) | T cell responses † | Antigen and HLA class I expression ‡ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LY6K | TTK | IMP‐3 | LY6K | TTK | IMP‐3 | HLA class | ||||
| 1 | SD | No | 240 | + | + | – | 1+ | 1+ | 1+ | 2+ |
| 2 | PD | No | 213 | + | + | – | 2+ | 1+ | 2+ | 2+ |
| 3 | SD | No | 168 | – | – | – | 1+ | 2+ | 1+ | 0 |
| 4 | SD | No | 163 | + | + | – | 2+ | 2+ | 2+ | 1+ |
| 5 | PD | Yes | 129 | + | + | + | 2+ | 2+ | 1+ | 2+ |
| 6 | PD | No | 286 | – | + | – | 0 | 2+ | 0 | 1+ |
| 7 | CR | Yes | 405 | + | + | + | 1+ | 2+ | 1+ | 2+ |
| 8 | PD | No | 88 | + | – | + | 1+ | 0 | 1+ | 2+ |
| 9 | PD | No | 73 | + | – | – | 2+ | 2+ | 2+ | 2+ |
| 10 | PD | No | 215 | + | + | + | 1+ | 2+ | 2+ | 2+ |
Positivity of antigen‐specific T‐cell response was defined as follows: the number of spots in specific peptide (20 µg/mL)‐pulsed TISI was > twice as many as that in HIV peptide‐pulsed TISI at day 42 after the first vaccination.
The intensity in the primary tumors was evaluated as 2+, dark brown staining in more than 50% of tumor cells completely obscuring cytoplasm; 1+, any lesser degree of brown staining appreciable in tumor cells; 0, no appreciable staining in tumor cells.
CR, complete response; IMP3, insulin‐like growth factor (IGF)‐II mRNA binding protein 3; LY6K, lymphocyte antigen 6 complex locus K; PD, progressive disease; RECIST, response evaluation criteria in solid tumors; SD, stable disease; TTK, TTK protein kinase.
To confirm whether T‐cells in PBLs could specifically recognize TTK, LY6K, and/or IMP‐3 antigens which are presented endogenously in a HLA‐A24‐restricted manner, we further analyzed by ELISPOT assay T‐cell activity against COS‐7 cells that were transfected with plasmids expressing each CTA, and/or HLA‐A*2402 or HLA‐A*0201. As shown by representative ELISPOT data using PBLs from case 5 (Fig. 1b), we detected specific and significant IFN‐γ spots against COS‐7 cells that were transfected with both HLA‐A*2402 expression vectors and vectors expressing one of TTK, LY6K, or IMP‐3, while T‐cells in PBLs did not immunologically respond to COS‐7 cells that were transfected with a combination of HLA‐A*0201 and CTA, or with CTA alone. Similar observations were observed when we used PBLs from all the remaining patients except case 3 (data not shown). These data indicate that cultured PBL cells derived from the vaccinated patients could recognize TTK, TY6K, and IMP‐3 antigens which are naturally processed and present on the cell surface in context with HLA‐A24.
Clinical responses and overall survivals. Among the 10 patients, one patient (case 7) revealed complete response (CR) in the multiple liver metastatic lesions after the vaccination (Fig. 2a,b) and three patients (cases 1, 3, and 4) showed stable disease (SD) for at least 2.5 months (Table 3). Disease control (CR or SD) was achieved in four of the 10 patients. Moreover, in case 5, all of the multiple lung metastatic legions markedly shrank (objectively responded) (Fig. 2c), but hepatic metastasis progressed (Fig. 2c). Hence, case 5 was judged as progressive disease (PD) by RECIST. Clinical responses including CR, SD, or objective response (case 5) were observed in half of the 10 vaccinated patients with advanced ESCC that was refractory to standard therapy. The median survival time after the first vaccination was 6.6 months in the 10 patients. The patient who achieved CR in liver metastases (case 7) showed the longest survival period (405 days; Table 3).
