Abstract
Curcumin (diferuloylmethane) has chemopreventive and chemotherapeutic potentials against various types of cancers. We have developed a series of curcumin analogs to improve its low bioavailability by enhancing its potentials. The newly synthesized analog GO‐Y030 [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl) penta‐1,4‐dien‐3‐one] showed a 30‐fold greater growth suppression in vitro via similar molecular mechanisms to curcumin. The availability of this analog was examined by using a mouse model harboring the germ‐line mutation of Apc, Apc580D/+, in vivo. Apc580D/+ mice had a very limited survival time with an intestinal obstruction due to polyposis. The average tumor number in mice fed GO‐Y030 was reduced to 61.2% of those that were fed the basal diet (P < 0.05). Compared with Apc580D/+ mice fed the basal diet (median survival time = 166.5 days), a significantly prolonged lifespan (213 days) was observed in Apc580D/+ mice fed GO‐Y030. The chemopreventive effect with GO‐Y030 was improved, compared with curcumin (191 days). The survival benefit corresponded to the diminished intestinal tumor incidence in Apc580D/+ mice fed GO‐Y030. No adverse reactions were observed, judging from body weight or biochemical data concerning liver and renal damage. Degradation of accumulated β‐catenin with curcumin is one of the major mechanisms of chemoprevention in colorectal carcinogenesis. It was demonstrated that the number of β‐catenin‐positive adenoma cells in Apc580D/+ mice fed GO‐Y030 was reduced. (Cancer Sci 2009; 100: 956–960)
Curcumin [1,7‐bis(4‐hydroxy‐3‐methoxyphenyl)‐1,6‐heptadiene‐3,5‐dione] is a dietary constituent extracted from turmeric. It is well known to have a very reasonable tumor suppressive ability.( 1 ) The mechanisms of tumor suppression are exerted by down‐regulation or ablation of a variety of oncogene products including transactivaters such as nuclear factor‐kappa B (NF‐κB), growth signal transducers such as the Wnt signal molecule, β‐catenin, and several growth factors and their receptors, and so on.( 2 ) Curcumin also has anti‐invasion, antimetastasis, and antiangiogenesis potential.( 3 , 4 ) This potential is desirable for new cancer therapeutic agents. β‐Catenin is a key molecule of APC (adenomatous polyposis coli)‐derived colorectal carcinogenesis( 5 ) that can be phosphorylated as a result of its association with APC, glycogen synthase kinase (GSK)‐3β, and then degraded in the ubiquitination pathway.( 6 ) Accumulated β‐catenin in the cytoplasm is transferred into the nucleus and acts together with lymphoid enhancer binding factor‐1/T‐cell‐factor family proteins as a transactivator of other oncogenes, including c‐myc and cyclin D1.( 7 , 8 , 9 ) The low toxicity of curcumin is also advantageous for clinical use; however, its hydrophobicity, low absorption, and easy degradation are disadvantageous.( 1 ) Recent clinical and preclinical studies of colorectal cancer patients and animals have suggested that curcumin bioavailability is limited.( 10 , 11 , 12 ) To improve this low bioavailability, we synthesized a series of curcumin analogs and successfully screened an analog named GO‐Y030 [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl)penta‐1,4‐dien‐3‐one] bearing a 30‐ to 50‐fold enhanced tumor suppressive potential against various types of cancers in vitro. ( 13 ) The molecular mechanisms of the tumor suppressive effects of GO‐Y030 were similar to those of curcumin, including cell cycle arrest, down‐regulation of oncogene activities such as NF‐κB and Wnt signal transactivation, and induction of caspase 3 activity. The familial adenomatous polyposis (FAP) mouse is a very good model, because it develops adenomas through the same molecular mechanism as in human colorectal carcinogenesis, including the APC tumor suppressor gene. The chemopreventive ability of curcumin was assessed in an FAP model mouse, Min, harboring a truncation mutation at codon 850 with the potential to prevent adenoma formation.( 12 , 14 ) To examine the bioavailability of synthesized analog and its toxicity, the FAP mouse model was applied. In this study, oral administration of GO‐Y030 improved chemopreventive ability in FAP mouse without apparent toxicities in vivo.
