Abstract
Loss or down‐regulation of human leukocyte antigen (HLA) class I expression has been demonstrated in a variety of solid tumors. To date, such altered HLA expression has not been studied extensively in freshly isolated leukemic blasts. If it occurs, leukemic cells could escape T‐cell surveillance as a consequence. Genotypes of nine leukemic cell lines were determined using a polymerase chain reaction for HLA classes I and II. Cells were also examined for HLA β2‐microglobulin, and allele‐specific HLA protein expression using flow cytometry. Next, 44 samples of freshly isolated leukemic blasts from 43 patients with malignant hematological diseases were examined for allele‐specific HLA expression using flow cytometry. Microsatellite analysis was performed to determine heterozygosity in the HLA region on chromosome 6. Genotype analysis for HLA class I together with microsatellite analysis demonstrated loss of HLA haplotype in HL‐60 cells. No loss of HLA haplotype was observed in 44 samples of freshly isolated leukemic blasts. As reported previously, flow cytometric analysis rarely demonstrated loss or down‐regulation of HLA expression at initial diagnosis (3/39; 7.7%); however, this was evident in two of five cases in relapse (40.0%), which contrasts with previous reports. In one patient with acute leukemia, HLA‐A2 cell surface expression was present at initial diagnosis, lost at relapse, and completely restored after 48 h of culture in the presence of interferon‐γ. These results suggest loss of allele‐specific HLA expression may be involved in the pathogenesis of relapse in patients with leukemia. The findings should be valuable in designing new strategies for clinical immunotherapy. (Cancer Sci 2007; 98: 102–108)
Human leukocyte antigen (HLA) molecules expressed on the cell surface are required in presenting antigens to T cells. HLA class I antigens are vital in recognition of tumor cells by tumor‐specific cytotoxic T lymphocytes (CTL), as tumor‐specific antigens are most often intracellular proteins expressed in association with membrane‐bound HLA class I molecules. Loss or down‐regulation of HLA class I antigens therefore represents a way by which tumors can escape T‐cell surveillance, adversely affecting the course of disease and the outcome of T‐cell‐based immunotherapy.( 1 , 2 , 3 ) To date, loss or down‐regulation of HLA expression has been demonstrated mainly in solid tumors, as opposed to hematological cancers. Therefore, the frequency of such loss in the course of leukemia is unknown. Marked heterogeneity in expression of HLA molecules is possible in leukemic cells, given their genetic instability. This may subvert immune surveillance against leukemic blasts.
Several leukemia‐associated or ‐specific antigens recognized by CTL have been identified recently, including BCR‐ABL,( 4 , 5 ) ETV6‐AML1,( 6 ) proteinase‐3,( 7 ) Wilms’ tumor gene (WT1),( 8 ) and telomerase reverse transcriptase.( 9 , 10 ) Clinical trials are underway for new specific immunotherapies including adoptive CTL transfer and peptide vaccination using leukemia‐specific antigens. However, the effectiveness of immunotherapy depends greatly on expression of the appropriate HLA class I molecules by the leukemic cells, so loss or down‐regulation of HLA expression could thwart specific immunotherapy against leukemia. Even the efficacy of conventional treatments such as allogeneic hematopoietic stem cell transplantation (allo‐HSCT) or donor lymphocyte infusion (DLI) may depend on HLA antigen expression. Considering this, clarification of the frequency and nature of HLA expression deficiencies in leukemic cells is needed.
In the current study we first investigated HLA class I molecules in nine leukemia/lymphoma cell lines using a polymerase chain reaction (PCR) to amplify the corresponding genomic DNA, and analyzed allele‐specific HLA class I surface expression using a broad panel of allele‐specific monoclonal antibodies (mAbs). Then we examined HLA class I, β2‐microglobulin (β2M), and allele‐specific HLA‐A expression in freshly isolated leukemic blasts from 43 patients with various hematological cancers.
Materials and Methods
Patients. We studied 43 adult patients with malignant hematological diseases who were treated at the Okayama University Hospital and Kameda General Hospital between November 2001 and December 2002. There were 29 men and 14 women, and their median age was 57 years (range, 18–83). Diseases included acute myeloid leukemia (AML; n = 22), acute lymphoblastic leukemia (ALL; n = 7), multiple myeloma (MM; n = 2), non‐Hodgkin lymphoma (NHL; n = 3), chronic lymphocytic leukemia (CLL; n = 1), plasma cell leukemia (PCL; n = 1), myelodysplastic syndrome (MDS; n = 6), and myeloproliferative disease (MPD; n = 1).
Cell lines. Nine leukemia/lymphoma cell lines were used: HL‐60, Jurkat, U937, CCRF‐CEM, Namalwa, MOLT‐4, NALM‐6, Ramos, and BV‐173. The cells were maintained in RPMI 1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal calf serum (FCS; Sigma), 100 U/mL penicillin, and 100 mg/mL streptomycin (Sigma) at 37°C in a humidified atmosphere containing 5% CO2.
Clinical samples. After informed consent was obtained, peripheral blood or bone marrow samples were collected from the 43 patients. Samples were obtained at initial diagnosis from 38 patients and at relapse from four patients. In one patient (case 39), samples were obtained at both initial diagnosis and relapse. Mononuclear cells (MNC) were separated by density‐gradient centrifugation using Ficoll‐Hypaque (Sigma), and then washed twice with phosphate‐buffered saline (PBS). These cells were subjected to flow cytometry and genomic DNA extraction.
DNA samples. Genomic DNA was extracted from MNC using a QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions.
