Abstract
Recent epidemiological studies have demonstrated that consumption of certain natural products can lower cancer risk in humans. For example, plant‐derived lignans have been shown to exert chemopreventive effects against cancer in vitro and in vivo. In the present study, the effects of three such lignans, termed arctiin, arctigenin, and nordihydroguaiaretic acid (NDGA), on the proliferation of Helicobacter pylori and the prevention of H. pylori‐associated gastric cancer were investigated in Mongolian gerbils. To examine the effects of arctigenin and NDGA on stomach carcinogenesis, specific pathogen‐free male, 5‐week‐old gerbils were infected with H. pylori, administered 10 p.p.m. N‐methyl‐N‐nitrosourea in their drinking water and fed diets containing various concentrations of lignans until they were killed after 52 weeks. At a dietary level of 0.25%, NDGA significantly decreased the incidence of gastric adenocarcinomas. Arctigenin, in contrast, failed to attenuate neoplasia at a level of 0.1%. Both NDGA and arctigenin significantly reduced serum 8‐hydroxy‐2′‐deoxyguanosine levels at doses of 0.25 and 0.05% (NDGA), and 0.1% (arctigenin). Administration of 0.25% NDGA significantly suppressed the formation of intestinal metaplasia both in the antrum and the corpus. Although all three lignans dose‐dependently inhibited the in vitro proliferation of H. pylori, there were no differences in the titers of anti‐H. pylori antibodies or the amount of the H. pylori‐specific urease A gene among all H. pylori‐infected groups. These results suggest that NDGA might be effective for prevention of gastric carcinogenesis. The possible mechanisms appear to be related to inhibitory effects on progression of gastritis and antioxidative activity rather than direct antimicrobial influence. (Cancer Sci 2007; 98: 1689–1695)
Lignans, one of the main groups of plant compounds classified as phytoestrogens, are characterized by possession of a diphenolic structure and have attracted interest as possible chemopreventive materials for cancer in recent years.( 1 ) A number of epidemiological, in vitro and animal model studies have provided evidence that naturally occurring plant products, including lignans, are effective for cancer prevention in parts of the body such as the breast, colon and prostate gland.( 1 , 2 , 3 ) The mechanisms of the anti‐carcinogenic effects of plant lignans may involve the hormonal influence on the estrogen‐mediated carcinogenic pathway, antioxidative activity to scavenge free radicals and block generation of carcinogenic precursors, and/or anti‐proliferative/pro‐apoptotic effects.( 1 )
Nordihydroguaiaretic acid (NDGA) is a plant lignan derived from the creosote bush (Larrea tridentata DC Coville, Zygophyllaceae), a common shrub of North America and traditionally used in folk medicine.( 4 ) NDGA is a potent antioxidant and has been used as an additive to preserve foods and oils. Several studies have demonstrated that NDGA can prevent tumor cell growth in vitro and inhibit in vivo carcinogenesis in the skin, bladder and colon.( 5 , 6 , 7 ) In addition to its antioxidative effects, NDGA has been shown to inhibit the activity of lipoxygenase, which is an important enzyme in the arachidonic acid cascade along with cyclooxygenase.( 8 ) While cyclooxygenase‐2 inhibitors have been reported to exert suppressive effects on gastric carcinogenesis in rodents,( 9 , 10 ) the influence of lipoxygenase inhibitors in animal models remains unclear. Arctiin and arctigenin are generally derived from Arctium and Artemisia species (Compositae) and possess similar structures. Several studies have indicated that they may exhibit inhibitory effects in vitro or in vivo on skin, pancreas and lung carcinogenesis.( 11 , 12 , 13 )
Helicobacter pylori is a major causative factor for gastric disorders and epidemiological evidence has accumulated that indicates a significant relationship with chronic active gastritis, peptic ulcers, atrophic gastritis, intestinal metaplasia, and lymphoma or cancer development.( 14 , 15 ) In 1994, the World Health Organization/International Agency for Research on Cancer concluded that H. pylori is a ‘definite carcinogen’ based on the epidemiological findings.( 16 ) Triple therapy with a proton pump inhibitor and two antimicrobials, amoxicillin and clarithromycin, is usually recommended as the general therapy for H. pylori eradication.( 17 ) However, considering the occurrence of strains resistant to these antimicrobial drugs and the persistence of gastric inflammation even after eradication of H. pylori, the search for new agents for alternative therapies continues to be very important.( 18 ) The major determining factor of stomach carcinogenesis is the severity of H. pylori‐induced gastritis. It has been suggested that oxidative stress associated with inflammation plays an important role in gastric carcinogenesis as a mediator of DNA damage and carcinogenic compound formation.( 19 ) Therefore, prevention of H. pylori‐induced gastritis and oxidative stress is a possible approach by which to inhibit gastric carcinogenesis.