Materials and Methods
Animal experiment. Genotyping of Apc580D/+ mice were carried out by the polymerase chain reaction method as described previously.( 15 ) Apc580D/+ mice were fed either the basal diet (HFD32; CLEA Japan, Tokyo, Japan) basal diet containing 0.1% (weight/weight) curcumin, or basal diet containing 0.1% or 0.5% GO‐Y030. All animal experiments were conducted in accordance with the guidelines set by Tohoku University.
Compound. GO‐Y030 was synthesized as previously described.( 13 )
Immunohistochemistry. The deparaffinized 4‐µm specimens were heated in 1 mM ethylenediaminetetraacetic acid (EDTA)/10 mM Tris buffer (pH = 9.0) at 95°C for 40 min. After washing the specimens in Tris‐buffered saline plus Tween 20 (0.15 M NaCl, 0.05 M Tris‐HCl [pH = 7.6], 0.1% Tween20) and treating them with 3% H2O2, anti‐β‐catenin antibody (1:1000; BD Biosciences, Mississauga, ON, Canada) was added at room temperature for 60 min. After being treated with peroxidase‐labeled polymer conjugated to goat antimouse immunoglobulin (K4007; Dako Cytomation, Carpinteria, CA, USA), the specimens were stained with 3,3′‐diaminobenzidine tetrahydrochloride and Mayer hematoxylin.
Biochemical analysis. Blood was sampled from the infraorbital venous plexus. The sera were applied to the test slides to measure total bilirubin (T‐Bil, TBIL‐PIII), creatinine (Cre, CRE‐PIII), blood urea nitrogen (BUN, BUN‐PII), and glutamic oxaloacetic transaminase (GOT, GOT/AST‐PIII), using Fuji Dri‐Chem 3500 V (Fujifilm, Tokyo, Japan) according to the manufacturer's instructions.
Statistical analysis. Pearson's correlation coefficient was determined, and linear regression, Kaplan–Meier analysis, the log‐rank test, and Student's t‐test were conducted using StatMate III version 3.14 (ATMS, Tokyo, Japan).
Results
Chemopreventive effects on intestinal tumorigenesis in FAP mice fed the new curcumin analog GO‐Y030. GO‐Y030 [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl)penta‐1,4‐dien‐3‐one], shown to have a very high potential to suppress tumor cell growth in vitro in our previous study,( 13 ) was used in the chemoprevention experiment (Fig. 1a). The FAP mouse model, Apc580D/+, harboring a heterozygous germline deletion of the exon 14, was established from embryonic stem (ES) cells harboring the same deletion by Cre/loxP recombination.( 13 ) The deletion of exon 14 results in the truncation of APC gene product at codon 580. The average intestinal tumor incidence of Apc580D/+ was 125.58 ± 50.28 at around 13 weeks of age, and almost all Apc580D/+ mice fed a standard diet, CE2 (CLEA Japan), were dead by 20 weeks of age. Causes of death in Apc580D/+ mice were hemorrhage from intestinal tumors and/or intestinal obstruction due to polyposis.( 16 ) Those were the typical phenotypes of the FAP model mice. Histological analysis showed that almost all tumors were adenomas (Fig. 1b). It was supposed that tumor formation became apparent at around 4 weeks of age when the mice started to eat solid foods (Fig. 1c). Because chemopreventive treatment was initiated when the mice were older than 8 weeks of age, it did not contribute to survival (data not shown); hence, we decided that 4 weeks would be the best time to start chemoprevention.
Figure 1.