HLA‐A, ‐B, ‐C, ‐DR, and ‐DQ locus‐specific PCR. Genotypes of nine leukemia/lymphoma cell lines were determined by PCR‐restriction fragment length polymorphism or PCR‐sequence‐specific primer (PCR‐SSP) methods, as described elsewhere.( 11 , 12 , 13 ) For the HLA‐B locus, the PCR‐sequence‐specific oligonucleotide probing method was used.( 14 )
Cytogenetic analysis. Cytogenetic analysis of HL‐60 cells was performed using standard G‐band karyotyping techniques. After cells were processed using a short‐term unstimulated culture (24 h), 10 banded metaphases were analyzed. Results were described according to the International System for Human Cytogenetic Nomenclature.( 15 )
Genotype determination at the HLA‐A locus in clinical samples. Genotypes at the HLA‐A locus were determined in clinical samples by PCR‐SSP methods (Dynal Biotech, Bromborough, UK) according to the manufacturer's protocol. Briefly, PCR was carried out with a GeneAmp 9600 thermal cycler (Applied Biosystems, Foster City, CA, USA) using a panel of primer solutions including an allele‐ or group‐specific primer pair, as well as an internal control primer pair. Fragments were separated by gel electrophoresis on a 2% agarose gel and visualized by staining with ethidium bromide. Banding patterns obtained were interpreted as described in the 12th International Histocompatibility Workshop Manual.
Monoclonal antibodies. The mAbs for allele‐specific HLA class I antigens used in the present study, listed in Table 1, were purchased from One Lambda (Canoga Park, CA, USA). Fluorescein isothiocyanate (FITC)‐conjugated anti‐HLA class I mAb were purchased from Beckman Coulter/Immunotech (Miami, FL, USA); FITC‐conjugated anti‐HLA class II and anti‐β2M mAb were purchased from One Lambda. A peridinin chlorophyll protein (PerCP)‐labeled anti‐CD45 antibody (Becton Dickinson, San Jose, CA, USA) was used to differentiate leukemic cells from normal lymphocytes in each case of acute leukemia.
Table 1.
Monoclonal antibodies for allele‐specific HLA class I antigens
| Monoclonal antibody | Isotype | Antigen specificity |
|---|---|---|
| 0544HA | IgM | HLA‐A1, A11, A26 + |
| 0475HA | IgM | HLA‐A2 |
| 0378HA | IgM | HLA‐A3 |
| 0497HA | IgM | HLA‐A23, A24 |
| 0273HA | IgM | HLA‐A30, A31 |
| 070HA | IgM | HLA‐B5, B49, B56 + |
| 0884HA | IgM | HLA‐B7, B27, B42 + |
| 0201HA | IgM | HLA‐B8 |
| 0044HA | IgG | HLA‐B13, B62, B15 |
| 0495HA | IgM | HLA‐B21, B70, B55 + |
| 0740HA | IgM | HLA‐B51, B14, B18 + |
| B9.12.1 | IgG2a | HLA class I |
| FH0002 | IgG2a | HLA class II |
| FH1078 | IgG1 | β2‐microglobulin MS |
HLA, human leukocyte antigen; Ig, immunoglobulin; MS, microsatellite.
Flow cytometric analysis. Flow cytometric analysis was performed as described previously. Briefly, 5 × 105 cells from cell lines or clinical samples were washed with PBS containing 5% heat‐inactivated FCS and 0.2% sodium azide and incubated for 20 min at room temperature with 7 mL of the appropriate mAb solution. Fluorescence was used to characterize cells with a FACSCalibur cytometer and CellQuest software (Becton Dickinson). Ten thousand cells were studied for each individual analysis. As negative controls, cells were incubated with an irrelevant mAb. When the mean fluorescence intensity of a sample was lower than that of the control, we evaluated the sample as “down‐regulated” regardless its homogeneity. To examine the effect of interferon (IFN)‐γ on HLA antigen expression, cells were treated in culture with IFN‐γ (200 U/mL) for 48 h, after which cell‐surface HLA class I antigen expression was examined by immunofluorescence as described above.
Microsatellite analysis. Microsatellite analysis was performed to determine heterozygosity of HL‐60 cells in the HLA region on chromosome 6. Seven highly informative microsatellite markers with more than 70% heterozygosity were selected; these are listed in Table 2.( 16 ) All forward primers used in this study were fluorescein‐labeled at the 5′ end. PCR amplification was performed in 25 mL of a reaction mixture containing 50 ng of genomic DNA, 5 pmol of each primer, 1.5 mM MgCl2 (Takara Bio, Otsu, Japan), dNTPs at 200 mM, and 1.25 units of Taq DNA polymerase (TaKaRa Taq; Takara). After a denaturation step (95°C, 5 min), amplification was performed in a GeneAmp 9600 thermal cycler (Applied Biosystems) for 25–30 cycles consisting of denaturation (95°C for 1 min), annealing (60°C for 1 min), and extension (72°C for 1 min). The final extension step was prolonged to 7 min. Then, 1 µL of each amplified product was mixed with 10 mL of HiDi formamide (Applied Biosystems) and 0.1 mL of size standard (HD400Rox, Applied Biosystems), heated at 95°C for 5 min, and electrophoresed using an ABI PRISM 3100 Genetic Analyzer. Fluorescence data were analyzed with GeneScan software (Applied Biosystems).
Table 2.