Mongolian gerbils can readily be infected with H. pylori, and the resultant chronic active gastritis, peptic ulcers, intestinal metaplasia, and gastric cancer resemble the lesions that are apparent in humans.( 20 , 21 ) The authors have previously demonstrated that a fruit‐juice concentrate of Japanese apricot has suppressive effects on H. pylori‐induced gastritis in the gerbil model.( 22 ) Several natural products, such as turmeric, garlic and green tea extract, have been also found to inhibit H. pylori‐associated gastric disorders.( 23 , 24 , 25 ) Therefore, in the present study, the effects of three plant lignans, arctiin, arctigenin, and NDGA, on H. pylori proliferation in vitro and H. pylori‐associated gastric carcinogenesis were investigated in Mongolian gerbils.
Materials and Methods
Lignans. NDGA was purchased from Cayman Chemicals (Ann Arbor, MI, USA; Fig. 1). Arctiin was kindly donated from Alps Pharmaceutical Ind. Co. Ltd. (Gifu, Japan), and arctigenin was obtained by acid hydrolysis of arctiin at Yomeishu Seizo, Co. Ltd. (Nagano, Japan; Fig. 1). Identification of isolated arctigenin was performed using high‐performance liquid chromatography (HPLC), infrared spectrometry, 1H/13C nuclear magnetic resonance analysis and thin‐layer chromatography and the purity was determined to be more than 98% using HPLC. The lignans were all prepared as 100 mM solutions in dimethyl sulfoxide (DMSO) immediately before use for in vitro experimentation. NDGA and arctigenin were pelleted into AIN93G diet (CLEA Japan, Tokyo, Japan) for the in vivo carcinogenesis experiment at the following concentrations: NDGA, 0.25%, 0.05%, and 0.01%; arctigenin, 0.1%.
Figure 1.
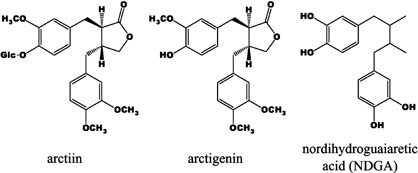
Chemical structures of the plant lignans used in the present study. Arctiin is a glycosidic form of arctigenin, a dibenzylbutyrolactone lignan. Nordihydroguaiaretic acid (NDGA) is a member of the dibenzylbutane lignans. Glc, glucose.
Bacterial culture. H. pylori were prepared using the same method as described previously.( 26 ) Briefly, H. pylori strain ATCC43504 (American Type Culture Collection, Rockville, MD, USA) was inoculated on Brucella agar (Merck, Darmstadt, Germany) plates containing 7% v/v heat‐inactivated fetal calf serum (FCS) and incubated at 37°C under microaerobic conditions using an Anaero Pack Campylo (Mitsubishi Gas Chemical Co. Inc. Tokyo, Japan) at high humidity. Two days later, the bacteria grown on the plates were introduced into Brucella broth (Becton Dickinson, Cockeysville, MD, USA) supplemented with 7% FCS and incubated under the same conditions for 24 h. The broth cultures of H. pylori were checked under a phase contrast microscope for bacterial shape and mobility.
Colony forming units (c.f.u.) of H. pylori. To assess the influence of lignans on H. pylori proliferation, the c.f.u. were determined for the various concentrations of lignans. H. pylori grown on Brucella agar plates for 2 days were introduced into Brucella broth with 7% FCS containing arctiin, arctigenin, or NDGA (1, 10 and 100 µM) or 0.1% DMSO as the vehicle control and incubated as mentioned above. After 24 h, serial diluted broth cultures were seeded on segregating agar plates for H. pylori (Nissui Pharmaceutical, Tokyo, Japan) and incubated as described above for 5 days. Then, the c.f.u. was determined for each group by counting numbers of colonies.