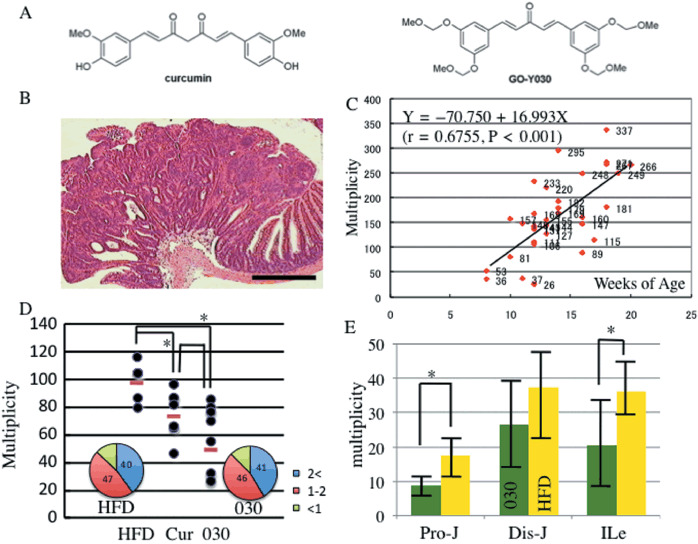
New curcumin analog and familial adenomatous polyposis (FAP) mouse model. (a) Chemical structures of curcumin and GO‐Y030 [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl)penta‐1,4‐dien‐3‐one]. (b) Histological appearance of intestinal tumor in Apc580D/+ mice. Scale bar = 400 µm. Histological features of these tumors correspond to those seen in adenomas. (c) Correlation between tumor incidence and aging of Apc580D/+ mice. Intestinal tumors are apparent at 8 weeks of age. The multiplicity reaches over 100 at around 12 weeks of age in almost all cases. Pearson's correlation coefficient (r) and linear regression equations were calculated. (d) Total tumor multiplicity in Apc580D/+ mice fed curcumin analogs. Apc580D/+ mice were fed the basal diet (HFD), curcumin (cur), or GO‐Y030. In the inset, the frequency of each tumor size: large (>2 mm diameter, blue), middle (1–2 mm, red), and small (<1 mm, green). *P < 0.05. (e) Multiplicity of adenomas in each intestinal segment in each diet group. Results are mean ± SD. *P < 0.05. Pro‐J, proximal jejunum; Dis‐J, distal jejunum; Ile, ileum.
As curcumin and its analog are very hydrophobic, we mixed the compound with HFD32, a basal diet oily enough to form a homogeneous paste. The mice were fed daily with 5 g of HFD32 containing 0.1% curcumin analog. We examined the suppressive effect of GO‐Y030 on the intestinal tumor incidence. The feeding of Apc580D/+ mice with the new curcumin analog was started at 4 weeks of age and continued until 8 weeks. Then, they were sacrificed to examine the tumors in the entire gastrointestinal tract, from the stomach to the anal cavity. In the age‐matched Apc580D/+ mouse model, the chemopreventive effect of curcumin analog on tumor incidence was apparent. The average number of the entire tumor multiplicity was 60.00 ± 23.65 in Apc580D/+ mice fed 0.1% GO‐Y030 (n = 7). The average tumor incidence in Apc580D/+ mice fed the basal diet (n = 6) was 98.83 ± 13.66 (Fig. 1d, Table 1). GO‐Y030 significantly suppressed the tumor incidence, where the P‐value was 0.0023, compared with the basal diet (Student's t‐test). The average tumor incidence in Apc580D/+ mice fed 0.1% curcumin (n = 6) was significantly lower (73.00 ± 18.89) than those fed the basal diet (P = 0.00953, Student's t‐test). GO‐Y030 suppressed tumor incidence more effectively than curcumin, but the P‐value between GO‐Y030 and curcumin was not significant (0.148, Student's t‐test) (Table 1).
Table 1.
Chemoprevention of intestinal tumorigenesis with curcumin analog
| Survival (median survival time, days) | Multiplicity of adenoma | |
|---|---|---|
| GO‐Y030 (0.1%) | 214.5* | 60.00 ± 23.65* |
| Curcumin (0.1%) | 191.0* | 73.00 ± 18.89 |
| HFD32 | 166.5 | 98.83 ± 13.66* |
P‐value (P < 0.05). Multiplicity of adenoma (mean ± SD).