Figure 2.

Computed tomography (CT) imaging in case 7 and case 5 before and after vaccination. (a,b) CT imaging of the liver metastasis in case 7 whose response to vaccination was judged as a complete response (CR). (c) CT imaging of the lung and liver metastases in case 5. All multiple lung metastases markedly regressed, while the hepatic metastases were not controlled by the vaccination.
Expression of TTK, LY6K, IMP‐3, and HLA class I molecules in the primary ESCC tumor. We semiquantitatively evaluated by immunohistochemical staining the expression levels of TTK, LY6K, IMP‐3, and MHC class I protein in the primary tumors from all of the 10 patients enrolled. Representative immunohistochemical staining is shown in Figure 3. We confirmed that the expression of TTK, LY6K, and IMP‐3 was observed frequently in ESCC tissues (90% for TTK, 90% for LY6K, and 90% for IMP‐3; Table 3), while no staining was observed in their adjacent esophageal tissues (Fig. 3). In addition, the strong expression of MHC class I (scored as 2+) was preserved in seven of the 10 cases, and it was low or absent (scored as 1+ and 0) in three cases (Table 3). The incidence of positivity for TTK, LY6K, IMP‐3, and HLA class I in primary ESCC was concordant with our recent report.( 14 , 15 ) Importantly, case 3, who lost MHC class I expression in the primary tumor, revealed no specific T‐cell responses against any of three antigens following the vaccination. The data suggest that the preservation of MHC class I was important for the induction of antigen‐specific T‐cell response by the vaccination.( 14 )
Figure 3.

(a) Representative immunohistochemical staining of TTK protein kinase (TTK), lymphocyte antigen 6 complex locus K (LY6K), and insulin‐like growth factor (IGF)‐II mRNA binding protein 3 (IMP‐3) in primary esophageal squamous cell carcinoma (ESCC) tissues and adjacent normal esophageal mucosa. (b) Immunohistochemical staining of HLA class I in primary ESCC tissues.
Monitoring of immunological response and tumor antigen/HLA class I expression in an ESCC patient who had CR and subsequent relapse after vaccination. To improve the clinical outcome for patients with cancer immunotherapy, reduction of the escape of tumor cells from the immune system is one of the most critical issues. To investigate the mechanism of tumor recurrence after the effective cancer vaccination, we focused on the clinical course of one patient (case 7) who achieved CR in this clinical study. Case 7 suffered from multiple liver metastases after receiving curative esophagectomy. Although this patient was treated with systemic chemotherapy including 5‐fluorouracil (5FU), cisplatin (CDDP), and docetaxel (DOC), hepatic metastases did not respond to these drugs and judged as PD. Two months after the peptide vaccination, the patient achieved CR in all the multiple liver metastatic legions (Fig. 2a,b). The antigen‐specific T‐cell responses against LY6K‐, TTK‐, and IMP3‐peptides were induced by the vaccination in this patient (2, 3). Although the CR condition lasted for 7 months, the patient suffered from relapse of hepatic metastases and died at day 405 after beginning the vaccine therapy. We therefore analyzed the antigen‐specific T‐cell immune responses using PBLs obtained at CR and refractory phases of tumors, and also detected expression status of the three CTAs and HLA class I in tumor tissues using clinical material obtained by initial surgical treatment before the cancer vaccination and by core needle biopsy of the liver metastatic lesion after the relapse from the CR condition. Immunohistochemical analysis indicated that the expression of MHC class I was significantly down‐regulated in the relapsed hepatic tumor, compared with the primary ESCC before vaccination, while the expression of LY6K, TTK, and IMP‐3 was likely to be preserved in the re‐growing liver tumor (Fig. 4). Furthermore, ELISPOT assay using PBLs after relapse from the vaccination showed that LY6K‐, TTK‐, and IMP3‐specific T‐cell responses were still preserved at the same levels as during the CR condition (Table 4). Taken together, the re‐growth of the liver metastasis in case 7 was considered to be the escape from the CTLs due to the significant reduction of the MHC class I molecule in the tumor cells.