In vivo carcinogenesis. The experimental design is illustrated in Fig. 2. A total of 178 specific pathogen‐free male, 5‐week‐old Mongolian gerbils (Meriones unguiculatus; MGS/Sea, Kyudo, Fukuoka, Japan) were used in the present study. They were housed in plastic cages on hardwood‐chip bedding in an air‐conditioned biohazard room with a 12‐h light/12‐h dark cycle, and were allowed free access to food and water. The gerbils were divided into eight groups (groups A–H). Animals of groups A–E were inoculated with 1.0 mL of broth culture containing H. pylori (1 × 108 c.f.u./mL) intragastrically and given a chemical carcinogen, N‐methyl‐N‐nitrosourea (MNU; Sigma Chemical, St Louis, MO, USA) in their drinking water at the concentration of 10 p.p.m. for 20 weeks, while gerbils of groups F–H were inoculated with Brucella broth. From weeks 8–52, the animals in groups A and F, B and G, C, and D received AIN93G diet containing 0.1% arctigenin, 0.25% NDGA, 0.05% NDGA, and 0.01% NDGA, respectively. Groups E and H were maintained on normal AIN93G diet. At week 52, all animals were killed under deep anesthesia and had their stomachs resected and blood samples collected from the inferior vena cava. Internal organs, including the liver, spleen, kidney, heart, lung, pancreas and testis of groups F–H were also excised for morphological observation. The experimental design was approved by the Animal Care Committee of the Aichi Cancer Center Research Institute, and the animals were cared for in accordance with institutional guidelines.
Figure 2.

Experimental design for in vivo carcinogenesis. Five‐week‐old male Mongolian gerbils were inoculated with Helicobacter pylori (ATCC43504; groups A–E) or broth (groups F–H). After 2 weeks, animals of groups A–E were administered 10 p.p.m. N‐methyl‐N‐nitrosourea (MNU) in their drinking water for 20 weeks. The animals were given AIN93G diets containing 0.1% arctigenin (groups A and F), 0.25% nordihydroguaiaretic acid (NDGA; groups B and G), 0.05% NDGA (group C) and 0.01% NDGA (group D) from weeks 8–52.
Histological and serological examination. The excised stomachs were fixed in 10% neutral‐buffered formalin and sliced along the longitudinal axis into 4–8 strips of equal width, and embedded in paraffin. Tissue sections were stained with HE. The degree of chronic active gastritis was graded according to criteria modified from the Updated Sydney System,( 27 ) by scoring the infiltration of neutrophils and mononuclear cells, intestinal metaplasia, and heterotopic proliferative glands, on a four‐point scale (0–3: 0, normal; 1, mild; 2, moderate; 3, marked). Blood samples were centrifuged and separated sera were stored at –80°C until use. The titers of anti‐H. pylori antibodies were measured as described earlier.( 28 ) The sera were also centrifuged (10 000 g for 50 min at room temperature) through centrifugal filter devices (Microcon YM‐10; Millipore, Bedford, MA, USA) and used for the measurement of 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG) using enzyme‐linked immunosorbent assay (high‐sensitive 8‐OHdG check; Japan Institute for the Control of Aging, Shizuoka, Japan).( 9 )
Real‐time polymerase chain reaction and relative quantitative analysis. Genomic DNA was extracted from glandular stomach mucosa at the border between the antrum and corpus using a DNeasy tissue kit (Qiagen, Hilden, Germany). For H. pylori quantification, relative quantitative real‐time polymerase chain reaction (PCR) of urease A was performed using a LightCycler system (Roche Diagnostics, Mannheim, Germany) with the gerbil‐specific glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene as an internal control. This was performed basically as described earlier, using QuantiTect SYBR Green PCR (Qiagen) with the optimal Mg2+ concentration at 2.5 mM.( 22 , 29 ) The primer sequences of each marker are listed in Table 1. Specificity of the PCR reaction was confirmed using a melting program provided with the LightCycler software. To further confirm that there was no obvious primer dimer formation or amplification of any extra bands, the samples were electrophoresed in 3% agarose gels and visualized with ethidium bromide after the LightCycler reaction. Relative quantification of the H. pylori urease A gene was performed as previously established using the internal control without the necessity for external standards.( 29 ) The amounts of the H. pylori urease A gene were expressed relative to 100% in the H. pylori‐infected control group (group E).
Table 1.
Primer sequences for relative quantitative real‐time polymerase chain reaction using the LightCycler
| Gene | Sequences | Product length (bp) | EMBL/GenBank/DDBJ Accession no. |
|---|---|---|---|
| GAPDH | 5′‐AACGGCACAGTCAAGGCTGAGAACG‐3′ | 118 | AB040445 |
| 5′‐CAACATACTCGGCACCGGCATCG‐3′ | |||
| Urease A | 5′‐TGTTGGCGACAGACCGGTTCAAATC‐3′ | 120 | M60398 |
| 5′‐GCTGTCCCGCTCGCAATGTCTAAGC‐3′ |
GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase.