GO‐Y030, [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl)penta‐1,4‐dien‐3‐one].
Next, we examined the preventive effect of GO‐Y030 in the proximal (proximal jejunum), middle (distal jejunum), and distal (ileum) segments of intestine. Tumor incidences were 8.86 ± 3.02 in the proximal jejunum, 26.71 ± 13.34 in the distal jejunum, and 20.71 ± 11.13 in the ileum, in the 0.1% GO‐Y030 diet group. On the other hand, it was 17.50 ± 6.16, 37.50 ± 11.47, and 36.17 ± 8.64, respectively, in the basal diet group (Fig. 1e). Even in the distal jejunum, tumor incidence was suppressed to level that was almost significance (P = 0.075, Student's t‐test). In the other segments, tumors were suppressed significantly. In the proximal jejunum, the P‐value was 0.0036 (Student's t‐test), and in the ileum it was 0.0094 (Student's t‐test). Chemoprevention with GO‐Y030 was observed in the entire small intestine. The size distribution of tumors was analyzed between GO‐Y030 and the basal HFD32. There were no fundamental differences between the basal and GO‐Y030 diet groups (Fig. 1d). These results indicated that 0.1% GO‐Y030 can prevent the adenoma formation, but not suppress its growth.
Effect of new curcumin analog on the survival of FAP mice. Then we examined whether the chemopreventive potential of the new analog contributed to the survival of FAP mice. Apc580D/+ mice were divided into three diet groups: (i) the basal diet group (n = 6); (ii) curcumin diet group (n = 6); and (iii) GO‐Y030 diet group (n = 6). The feeding started at 4 weeks old and continued until they became moribund. The Apc580D/+ mice fed the basal diet survived for a minimum of 95 days and a maximum of 248 days, and the median survival time (MST) was estimated to be 166.5 days. Apc580D/+ mice fed the curcumin diet survived for a minimum of 141 days and a maximum of 218 days, and the MST was estimated to be 191 days. Apc580D/+ mice fed GO‐Y030 diet survived for a minimum of 174 days and a maximum of 241 days, and the MST was estimated to be 214.5 days. The MST of the GO‐Y030 diet group was prolonged compared with the basal and curcumin diet groups. The Kaplan–Meier analyses and the log‐rank test showed that the survival benefit of GO‐Y030 was significantly superior to curcumin (P = 0.048) (Fig. 2). However, compared with HFD32, the survival benefit of GO‐Y030 was not significant (P = 0.641, log‐rank test) (Fig. 2). Curcumin itself had a small but not significant survival benefit compared with the basal diet in this study (P = 0.987). It was observed that the 0.1% GO‐Y030 diet had an improved positive effect on the survival of FAP mice to some extent.
Figure 2.
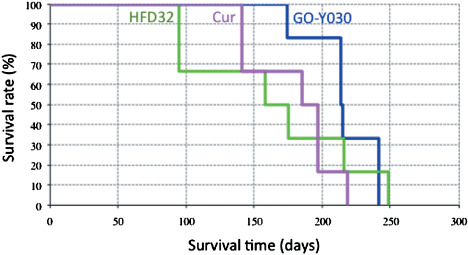
Chemopreventive effects of curcumin analogs. Kaplan–Meier analysis of Apc580D/+ mice fed the curcumin analog. Green line shows the basal diet (HFD32); magenta line, curcumin (Cur); blue line, GO‐Y030 [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl)penta‐1,4‐dien‐3‐one]. P‐value between GO‐Y030 and curcumin is 0.048 (HR = 0.39, 95% confidence interval, 0.14–2.18).