Microsatellite markers used to determine heterozygosity of HL‐60 cells in the HLA region on chromosome 6
| MS marker | Size (bp) | Alleles | Heterozyg (%) | Repeat | Primer |
|---|---|---|---|---|---|
| D6S311 | 230–276 | 18 | 91 | CA | 5′‐ATGTCCTCATTGGTGTTGTG‐3′ 5′‐GATTCAGAGCCCAGGAAGAT‐3′ |
| C1.2.5 | 178–220 | 20 | 89 | CA | 5′‐CAGTAGTAAGCCAGAAGCTATTAC‐3′ 5′‐AAGTCAAGCATATCTGCCATTTGG‐3′ |
| C3.2.11 | 187–229 | 17 | 85 | GA | 5′‐AGATGGCATTTGGAGAGTGCAG‐3′ 5′‐TCCTTACAGCAGAGATATGTGG‐3′ |
| D6S276 | 198–230 | 14 | 84 | CA | 5′‐TCAATCAAATCATCCCCAGAAG‐3′ 5′‐GGGTGCAACTTGTTCCTCCT‐3′ |
| D6S265 | 118–140 | 8 | 79 | CA | 5′‐ACGTTCGTACCCATTAACCT‐3′ 5′‐ATCGAGGTAAACAGCAGAAA‐3′ |
| D6S273 | 120–140 | 8 | 77 | CA | 5′‐GCAACTTTTCTGTCAATCCA‐3′ 5′‐ACCAAACTTCAAATTTTCGG‐3′ |
| D6S291 | 198–210 | 7 | 72 | CA | 5′‐CTCAGAGGATGCCATGTCTAAAATA‐3′ 5′‐GGGGATGACGAATTATTCACTAACT‐3′ |
HLA, human leukocyte antigen; MS, microsatellite; Heterozyg, heterozygosity.
Results
HLA genotypes of leukemic cell lines. Table 3 summarizes HLA class I genotypes of nine leukemia/lymphoma cell lines. HLA antigens from both alleles were detected in six cell lines. However, only one allele was amplified in Jurkat cells and Ramos cells for HLA‐A and for HLA‐A and ‐C, respectively. Further, only one allele was amplified in HL‐60 cells for HLA‐A, ‐B, ‐C, ‐DRB1, and ‐DQB1 (A*0101, B*5701, Cw*0602, DRB1*0701, DQB1*03032), strongly suggesting HLA haplotype loss in this cell line.
Table 3.
Human leukocyte antigen (HLA) class I genotypes of nine leukemia/lymphoma cell lines
| Cell line | Locus A | Locus B | Locus Cw |
|---|---|---|---|
| HL‐60 | *0101 | *5701 | *0602 |
| Jurkat | *0301 | *0702, *3503 | *0401, *0702 |
| U937 | *0301, *31012 | *1801, *5101 | *0102, *0701 |
| CCRF‐CEM | *0101, *31012 | *0801, *4001 | *0304, *0701 |
| Namalwa | *0301, *6802 | *0702, *4901 | *0701, *0702 |
| MOLT‐4 | *0101, *2501 | *1801, *5801 | *0602, *1203 |
| NALM‐6 | *0101, *0201 | *0801, *1501 | *0401, *0701 |
| Ramos | *0301 | *4403, *5101 | *1601 |
| BV173 | *0201, *3001 | *1510, *1801 | *0304, *1203 |
HLA haplotype loss in HL‐60 cells. As HLA genotype analysis strongly suggested that HL‐60 cells had lost one complete HLA haplotype, we performed G‐banding analysis of these cells, which proved to have the karyotype: 45–46, X: ‐X, del(4)(q?), der(5)t(5;17) (q11;q11), +7, add(8)(q24), der(8)(q13q22), add(9)(p11), add(10)(p11), −11, i(11)(q10), add(14)(q24), add(16)(q22), der(16)t(11;16) (q13;q22)ins(16;?) (q22;?), −17, +18, +mar1, +0~1mar. No apparent deletion in chromosome 6 was observed in any metaphases analyzed. However, these results could not completely rule out the possibility of HLA haplotype loss in HL‐60 cells.
We next analyzed HL‐60 for heterozygosity at seven different loci in the HLA region using microsatellite markers, as no other samples such as normal tissue or peripheral blood mononuclear cells (PBMC) were available for the patient from whom HL‐60 was derived. Previous studies have shown heterozygosity frequencies of these seven microsatellite markers to range from 72% to 91% (Table 2).( 16 ) As shown in Figure 1, HL‐60 cells showed homozygosity at the D6S265, C3.2.11, C1.2.5, D6S311 and D6S273 loci, which are very close to the HLA class I gene; heterozygosity was retained at the D6S276 and D6S291 loci. Taken together, these findings strongly suggest that HL‐60 cells had lost one complete HLA haplotype.
Figure 1.
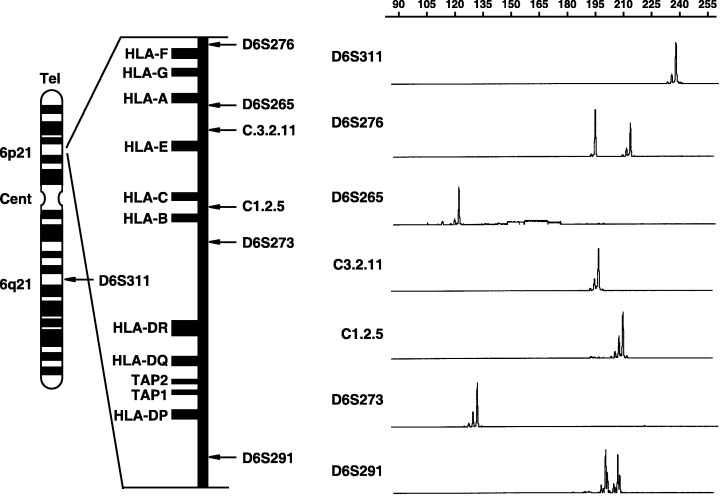
Schematic presentation of the location of microsatellite (MS) markers on chromosome 6 and GeneScan images of heterozygosity analysis at each MS locus. Positions of MS loci relative to genes within the human leukocyte antigen (HLA) class I/II region also are shown. In GeneScan images, a fragment with two high peaks represents heterozygosity, and one with a single high peak represents homozygosity. The pattern of homozygosity despite use of highly heterologous MS markers strongly suggests that HL‐60 cells have loss of one haplotype of the HLA region. Cent, centromere; Tel., telomere.