Statistical analysis. Fisher's exact test was used to assess the incidence of gastric adenocarcinomas. The Mann–Whitney U‐test was applied to determine the significance of differences in the c.f.u., microscopic scores for gastritis, body weights, serological results, and the amount of H. pylori genomic DNA using urease A locus. P‐values <0.05 were considered to be statistically significant.
Results
Inhibitory effects of lignans on H. pylori proliferation. All three lignans decreased the numbers of H. pylori colonies in a dose‐dependent manner, and the suppressive effects of each lignan were significant at the dose of 100 µM (Fig. 3). Arctigenin showed the highest inhibitory effect of all three lignans, and colony formation was also significantly inhibited at the dose of 10 µM. Inhibition by NDGA was slightly stronger than that by arctiin.
Figure 3.
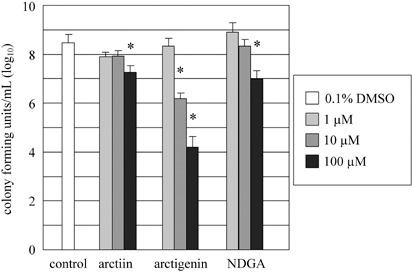
Inhibitory effects of arctiin, arctigenin and nordihydroguaiaretic acid (NDGA) on Helicobacter pylori proliferation, as assessed by counting the numbers of c.f.u. Each value represents the mean ± SD of three independent experiments. *P < 0.05 compared with vehicle control (0.1% dimethyl sulfoxide [DMSO]).
Average body weights, total lignan intake, titers of anti‐H. pylori antibodies and serum 8‐OHdG levels in each experimental group. Data for average body weights at week 52, total lignan intake, titers of anti‐H. pylori antibodies and serum 8‐OHdG levels are summarized in Table 2. The average body weights for 0.25% NDGA‐treated groups (groups B and G) were significantly lower than those of the corresponding control groups (groups E and H, respectively). Total lignan intake by each group essentially corresponded to the proportion of lignan in their food. All H. pylori‐infected groups (groups A–E) demonstrated significantly higher values for anti‐H. pylori antibody titers than the non‐infected groups (groups F–H). There were no significant differences between the H. pylori‐infected groups. Serum 8‐OHdG levels in the 0.1% arctigenin‐treated and H. pylori‐infected group (group A), 0.25% NDGA‐treated and H. pylori‐infected group (group B) and 0.05% NDGA‐treated and H. pylori‐infected group (group C) were significantly lower than that in the H. pylori‐infected control group (group E; P < 0.01).
Table 2.
Summary of general data and incidences of gastric carcinomas in Mongolian gerbils
| Group | Treatment | Effective number | Body weight (g) | Total lignan intake (g) | Anti‐Hp IgG titer (AI) | Serum 8‐OHdG level (ng/mL) | Carcinoma† | ||
|---|---|---|---|---|---|---|---|---|---|
| Differentiated | Undifferentiated | Incidence (%) | |||||||
| A | Hp + MNU + 0.1% Arctigenin | 30 | 91.8 ± 14.0 | 0.942 ± 0.036 | 370.8 ± 35.6 | 0.448 ± 0.086** | 13 | 4 | 17/30 (56.7) |
| B | Hp + MNU + 0.25% NDGA | 33 | 79.0 ± 12.7* | 2.078 ± 0.054 | 306.0 ± 20.0 | 0.467 ± 0.123** | 11 | 2 | 13/33 (39.4)* |
| C | Hp + MNU + 0.05% NDGA | 29 | 90.5 ± 15.2 | 0.513 ± 0.056 | 280.9 ± 18.9 | 0.449 ± 0.064** | 15 | 1 | 16/29 (55.2) |
| D | Hp + MNU + 0.01% NDGA | 28 | 95.2 ± 14.2 | 0.108 ± 0.012 | 258.9 ± 38.9 | 0.583 ± 0.233 | 16 | 1 | 17/28 (60.7) |
| E | Hp + MNU | 29 | 92.0 ± 14.8 | 0 | 278.4 ± 25.5 | 0.590 ± 0.132*** | 17 | 2 | 19/29 (65.5) |
| F | Broth + 0.1% Arctigenin | 7 | 108.5 ± 10.6 | 1.094 ± 0.201 | 2.51 ± 0.81 | n.d. | 0 | 0 | 0/7 (0) |
| G | Broth + 0.25% NDGA | 8 | 94.8 ± 6.70*** | 2.573 ± 0.105 | 1.85 ± 0.19 | n.d. | 0 | 0 | 0/8 (0) |
| H | Broth | 14 | 106.6 ± 7.46 | 0 | 1.54 ± 0.29 | 0.485 ± 0.107 | 0 | 0 | 0/14 (0) |
Classification based on the histopathology. ‘Differentiated’ includes tubular types, whereas ‘undifferentiated’ consists of signet‐ring cell and poorly differentiated types.