β‐Catenin‐positive adenomas were decreased with the treatment of GO‐Y030. β‐Catenin is a key molecule of APC‐induced colorectal carcinogenesis and it is one of the targets of curcumin as well as the new curcumin analog GO‐Y030 in vitro. ( 13 ) To test the action mechanism of GO‐Y030 in vivo, 13‐week‐old Apc580D/+ mice were fed either the basal diet alone or a diet containing 0.5% GO‐Y030 for 10 days. Along the longitudinal axis of the proximal jejunum, the 4‐µm‐thick paraffin specimen were dissected. The number of the β‐catenin‐positive adenomas was examined along this axis. The multiplicity of the β‐catenin‐positive adenomas in mice fed 0.5% GO‐Y030 (n = 3) was 21.67 ± 1.53, whereas the multiplicity in the basal diet group (n = 3) was 43.00 ± 4.00 (Fig. 3a). Tumor incidence in the 0.5% GO‐Y030 diet group was significantly reduced by 49.6% compared with the basal diet group (P < 0.001). Moreover, in the 0.5% GO‐Y030 diet group, the frequency of tumors larger than 1.0 mm in diameter reached at as many as 5% of the total tumors examined, whereas this value reached at as many as 38% of the examined tumors in the basal diet group (Fig. 3a). These results suggest that 0.5% GO‐Y030 may suppress tumor growth as well as the incidence of tumors in vivo. An immunohistchemical analysis with the anti‐β‐catenin antibody was carried out. Classification according to the immunoreactivity and the structure described below was undertaken. The β‐catenin‐positive adenomas in their cytoplasm were divided into two groups: strong positive (++) and positive (+). The strong positive group was further divided into two groups comprising cells with (+) or without (–) loss of polarity (LOP) (Fig. 3c). LOP is also known as the folded layers formation and it is one of the pathological features of more advanced adenomas.( 17 , 18 ) Using this molecular pathological approach, the β‐catenin‐positive adenomas were categorized as follows: β‐catenin (++) and LOP (+) (Grade 3 [G‐3]), Figure 3(d); β‐catenin (++) and LOP (–) (Grade 2 [G‐2], Figure 3(e); and β‐catenin (+) and LOP (+) (Grade 1 [G‐1], Figure 3(f). In particular, the G‐3 tumors were apparently reduced in mice fed 0.5% GO‐Y030, compared with those fed the basal diet (15.0%vs 36.4%, Fig. 3c). It was observed that the immunoreactivity to β‐catenin in the tumors, as well as the tumor incidence, was reduced in the 0.5% GO‐Y030 diet group.
Figure 3.
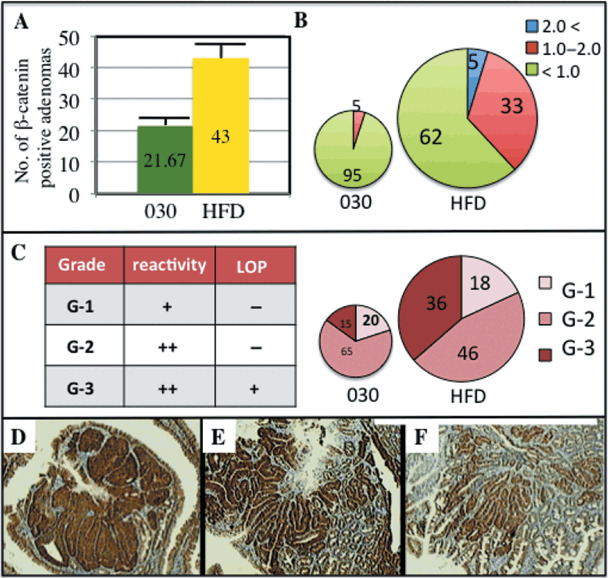
Expression of β‐catenin in adenomas treated with the curcumin analog GO‐Y030 [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl)penta‐1,4‐dien‐3‐one]. Immunohistchemical analysis for β‐catenin in each adenoma. (a) Multiplicity of the β‐catenin‐positive adenomas in each diet group. Results are mean ± SD. HFD, basal diet HFD32. (b) Frequency of each size of the adenomas in each diet group is shown in the pie graph. Blue, >2 mm diameter; red, 1–2 mm; green, <1 mm. The size of the graph corresponds to the magnitude of the adenoma multiplicity in each diet group. (c) Immunohistopathological grades (Grade 1–3) classified by the β‐catenin reactivity and the histological features of the adenomas in each diet group. The immunohistopathological grades are shown in the table. Frequency of each grade is shown in the pie graph. The size of the graph corresponds to the magnitude of adenoma multiplicity in each diet group. LOP, loss of polarity. (d) Grade 3 adenoma forming folded layers; (e) grade 2 adenoma; (f) Grade 1 adenoma.