Figure 4.

Immunohistochemical staining of TTK protein kinase (TTK), lymphocyte antigen 6 complex locus K (LY6K), insulin‐like growth factor (IGF)‐II mRNA binding protein 3 (IMP‐3), and HLA class I using primary esophageal squamous cell carcinoma (ESCC) after surgery and relapsed liver metastasis that was refractory to cancer vaccine treatment, both of which were obtained from case 7.
Table 4.
Antigen‐specific T‐cell responses evaluated by ELISPOT assay using PBLs after relapse from the vaccination in case 7
| TTK | LY6K | IMP‐3 | Control | |
|---|---|---|---|---|
| Number of spots | 178.1 ± 5.9 | 143.3 ± 9.8 | 141.7 ± 6.3 | 69.3 ± 5.9 |
The number of spots on ELISPOT assay was demonstrated as mean ± SD for TISI pulsed with TTK, LY6K, IMP3, or HIV (control) peptide (20 µg/mL).
ELISPOT, enzyme‐linked immunospot; IMP3, insulin‐like growth factor (IGF)‐II mRNA binding protein 3; LY6K, lymphocyte antigen 6 complex locus K; PBL, TTK, TTK protein kinase.
Discussion
In this clinical trial, the cancer vaccination using a combination of multiple peptides (TTK, LY6K, and IMP‐3) that were mixed with IFA was well tolerated, without any major side effects in patients with advanced or recurrent ESCC. The treatment‐associated adverse events such as mild redness and swelling were limited to the injection sites. We found that the vaccination could induce antigen‐specific T‐cell responses in nine of the 10 patients, in whom no detectable T‐cell responses were observed before the vaccination. We recently described the presence of precursor T‐cells specific to these tumor antigens, LY6K, TTK, or IMP‐3 in the lymph node, and PBLs of ESCC patients.( 14 ) Our data reported here strongly imply that peptide vaccination therapy for ESCC patients could boost and expand the precursor T‐cells specific to LY6K, TTK, or IMP‐3, and enabled us to detect and monitor the levels of the immune responses after the vaccine treatment. Our data also suggest the very strong immunogenicity of the peptides derived from three CTAs. More importantly, the vaccination used in this study could reveal promising clinical responses including CR, SD, and the objective response in half of the 10 patients who had been resistant to chemo‐ and/or ‐radiotherapy.
In case 3 without MHC class I expression in the primary tumor cells, we detected no specific T‐cell response against any of three antigens following the vaccination. We previously reported the correlation between the presence or absence of precursor T‐cells specific to CTAs, and MHC class I expression in the primary tumor on ESCC.( 14 ) Since precursor T‐cells specific to TTK, LY6K, or IMP‐3 were less frequent in the patients with ESCC in which expression levels of MHC class I were none or low, it might be difficult to boost the antigen‐specific T‐cells in the present immunization protocol. In order to further evaluate the proportion of precursor T‐cells specific to CTAs and their boosting by the vaccination in the present study, different assay protocols including in vitro sensitization may help our understanding of the CTA‐specific T‐cell response. Furthermore, it also remains unclear whether CTA‐specific T‐cell response was not induced due to the absence of MHC class I expression in the primary tumor.