P < 0.05 versus group E,
P < 0.01 versus group E,
P < 0.05 versus group H. Values for results are expressed as averages ± SD. 8‐OHdG, 8‐hydroxy‐2′‐deoxyguanosine; AI, arbitrary index; Hp, Helicobacter pylori; MNU, N‐methyl‐N‐nitrosourea; n.d., not determined; NDGA, nordihydroguaiaretic acid.
Incidences of glandular stomach adenocarcinomas. The incidences of gastric adenocarcinomas are summarized in Table 2. The value for group B (39.4%) was significantly lower than that in group E (65.5%, P < 0.05). In contrast, there was no significant difference in the incidence between groups A and E. In groups F–H, no gastric tumors were observed. Both differentiated and undifferentiated adenocarcinomas were found in groups A–E (Fig. 4). All of the glandular stomach adenocarcinomas generated in the present study developed in the pyloric gland area. No macroscopic lesions were observed in liver, spleen, kidney, heart, lung, pancreas and testis of all groups. In the histological examination for groups F–H, no pathological findings were recognized in the internal organs except for a renal hemangioma in 0.25% NDGA‐treated group (group G).
Figure 4.
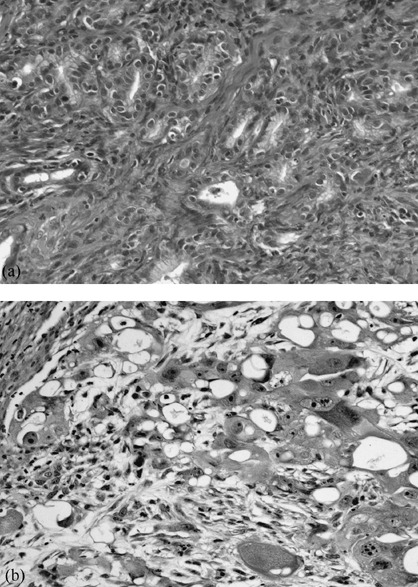
Histology of gastric adenocarcinomas. (a) Well differentiated adenocarcinoma and (b) poorly differentiated adenocarcinoma from group E.
Status of gastritis. Data for the status of gastritis in each group are summarized in Table 3. The gastric mucosa of groups A–E was generally thickened and edematous, occasionally with erosions, ulcers, and polypoid lesions. Such macroscopic findings were not recognized in the stomachs of groups F–H. Groups A–E showed significantly higher scores for infiltration of neutrophils and mononuclear cells, intestinal metaplasia, and heterotopic proliferative glands than those of groups F–H. Scores for intestinal metaplasia both of antrum and corpus in group B and that for heterotopic proliferative glands of antrum in group A were significantly lower than those of group E (P < 0.05). There were no significant differences in scores for infiltration of neutrophils and mononuclear cells between lignan‐treated groups (groups A–D) and group E.
Table 3.
Histopathological evaluation of gastritis
| Group | Effective number | Infiltration of neutrophils | Infiltration of mononuclear cells | Intestinal metaplasia | Heterotopic proliferative glands | ||||
|---|---|---|---|---|---|---|---|---|---|
| Antrum | Corpus | Antrum | Corpus | Antrum | Corpus | Antrum | Corpus | ||
| A | 30 | 2.37 ± 0.11 | 2.40 ± 0.11 | 2.97 ± 0.03 | 3.00 ± 0.00 | 1.90 ± 0.15 | 1.87 ± 0.13 | 2.57 ± 0.10* | 2.87 ± 0.06 |
| B | 33 | 2.61 ± 0.12 | 2.45 ± 0.14 | 2.91 ± 0.05 | 2.91 ± 0.05 | 1.40 ± 0.14* | 1.12 ± 0.09* | 2.73 ± 0.08 | 2.70 ± 0.08 |
| C | 29 | 2.66 ± 0.10 | 2.79 ± 0.09 | 3.00 ± 0.00 | 3.00 ± 0.00 | 2.00 ± 0.16 | 1.62 ± 0.13 | 2.90 ± 0.06 | 2.86 ± 0.07 |
| D | 28 | 2.43 ± 0.11 | 2.71 ± 0.10 | 2.93 ± 0.07 | 2.93 ± 0.05 | 1.54 ± 0.14 | 1.61 ± 0.15 | 2.79 ± 0.09 | 2.79 ± 0.08 |
| E | 29 | 2.38 ± 0.15 | 2.48 ± 0.15 | 3.00 ± 0.00 | 3.00 ± 0.00 | 1.97 ± 0.14 | 1.79 ± 0.13 | 2.90 ± 0.06 | 2.83 ± 0.07 |
| F | 7 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| G | 8 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| H | 14 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
P < 0.05 versus group E. Values for results are expressed as averages ± SD.