No adverse reactions were observed with the new curcumin analog. As previously described,( 13 ) the appearance and body weight of Apc580D/+ mice fed GO‐Y030 were not distinguishable from mice fed the basal diet. Moreover, biochemical analyses of serum levels of T‐Bil, GOT, BUN, and Cre were conducted to examine the adverse reaction of GO‐Y030. Serum T‐Bil and GOT are the indicators of hepatic damage and BUN and Cre are the indicators of renal dysfunction (Fig. 4). The blood samples were collected from the mice that were sacrificed at the time of examination of the tumor incidence. Serum T‐Bil levels ranged from 0.6 to 1.2 mg/dL in Apc580D/+ mice fed GO‐Y030 (n = 6), while it ranged from 0.6 to 0.8 mg/dL in Apc580D/+ mice fed the basal diet alone (n = 6) and 0.7–0.8 mg/dL in those fed curcumin (n = 6). There were no significant variances of serum T‐Bil values among the groups (Fig. 4a). Serum GOT levels ranged from 58 to 118 U/L in the GO‐Y030 group, 36–95 U/L in the basal diet group, and 52–97 U/L in the curcumin group. There were no significant variances of the GOT values among the groups (Fig. 4b). Serum Cre levels ranged from 0.3 to 0.4 mg/dL in the GO‐Y030 group, 0.3–0.4 mg/dL in the basal diet group, and 0.3–0.4 mg/dL in the curcumin group. Serum BUN levels ranged from 22.6 to 58.2 mg/dL in the GO‐Y030 group, 26.2–49.2 mg/dL in the basal diet group, and 34.5–38.8 mg/dL in the curcumin group. No significant variances were apparent among the groups (Fig. 4c,d). These analyses indicated GO‐Y030 had no adverse reaction in vivo during observation as well as in vitro. ( 13 )
Figure 4.
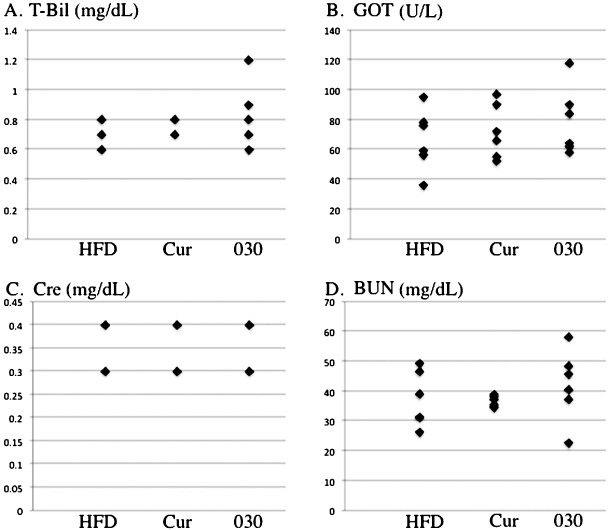
Adverse reactions of curcumin analogs. Serum levels of total bilirubin (T‐Bil) (a), glutamic oxaloacetic transaminase (GOT) (b), creatinine (Cre) (c), and blood urea nitrogen (BUN) (d) in Apc580D/+ mice fed the curcumin analog. There were no difference in any of the values among the Apc580D/+ mice fed a basal diet HFD32 (HFD), curcumin (Cur), or GO‐Y030 [(1E, 4E)‐1,5‐bis‐(3,5(‐bismethoxymethoxyphenyl)penta‐1,4‐dien‐3‐one].