Although treatments with several cancer vaccines have shown an increase of circulating tumor antigen‐specific T‐cells, the proportion of patients showing positive responses with long‐term cancer remission has been very low (less than 5%).( 16 ) Recent studies have suggested several mechanisms of the immune escape of cancer cells from antigen‐specific CTLs:( 1 ) T‐cell anergy,( 2 ) influence of CD4+ CD25+ regulatory T‐cells,( 3 ) elevated expression of inhibitory ligands such as PD‐L1,( 4 ) activation of nutrient‐catabolizing enzymes such as indoleamine 2,3‐dioxygenase, and( 5 ) antigen‐loss and/or down‐regulation of HLA class I in tumor cells.( 16 , 17 , 18 ) Since TTK, LY6K, and IMP‐3 appeared to have essential functions for cancer cell survival,( 13 , 19 , 20 ) it is very unlikely that a subpopulation of tumor cells lacking TTK, LY6K, or IMP‐3 expression can survive and escape from the cytotoxic T‐cells specific to either of these molecules. Therefore, vaccination using TTK, LY6K, and IMP‐3 peptides should reduce the risk of emergence of immune escape variants. High disease control rate in this phase I study might support this hypothesis. Interestingly, the expression of MHC class I was significantly down‐regulated in the relapsed hepatic tumor obtained from one patient (case 7) who achieved CR after the cancer vaccination, compared with prevaccinated primary tumor cells from the same patient, while the expression of LY6K, TTK and IMP‐3 was preserved in the relapsed liver tumor. In addition, antigen‐specific T‐cell responses were still detected in PBLs in this patient after tumor relapse. The data also implied that our cancer vaccine regimen did not cause T‐cell anergy, but the reduced expression of MHC class I significantly affected the clinical response in this patient. Considering the mechanisms of the immune escape of cancer cells, it is important to design rational strategies aiming to preserve or restore MHC class I in combination with the present vaccination therapy that may be desirable for the further success of the cancer vaccine treatment.
Although there is no curative therapy for ESCC patients with inoperable tumor or recurrence after surgery, platinum‐based regimens including 5FU and CDDP have been generally used as a first‐line chemotherapy, and a median survival time of patients treated with this regimen was 7–8 months.( 21 ) However, there is no effective treatment available for patients with ESCC that are refractory to the first‐line chemotherapy. For example, second‐line chemotherapies using DOC or the receptor tyrosine kinase inhibitor gefitinib in patient groups similar to patients enrolled in this cancer vaccination study had a median survival period of 5.1 and 5.5 months, respectively.( 22 , 23 ) Although the number in this study was very small, the median survival time was 6.6 months and the observed disease control ratio was 40% without any severe adverse reactions. Hence, we assume that our protocol is encouraging and promising for improvement of the prognosis and quality of life for ESCC patients.
In conclusion, the phase I vaccination study using peptides derived from TTK, LY6K, and IMP‐3 for ESCC patients indicated that the vaccine treatment was well tolerated and feasible, and that antigen‐specific T‐cell responses were strongly induced by the vaccination with some objective clinical responses. The evidence in this study supports the recommendation of moving forward to the next‐stage trial. We are currently initiating the randomized phase II clinical vaccination study for the same cohort with ESCC to evaluate the survival benefit of the cancer vaccination.
Acknowledgment
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 20591566).