HLA class I/II and β2‐microglobulin expression by leukemic cell lines. We next analyzed HLA class I/II and β2M cell‐surface expression patterns in the nine leukemia/lymphoma cell lines using a broad panel of allele‐specific mAbs. Flow cytometric analysis displayed a limited to high degree of monomorphic HLA class I expression in all cell lines (Fig. 2). HLA‐A or B allele‐specific loss or down‐regulation also was observed in all cell lines. Further analysis of cell surface HLA antigen expression using allele‐specific mAbs was therefore required. Flow cytometric analysis demonstrated some β2M expression in all cell lines and high expression in some lines; however, monomorphic HLA class II expression was detected in five of the nine cell lines.
Figure 2.
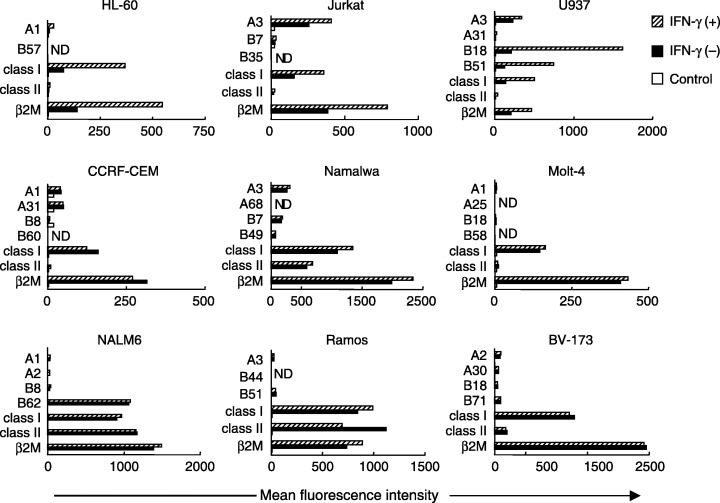
Flow cytometric analysis of human leukocyte antigen (HLA) class I/II and β2‐microglobulin expression in nine human leukemia/lymphoma cell lines. Cells were either untreated or treated with interferon (IFN)‐γ (200 U/mL, 48 h). Mean fluorescence intensity of each HLA molecule was analyzed by flow cytometry.
Down‐regulation of HLA antigen expression in cultures of some tumor cells can be reversed by exogenous IFN‐γ, which up‐regulates both HLA class I and II expression in various types of tumor cells. We therefore tested the ability of this cytokine to up‐regulate HLA class I and allele‐specific HLA expression in the nine leukemia/lymphoma cell lines. Three lines showed up‐regulation of HLA class I and allele‐specific HLA expression by IFN‐γ.
HLA class I expression on freshly isolated leukemic blasts compared with normal lymphocytes. To determine whether our observations in cultured cell lines were consistent with freshly isolated leukemic cells, we examined expression of HLA class I, HLA‐A, and β2M in 44 blast samples from 43 patients with leukemia. We first determined the genotype at the HLA‐A locus by PCR‐SSP, and then analyzed cell‐surface expression of these molecules using allele‐specific mAbs and flow cytometry. As summarized in Table 4, loss or down‐regulation of HLA expression was observed in three of 39 (7.7%) cases at initial diagnosis and in two of five (40.0%) cases in relapse. In four of the relapsed cases (80.0%), down‐regulation of HLA expression was accompanied by down‐regulation of β2M expression. Figure 3 shows representative results of flow cytometric analysis for samples with preserved (case 35) and down‐regulated (case 41) HLA expression.
Table 4.