Discussion
In this study, it was proven that the new curcumin analog GO‐Y030, as screened by the criterion of in vitro tumor growth suppressive potential, actually showed an improved chemopreventive effect on intestinal tumorigenesis. Chemoprevention of the adenoma formation with 0.1% GO‐Y030 was shown to be achievable. Compared with the basal diet, the average incidence of tumor formation decreased from 98.83 to 60, showing a 39% drop in the mice fed 0.1% GO‐Y030. This result was consistent with the MST of the mice fed GO‐Y030, which was prolonged from 166.5 days to 214.5 days (128.8% of the basal diet). As for the enhancement of the potential of GO‐Y030 at 0.1% compared with curcumin at 0.1%, the superiority of GO‐Y030 was observed, but it was not statistically significant. For example, the average incidence of tumor formation was 73 in the curcumin diet group against 60 in GO‐Y030 group; however, the P‐value between GO‐Y030 and curcumin is 0.148 (Student's t‐test). The MST was, however, estimated to be 191 days in the curcumin diet group against 214.5 days in the GO‐Y030 group. In this case, the log‐rank test indicated a slight significance (P = 0.048).
Among previous studies conducted on ApcMin/+ mice fed 0.1% curcumin, Bertagnolli's group reported that tumor multiplicity was suppressed by 64%.( 14 ) However, in another study by Gescher's group, 0.1% curcumin did not reduce the multiplicity; however, at 0.2 and 0.5%, it reduced the multiplicity by 39 and 40%, respectively, in ApcMin/+ mice.( 12 ) In our study, the multiplicity was suppressed by 26% in the 0.1% curcumin diet group and by 39% in the 0.1% GO‐Y030 diet group. Our results obtained with 0.1% curcumin were similar to those of Gescher's group. The differences in results may have been due to the differences of the housing condition and/or the phenotypes between ApcMin/+ and Apc580D/+. Apc580D/+ mice generally exhibit a severe polyposis phenotype. For example, the average multiplicity of intestinal tumor ranges from 30 to 120 in ApcMin/+, whereas it ranges from 90 to 120 in Apc580D/+ mice. The observation periods varied among these studies. For example, Bertagnolli's group started experiments in 5‐week‐old mice and analyzed them at 16 weeks, and Gescher's group started the examination in 4‐week‐old mice and analyzed them at 18 weeks.( 12 , 14 ) In contrast, our study started with 4‐week‐old mice and analyzed them at 12 weeks. This was early for determining differences between the control and study groups.
Among the different studies there were also big differences in the basal diets. Bertagnolli's group fed ApcMin/+ mice with a AIN‐76 diet and the average multiplicity of the control was as small as 33.2, whereas Gescher's group fed ApcMin/+ mice with a RM3 diet and the average multiplicity of the control was around 120.( 12 , 14 ) The latent effect in the basal diet could not be ignored. It was true in case of Apc580D/+. When Apc580D/+ were fed HFD32, the average multiplicity was around 90, but it reached around 120 in Apc580D/+ mice fed CE‐2. Gescher's group showed that dose escalation of curcumin from 0.1% to 0.2% was effective.( 12 ) In our study, higher dose administration of GO‐Y030 by as much as 0.5% for only 10 days effectively suppressed adenoma multiplicity by 49.6% in mice older 13 weeks of age.
GO‐Y030 at 0.5% could retard the adenoma formation as well as prevent it. It was observed that the number of β‐catenin‐positive adenomas and their immunoreavtivity for β‐catenin were diminished in this case. It was speculated that the prevention and retardation of adenoma formation with GO‐Y030 might be due to the degradation of β‐catenin. The degradation of β‐catenin shown in vitro was also observed in vivo by oral administration of GO‐Y030. Oral administration of 0.1% GO‐Y030 induced neither liver damage nor renal damage during 2 months of observation. In addition, judging from the body weight and general condition, the toxicity of 0.1% GO‐Y030 appeared to be acceptable. In the case of 0.5% GO‐Y030 treatment for 10 days, there were no adverse reactions; however, the issues about long‐term toxicity remain to be elucidated. In this study, it was demonstrated that the newly synthesized curcumin analog GO‐Y030 was effective as a chemopreventive agent against intestinal tumorigenesis without any adverse reactions.