Clinical trial registration: ClinicalTrials.gov, number NCT00682227
References
- 1. Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol 2007; 5: 4110–7. [DOI] [PubMed] [Google Scholar]
- 2. Ando N, Iizuka T, Ide H et al . Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study – JCOG9204. J Clin Oncol 2003; 21: 4592–6. [DOI] [PubMed] [Google Scholar]
- 3. Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994; 220: 364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bender A, Karbach J, Neumann A et al . LUD 00‐009: phase I study of intensive course immunization with NY‐ESO‐1 peptides in HLA‐A2 positive patients with NY‐ESO‐1‐expressing cancer. Cancer Immun 2007; 7: 16–23. [PMC free article] [PubMed] [Google Scholar]
- 5. Germeau C, Ma W, Schiavetti F et al . High frequency of antitumor T cells in the blood of melanoma patients before and after vaccination with tumor antigens. J Exp Med 2005; 201: 241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nestle FO, Alijagic S, Gilliet M et al . Vaccination of melanoma patients with peptide‐ or tumor lysate‐pulsed dendritic cells. Nat Med 1998; 4: 328–32. [DOI] [PubMed] [Google Scholar]
- 7. Uemura H, Fujimoto K, Tanaka M et al . A phase I trial of vaccination of CA9‐derived peptides for HLA‐A24‐positive patients with cytokine‐refractory metastatic renal cell carcinoma. Clin Cancer Res 2006; 12: 1768–75. [DOI] [PubMed] [Google Scholar]
- 8. Letsch A, Keilholz U, Fluck M et al . Peptide vaccination after repeated resection of metastases can induce a prolonged relapse‐free interval in melanoma patients. Int J Cancer 2005; 114: 936–41. [DOI] [PubMed] [Google Scholar]
- 9. Sato Y, Shomura H, Maeda Y et al . Immunological evaluation of peptide vaccination for patients with gastric cancer based on pre‐existing cellular response to peptide. Cancer Sci 2003; 94: 802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kono K, Takahashi A, Sugai H et al . Dendritic cells pulsed with HER‐2/neu‐derived peptides can induce specific T‐cell responses in patients with gastric cancer. Clin Cancer Res 2002; 8: 3394–400. [PubMed] [Google Scholar]
- 11. Suda T, Tsunoda T, Daigo Y, Nakamura Y, Tahara H. Identification of human leukocyte antigen‐A24‐restricted epitope peptides derived from gene products upregulated in lung and esophageal cancers as novel targets for immunotherapy. Cancer Sci 2007; 98: 1803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamabuki T, Daigo Y, Kato T et al . Genome‐wide gene expression profile analysis of esophageal squamous cell carcinomas. Int J Oncol 2006; 28: 1375–84. [PubMed] [Google Scholar]
- 13. Ishikawa N, Takano A, Yasui W et al . Cancer‐testis antigen lymphocyte antigen 6 complex locus K is a serologic biomarker and a therapeutic target for lung and esophageal carcinomas. Cancer Res 2007; 67: 11601–11. [DOI] [PubMed] [Google Scholar]
- 14. Mizukami Y, Kono K, Daigo Y et al . Detection of novel cancer‐testis antigen‐specific T‐cell responses in TIL, regional lymph nodes, and PBL in patients with esophageal squamous cell carcinoma. Cancer Sci 2008; 99: 1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mizukami Y, Kono K, Maruyama T et al . Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer 2008; 99: 1462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spiotto MT, Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer‐specific antigens cross‐presented by stromal cells. Cancer Immun 2005; 5: 8–14. [PubMed] [Google Scholar]
- 17. Spiotto MT. Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med 2004; 10: 294–8. [DOI] [PubMed] [Google Scholar]
- 18. Maeurer MJ, Gollin SM, Martin D et al . Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP‐1 and loss of expression of the immunodominant MART‐1/Melan‐A antigen. J Clin Invest 1996; 98: 1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Cárcer G, De Castro IP, Malumbres M. Targeting cell cycle kinases for cancer therapy. Curr Med Chem 2007; 14: 969–85. [DOI] [PubMed] [Google Scholar]
- 20. Vikesaa J, Hansen TV, Jønson L, Borup R, Wewer UM, Christiansen J, Nielsen FC. RNA‐binding IMPs promote cell adhesion and invadopodia formation. EMBO J 2006; 25: 1456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349: 2241–52. [DOI] [PubMed] [Google Scholar]
- 22. Janmaat ML, Gallegos‐Ruiz MI et al . Predictive factors for outcome in a phase II study of gefitinib in second‐line treatment of advanced esophageal cancer patients. J Clin Oncol 2006; 24: 1612–9. [DOI] [PubMed] [Google Scholar]
- 23. Yamazaki K, Hironaka S, Boku N et al . A retrospective study of second‐line chemotherapy for unresectable or recurrent squamous cell carcinoma of the esophagus refractory to chemotherapy with 5‐fluorouracil plus platinum. Int J Clin Oncol 2008; 13: 150–5. [DOI] [PubMed] [Google Scholar]