Expression of HLA class I, HLA‐A, and β2M in 44 blast samples from 43 patients with leukemia
| Case no. | Age (years) | Sex | Disease | Status | HLA‐A | Surface expression | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 2 | Allele 1 | Allele 2 | Class I | β2M | |||||
| 1 | 59 | M | MDS/AML | I | A24 | A26 | + | + | + | + |
| 2 | 64 | M | AML | I | A2 | A24 | + | + | + | + |
| 3 | 78 | F | AML | I | A2 | A11 | + | + | + | + |
| 4 | 54 | F | ALL | I | A1 | A2 | + | + | + | + |
| 5 | 83 | F | FL | I | A2 | A24 | + | + | + | + |
| 6 | 49 | M | ALL | I | A11 | A24 | + | + | + | + |
| 7 | 70 | M | AML | I | A24 | A26 | + | + | + | + |
| 8 | 50 | M | AML | I | A24 | A31 | + | + | + | + |
| 9 | 80 | M | MDS/AML | I | A2 | A2 | + | + | + | ND |
| 10 | 75 | M | AML | I | A2 | A2 | + | + | + | ND |
| 11 | 53 | M | AML | I | A24 | A31 | + | ND | + | + |
| 12 | 75 | M | MDS/AML | I | A2 | A31 | + | + | + | + |
| 13 | 58 | M | AML | I | A24 | A24 | + | + | + | + |
| 14 | 41 | F | AML | A24 | A26 | + | + | + | + | |
| 15 | 76 | M | MCL | I | A24 | A24 | + | + | + | + |
| 16 | 57 | M | AML | I | A11 | A 24 | + | + | + | + |
| 17 | 18 | M | AML | I | A2 | A24 | + | + | + | + |
| 18 | 70 | M | PCL | I | A2 | A24 | + | + | + | + |
| 19 | 77 | F | AML | I | A24 | A24 | + | + | + | + |
| 20 | 37 | F | ALL | I | A11 | A24 | + | + | + | + |
| 21 | 75 | M | MDS/AML | I | A11 | A26 | ↓ | ↓ | ↓ | ↓ |
| 22 | 64 | M | CLL | I | A2 | A24 | + | + | + | + |
| 23 | 46 | F | ALL | I | A2 | A31 | + | + | + | + |
| 24 | 49 | M | AML | I | A11 | A11 | + | + | + | + |
| 25 | 65 | M | MM | I | A24 | A24 | + | + | + | + |
| 26 | 39 | M | MDS/AML | I | A2 | A26 | + | + | + | ND |
| 27 | 74 | M | MPD/AML | I | A2 | A24 | + | + | + | ND |
| 28 | 66 | F | MM | I | A24 | A24 | + | + | + | ND |
| 29 | 62 | F | AML | I | A11 | A24 | + | + | + | + |
| 30 | 35 | M | AML | I | A2 | A33 | + | ND | + | + |
| 31 | 34 | F | ALL | I | A26 | A33 | + | ND | + | + |
| 32 | 32 | M | AML | I | A24 | A26 | + | + | + | + |
| 33 | 54 | M | MDS/AML | I | A24 | A31 | + | + | + | + |
| 34 | 26 | F | ALL | I | A1 | A24 | + | + | + | + |
| 35 | 61 | M | AML | I | A11 | A11 | + | + | + | + |
| 36 | 19 | M | AML | I | A2 | A11 | ↓ | ↓ | ↓ | ↓ |
| 37 | 66 | F | MCL | I | A2 | A24 | + | + | + | + |
| 38 | 53 | F | AML | I | A11 | A24 | ↓ | ↓ | ↓ | ↓ |
| 39 | 18 | F | AML | I | A2 | A33 | + | ND | + | + |
| R | – | ND | + | + | ||||||
| 40 | 70 | M | AML | R | A2 | A2 | + | + | + | + |
| 41 | 27 | M | ALL | R | A24 | A33 | – | ND | ↓ | ↓ |
| 42 | 28 | M | AML | R | A24 | A24 | + | + | + | + |
| 43 | 38 | M | AML | R | A24 | A24 | + | + | + | + |
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; β2M, β2‐microglobulin; CLL, chronic lymphocytic leukemia; F, female; FL, follicular lymphoma; HLA, human leukocyte antigen; I, at initial diagnosis; M, male; MCL, mantle cell lymphoma; MDS/AML, myelodysplastic syndrome overt leukemia; MM, multiple myeloma; MPD/AML, myeloproliferative disease overt leukemia; ND, not done; PCL, plasma cell leukemia; R, at relapse. +, present; –, absent; ↓, decrease.
Figure 3.
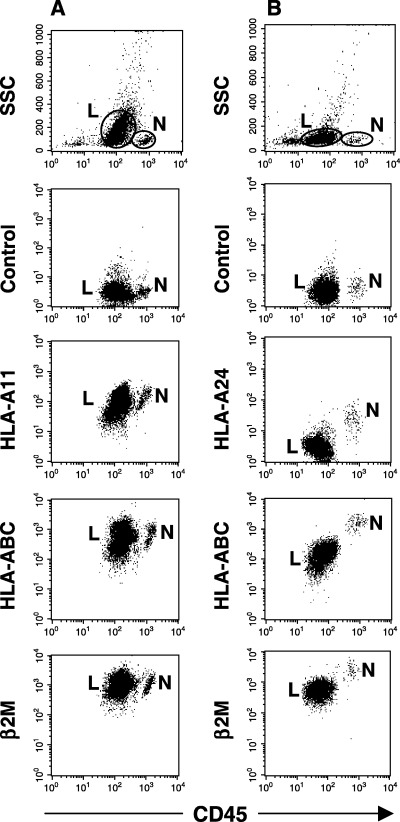
Cell surface expression of human leukocyte antigen (HLA) class I on freshly isolated leukemic blasts from (A) case 35 with acute myeloblastic leukemia (AML) and (B) case 41 with acute lymphoblastic leukemia (ALL). In case 35, no loss or down‐regulation in HLA class I, HLA‐A11, or β2‐microglobulin (β2M) expression on AML blasts (L) is evident in comparison with normal lymphocytes (N). In case 41, down‐regulation of HLA class I, HLA‐A24, and β2M expression on ALL blasts is seen. Leukemic blasts were differentiated from normal lymphocytes by lower CD45 fluorescence.
Allele‐specific loss of HLA class I expression: Association with relapse and restoration by IFN‐γ. In one patient (case 39) we could examine HLA expression on freshly isolated blasts obtained both at initial diagnosis and at relapse (Fig. 4). Flow cytometric analysis showed loss of HLA‐A2 cell surface expression at relapse, although this molecule had been expressed at initial diagnosis. Expression of HLA class I and β2M were preserved at both initial diagnosis and relapse. After 48 h of culture in the presence of IFN‐γ, HLA‐A2 cell‐surface expression was completely restored in blasts obtained at relapse (Fig. 4).
Figure 4.