Acknowledgments
Grants‐in‐aid were received from the HIROMI Medical Reserarch Foundation, Sendai, Japan (H. Shibata) and Miyagi Health Service Association (C. Ishioka). Grants‐in‐Aid for Scientific Research on Priority Areas (Cancer, No. 17015002) were received from the Ministry of Education, Culture, Sports, Science and Technology, Japan (H. Shibata and C. Ishioka). The immunohistchemical analysis of β‐catenin was conducted at Kotobiken Medical Laboratories, Tokyo, Japan.
References
- 1. Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol 2007; 75: 787–809. [DOI] [PubMed] [Google Scholar]
- 2. Singh YJ, Aggarwal BB. Activation of transcription factor NF‐κB is suppressed by curcumin (diferuloymethane). J Biol Chem 1995; 270: 24995–5000. [DOI] [PubMed] [Google Scholar]
- 3. Menon LG, Kuttan R, Kuttan G. Anti‐metastatic activity of curcumin and catechin. Cancer Lett 1999; 141: 159–65. [DOI] [PubMed] [Google Scholar]
- 4. Hahm ER, Gho YS, Park S, Park C, Kim KW, Yang CH. Synthetic curcumin analogs inhibit activation protein‐1 transcription and tumor‐induced angiogenesis. Biochem Biophys Res Commun 2004; 321: 337–44. [DOI] [PubMed] [Google Scholar]
- 5. Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β‐catenin levels by the adenomatous polyposis coli (APC) tumor‐suppressor protein. Proc Natl Acad Sci USA 1995; 92: 3046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation‐regulated ubiquitination and degradation of β‐catenin. J Biol Chem 1997; 272: 24735–8. [DOI] [PubMed] [Google Scholar]
- 7. Morin PJ, Sparks AB, Korinek V et al . Activation of β‐catenin‐Tcf signaling in colon cancer by mutations in beta‐catenin or APC. Science 1997; 275: 1787–90. [DOI] [PubMed] [Google Scholar]
- 8. He TC, Sparks AB, Rago C et al . Identification of c‐MYC as a target of the APC pathway. Science 1998; 281: 1509–12. [DOI] [PubMed] [Google Scholar]
- 9. Tetsu O, McCormick F. β‐catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999; 398: 422–6. [DOI] [PubMed] [Google Scholar]
- 10. Sharma R, Gescher AJ, McLelland HR et al . Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 2001; 7: 1894–900. [PubMed] [Google Scholar]
- 11. Ireson CR, Jones DJ, Orr S et al . Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev 2002; 11: 105–11. [PubMed] [Google Scholar]
- 12. Perkins S, Verschoyle RD, Hill K, Parveen I et al . Chemopreventive efficacy and pharmacokinetics of curcumin in the Min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol, Biomarkers Prevention 2002; 11: 535–40. [PubMed] [Google Scholar]
- 13. Ohori H, Yamakoshi H, Tomizawa M et al . Synthesis and biological analysis of new curcumin analogs bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther 2006; 5: 2563–71. [DOI] [PubMed] [Google Scholar]
- 14. Mahmoud NN, Carothers AM, Grunberger D et al . Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis 2000; 21: 921–7. [DOI] [PubMed] [Google Scholar]
- 15. Shibata H, Toyama K, Shioya H et al . Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 1997; 278: 120–3. [DOI] [PubMed] [Google Scholar]
- 16. Shibata H, Takano H, Ito M et al . α‐Catenin is essential in intestinal adenoma formation. Proc Natl Acad Sci USA 2007; 104: 18199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oshima H, Oshima M, Kobayashi M, Tsutsumi M, Taketo MM. Morphological and molecular processes of polyp formation in ApcΔ716 knockout mice. Cancer Res 1997; 57: 1644–9. [PubMed] [Google Scholar]
- 18. Petrova TV, Nykänen A, Norrmén C et al . Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 2008; 13: 407–19. [DOI] [PubMed] [Google Scholar]