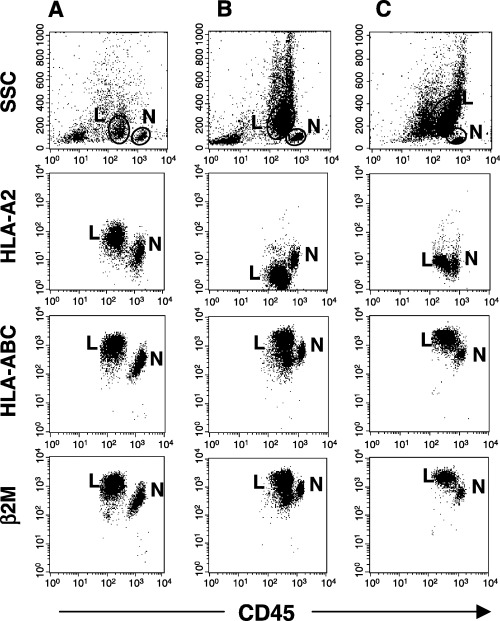
Down‐regulation of human leukocyte antigen (HLA) class I expression at relapse, and restoration by interferon (IFN)‐γ. In one patient with acute myeloid leukemia (case 39), freshly isolated bone marrow cells were obtained both (A) at initial diagnosis and (B) at relapse after transplantation. In a sample obtained at initial diagnosis, HLA class I molecule expression is preserved. After multiple courses of chemotherapy, the patient received an allogeneic peripheral blood stem cell transplant from her uncle. A 19‐month complete remission ended in relapse. A sample obtained at relapse showed loss of HLA‐A2 expression by leukemic blasts. Chimerism analysis confirmed that these blasts were of recipient, not donor, origin. (C) Cell incubation with 200 U/mL of IFN‐γ for 48 h completely restored HLA‐A2 expression.
Discussion
Loss or down‐regulation of HLA class I antigen expression has been demonstrated in a variety of tumors, and can have several causes: absence of β2M or transporter associated with antigen processing (TAP) expression;( 17 , 18 , 19 , 20 ) loss of heterozygosity, large deletions, or mitotic recombination in chromosome 6;( 18 , 21 , 22 ) transcriptional down‐regulation;( 23 , 24 , 25 ) and point mutation, partial deletion, or somatic recombination.( 18 , 26 , 27 ) We and other investigators have demonstrated loss of HLA haplotype secondary to loss of heterozygosity mainly in cell lines and tissue sections from solid tumors.( 22 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ) We know of no report concerning HLA haplotype deletion in leukemic cells. In the current study we found for the first time that allelic loss caused by a deletion of a complete HLA haplotype can occur in leukemic cell lines at a low frequency (11.1%). Our results suggest that haplotype loss of HLA represents a likely immune escape mechanism in leukemic cells, as it does in solid tumors. However, because the freshly isolated leukemic cells from which HL‐60 was derived were not available for investigation, we could not rule out the possibility that HLA haplotype loss might have occurred during culture. We therefore examined 44 samples of freshly isolated blasts obtained at initial diagnosis and/or at relapse. Although genotyping showed HLA‐A homozygosity in 11 of 44 samples, microsatellite analysis showed no haplotype loss of the HLA genome (data not shown). These results suggest that haplotype loss of the HLA gene occurs less often in leukemic cells than in solid tumors.
To date, loss and down‐regulation of HLA expression has not been extensively studied in freshly isolated leukemic blasts. Loss of HLA class I expression has been reported in lymphoblastoid cell lines and in B‐cell lymphoma( 36 , 37 ) but uncertainty prevails as to whether this event is rare or frequent. We therefore determined the frequency of locus‐specific or allele‐specific down‐regulation in freshly isolated leukemic blasts, observing down‐regulation of HLA class I expression in four of 44 samples (9.1%). As suggested by a previous report( 38 ) the frequency of loss or down‐regulation of HLA expression in blasts is less than with solid tumors: 20–70% in melanoma and colonic, renal cell and laryngeal carcinomas;( 34 , 39 , 40 ) 38% in high‐grade breast cancer; and 90% in cervical cancer.( 31 ) The reason for this difference in frequency of loss or down‐regulation of HLA expression between solid tumors and leukemia remains unknown. This phenomenon may explain why leukemic cells are immunogenic to some degree with allo‐HSCT or DLI in the clinical setting.
To examine whether relapse of leukemia was associated with increased occurrence of loss and down‐regulation of HLA expression, we studied five samples representing acute leukemia in relapse. Although the number of samples was small, two of five (40.0%) showed loss or down‐regulation of HLA class I, compared with three of 39 (7.7%) samples obtained at initial diagnosis. Our results suggest that loss of HLA antigen expression is involved in the pathogenesis of relapse in patients with acute leukemia and plays an important role in relapsed leukemia after allo‐HSCT or failure of DLI. However, we did not examine the expression of HLA‐B or C allele at initial diagnosis and relapse. Relapsed leukemic cells with HLA‐A allelic loss could be recognized by host cellular immune system in the context of HLA‐B or C molecules. In addition, our results differ from those reported by Brouwer et al. and Wetzler et al. ( 38 , 41 ) who found expression loss at relapse in only two of 25 (8.0%) and none of five (0.0%) cases, respectively. The reason for this disagreement is unknown. Further study in a large number of patients should provide definitive data concerning prevalence and significance of lost HLA expression in leukemic relapse.
Interestingly, the current study also demonstrated loss of HLA‐A2 expression at relapse that had been present at initial diagnosis in case 39. This change supports our hypothesis that loss of HLA antigen expression may contribute to relapse of leukemia. To our knowledge, such a sequence of events has not been reported previously for leukemia. Of note, loss of HLA expression at relapse was completely restored by IFN‐γ. This result implies that the HLA‐allele may be preserved at the genome level and that the cell surface expression could be suppressed by transcriptional down regulation. It is well known that IFN‐γ up‐regulates major histocompatibility class I transcription in an IFN regulatory factor‐1 (IRF‐1) dependent manner.( 42 ) Together, our results suggest a new treatment strategy such as concurrent cytokine‐CTL therapy might terminate relapses in acute leukemia patients.
In conclusion, we found allelic loss caused by deletion of a complete HLA haplotype in a leukemic cell line. By flow cytometric analysis of freshly isolated leukemic blasts, we confirmed a relatively low frequency of defective HLA class I expression in leukemia as previously reported. However, loss or down‐regulation of HLA class I antigen expression was observed more frequently at relapse, a finding in disagreement with previous reports.( 38 , 41 ) Furthermore, we demonstrated for the first time that loss of allele‐specific HLA‐A locus expression was associated with relapse in acute leukemia and was restored in vitro by IFN‐γ. These results suggest that loss of HLA antigen expression may be involved in the pathogenesis of relapse in patients with leukemia. Because surface expression of HLA class I and HLA class I alleles on leukemic cells is a prerequisite for successful T‐cell‐mediated immunotherapy against leukemia, our results should be of help in designing new modalities of clinical immunotherapy.
Acknowledgment
We thank Dr Tomofumi Yano for providing clinical information concerning one of the patients studied.
References
- 1. Garrido F, Cabrera T, Concha A, Glew S, Ruiz‐Cabello F, Stern PL. Natural history of HLA expression during tumour development. Immunol Today 1993; 14: 491–9. [DOI] [PubMed] [Google Scholar]
- 2. Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today 1995; 16: 487–94. [DOI] [PubMed] [Google Scholar]
- 3. Garrido F, Ruiz‐Cabello F, Cabrera T et al. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 1997; 18: 89–95. [DOI] [PubMed] [Google Scholar]
- 4. Yotnda P, Firat H, Garcia‐Pons F et al. Cytotoxic T cell response against the chimeric p210 BCR‐ABL protein in patients with chronic myelogenous leukemia. J Clin Invest 1998; 101: 2290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falkenburg JH, Wafelman AR, Joosten P et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia‐reactive cytotoxic T lymphocytes. Blood 1999; 94: 1201–8. [PubMed] [Google Scholar]
- 6. Yotnda P, Garcia F, Peuchmaur M et al. Cytotoxic T cell response against the chimeric ETV6‐AML1 protein in childhood acute lymphoblastic leukemia. J Clin Invest 1998; 102: 455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Molldrem JJ, Clave E, Jiang YZ et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony‐forming units. Blood 1997; 90: 2529–34. [PubMed] [Google Scholar]
- 8. Oka Y, Udaka K, Tsuboi A et al. Cancer immunotherapy targeting Wilms’ tumor gene WT1 product. J Immunol 2000; 164: 1873–80. [DOI] [PubMed] [Google Scholar]
- 9. Minev B, Hipp J, Firat H, Schmidt JD, Langlade‐Demoyen P, Zanetti M. Cytotoxic T cell immunity against telomerase reverse transcriptase in humans. Proc Natl Acad Sci USA 2000; 97: 4796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nair SK, Heiser A, Boczkowski D et al. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med 2000; 6: 1011–17. [DOI] [PubMed] [Google Scholar]
- 11. Browning MJ, Krausa P, Rowan A, Bicknell DC, Bodmer JG, Bodmer WF. Tissue typing the HLA‐A locus from genomic DNA by sequence‐specific PCR: comparison of HLA genotype and surface expression on colorectal tumor cell lines. Proc Natl Acad Sci USA 1993; 90: 2842–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bunce M, O’Neill CM, Barnardo MC et al. Phototyping: comprehensive DNA typing for HLA‐A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence‐specific primers (PCR‐SSP). Tissue Antigens 1995; 46: 355–67. [DOI] [PubMed] [Google Scholar]
- 13. Moribe T, Kaneshige T, Inoko H. Complete HLA‐A DNA typing using the PCR‐RFLP method combined with allele group‐ and sequence‐specific amplification. Tissue Antigens 1997; 50: 535–45. [DOI] [PubMed] [Google Scholar]
- 14. Yoshida M, Kimura A, Numano F, Sasazuki T. Polymerase‐chain‐reaction‐based analysis of polymorphism in the HLA‐B gene. Hum Immunol 1992; 34: 257–66. [DOI] [PubMed] [Google Scholar]
- 15. Mitelman F, ed. International System for Human Cytogenetic Nomenclature. Basel: S. Karger, 1995. [Google Scholar]
- 16. Foissac A, Salhi M, Cambon‐Thomsen A. Microsatellites in the HLA region: 1999 update. Tissue Antigens 2000; 55: 477–509. [DOI] [PubMed] [Google Scholar]
- 17. Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. β2‐Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest 1998; 101: 2720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Browning M, Petronzelli F, Bicknell D et al. Mechanisms of loss of HLA class I expression on colorectal tumor cells. Tissue Antigens 1996; 47: 364–71. [DOI] [PubMed] [Google Scholar]
- 19. D’Urso CM, Wang ZG, Cao Y, Tatake R, Zeff RA, Ferrone S. Lack of HLA class I antigen expression by cultured melanoma cells FO‐1 due to a defect in B2m gene expression. J Clin Invest 1991; 87: 284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spies T, DeMars R. Restored expression of major histocompatibility class I molecules by gene transfer of a putative peptide transporter. Nature 1991; 351: 323–4. [DOI] [PubMed] [Google Scholar]
- 21. Koopman LA, Mulder A, Corver WE et al. HLA class I phenotype and genotype alterations in cervical carcinomas and derivative cell lines. Tissue Antigens 1998; 51: 623–36. [DOI] [PubMed] [Google Scholar]
- 22. Torres MJ, Ruiz‐Cabello F, Skoudy A et al. Loss of an HLA haplotype in pancreas cancer tissue and its corresponding tumor derived cell line. Tissue Antigens 1996; 47: 372–81. [DOI] [PubMed] [Google Scholar]
- 23. Versteeg R, Kruse‐Wolters KM, Plomp AC et al. Suppression of class I human histocompatibility leukocyte antigen by c‐myc is locus specific. J Exp Med 1989; 170: 621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peltenburg LT, Schrier PI. Transcriptional suppression of HLA‐B expression by c‐Myc is mediated through the core promoter elements. Immunogenetics 1994; 40: 54–61. [DOI] [PubMed] [Google Scholar]
- 25. Soong TW, Hui KM. Locus‐specific transcriptional control of HLA genes. J Immunol 1992; 149: 2008–20. [PubMed] [Google Scholar]
- 26. Koopman LA, Van Der Slik AR, Giphart MJ, Fleuren GJ. Human leukocyte antigen class I gene mutations in cervical cancer. J Natl Cancer Inst 1999; 91: 1669–77. [DOI] [PubMed] [Google Scholar]
- 27. Browning MJ, Krausa P, Rowan A et al. Loss of human leukocyte antigen expression on colorectal tumor cell lines: implications for anti‐tumor immunity and immunotherapy. J Immunother 1993; 14: 163–8. [DOI] [PubMed] [Google Scholar]
- 28. Grandis JR, Falkner DM, Melhem MF, Gooding WE, Drenning SD, Morel PA. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clin Cancer Res 2000; 6: 2794–802. [PubMed] [Google Scholar]
- 29. Feenstra M, Verdaasdonk M, Van Der Zwan AW, De Weger R, Slootweg P, Tilanus M. Microsatellite analysis of microdissected tumor cells and 6p high density microsatellite analysis in head and neck squamous cell carcinomas with down‐regulated human leukocyte antigen class I expression. Lab Invest 2000; 80: 405–14. [DOI] [PubMed] [Google Scholar]
- 30. Maleno I, Lopez‐Nevot MA, Cabrera T, Salinero J, Garrido F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol Immunother 2002; 51: 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koopman LA, Corver WE, Van Der Slik AR, Giphart MJ, Fleuren GJ. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J Exp Med 2000; 191: 961–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hiraki A, Kaneshige T, Kiura K et al. Loss of HLA haplotype in lung cancer cell lines: implications for immunosurveillance of altered HLA class I/II phenotypes in lung cancer. Clin Cancer Res 1999; 5: 933–6. [PubMed] [Google Scholar]
- 33. Hiraki A, Ikeda K, Yoshino T et al. Tumor‐specific cytotoxic T lymphocyte responses against chondrosarcoma with HLA haplotype loss restricted by the remaining HLA class I allele. Biochem Biophys Res Commun 2001; 286: 786–91. [DOI] [PubMed] [Google Scholar]
- 34. Metzelaar‐Blok JA, Jager MJ, Moghaddam PH, Van Der Slik AR, Giphart MJ. Frequent loss of heterozygosity on chromosome 6p in uveal melanoma. Hum Immunol 1999; 60: 962–9. [DOI] [PubMed] [Google Scholar]
- 35. Marincola FM, Shamamian P, Alexander RB et al. Loss of HLA haplotype and B locus down‐regulation in melanoma cell lines. J Immunol 1994; 153: 1225–37. [PubMed] [Google Scholar]
- 36. Ohlen C, Bejarano MT, Gronberg A et al. Studies of sublines selected for loss of HLA expression from an EBV‐transformed lymphoblastoid cell line. Changes in sensitivity to cytotoxic T cells activated by allostimulation and natural killer cells activated by IFN or IL‐2. J Immunol 1989; 142: 3336–41. [PubMed] [Google Scholar]
- 37. Riemersma SA, Jordanova ES, Schop RF et al. Extensive genetic alterations of the HLA region, including homozygous deletions of HLA class II genes in B‐cell lymphomas arising in immune‐privileged sites. Blood 2000; 96: 3569–77. [PubMed] [Google Scholar]
- 38. Brouwer RE, Van Der Heiden P, Schreuder GM et al. Loss or downregulation of HLA class I expression at the allelic level in acute leukemia is infrequent but functionally relevant, and can be restored by interferon. Hum Immunol 2002; 63: 200–10. [DOI] [PubMed] [Google Scholar]
- 39. Jimenez P, Canton J, Collado A et al. Chromosome loss is the most frequent mechanism contributing to HLA haplotype loss in human tumors. Int J Cancer 1999; 83: 91–7. [DOI] [PubMed] [Google Scholar]
- 40. Cabrera T, Salinero J, Fernandez MA, Garrido A, Esquivias J, Garrido F. High frequency of altered HLA class I phenotypes in laryngeal carcinomas. Hum Immunol 2000; 61: 499–506. [DOI] [PubMed] [Google Scholar]
- 41. Wetzler M, Baer MR, Stewart SJ et al. HLA class I antigen cell surface expression is preserved on acute myeloid leukemia blasts at diagnosis and at relapse. Leukemia 2001; 15: 128–33. [DOI] [PubMed] [Google Scholar]
- 42. Chang CH, Hammer J, Loh JE, Fodor WL, Flavell RA. The activation of major histocompatibility complex class I genes by interferon regulatory factor‐1 (IRF‐1). Immunogenetics 1992; 35: 378–84. [DOI] [PubMed] [Google Scholar]