Quantification of H. pylori. Average relative urease A gene levels in the glandular stomachs in each group are shown in Fig. 5. There were no significant differences in the amount of H. pylori genomic DNA levels at the urease A gene locus among groups A–E. No amplification of the urease A gene was detected in groups F–H.
Figure 5.
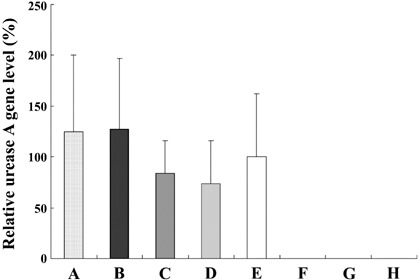
Quantitation of the Helicobacter pylori using DNA specific for urease A in glandular stomachs of Mongolian gerbils. The value was set at 100% in group E and data are means ± SE.
Discussion
In the present study, it was demonstrated that arctigenin and NDGA exert inhibitory effects on H. pylori proliferation in vitro, and NDGA was found to decrease the incidence of H. pylori‐associated gastric adenocarcinomas in Mongolian gerbils. In in vitro culture, all three lignans reduced the c.f.u. of H. pylori in a dose‐dependent manner, with arctigenin being the most effective. Arctiin is a glycosidic form of arctigenin and its relatively low effect might be explained by low permeability due to the conjugated glucose. The present result is consistent with previous reports that arctigenin has stronger suppressive effects than arctiin on heat shock responses in mammalian cells and 4‐nitroquinoline‐N‐oxide/glycerol‐induced mouse pulmonary tumors.( 11 , 30 ) The weak suppressive effect of arctiin at the highest concentration might reflect spontaneous hydrolysis and conversion to arctigenin rather than actual influence of the glycosidic form.
Based on the results of inhibitory effects in vitro, the chemopreventive effects of NDGA and arctigenin on H. pylori‐associated gastric carcinogenesis in Mongolian gerbils were investigated, and it was found that administration of a 0.25% NDGA diet significantly decreased the incidence of gastric adenocarcinomas at week 52. NDGA concentrations in the non‐toxic range of ≤0.25% were chosen because a previous study in rats demonstrated no significant toxicity at 0.5% and 1.0% NDGA given for 2 years.( 31 ) We also set a dose of 0.1% for arctigenin, based on the amount in the dried seed of Arctium lappa (0.05–0.6%), which is traditionally used as a folk medicine.( 13 , 30 ) To the authors’ knowledge, this is the first demonstration that NDGA can prevent stomach carcinogenesis. Although the basic mechanisms for the inhibitory effects of NDGA remain unclear, the present results are in line with previous epidemiological studies suggesting that antioxidants reduce the risk of gastric cancer.( 32 , 33 )
NDGA has a long history as an antioxidant to preserve foods and oils and is known to be a potent scavenger of peroxynitrite, singlet oxygen, hydroxyl radicals, and hypochlorous acid.( 34 ) H. pylori infection has been demonstrated to cause production of reactive oxygen species in human gastric epithelial cells, and antioxidative supplements such as vitamin C or E lead to protective effects on H. pylori‐induced gastric lesions.( 35 , 36 ) Several studies have pointed to the suppressive effects of NDGA on in vivo carcinogenesis in the skin, urinary bladder, kidney, and colon in rodent models.( 4 , 5 , 6 , 37 ) In these studies, the antioxidative ability of NDGA was thought to be responsible for the cancer preventive effect. In the present case, although both NDGA and arctigenin reduced the serum 8‐OHdG levels, a marker of oxidative stress, only 0.25% NDGA‐treatment exhibited a preventive effect on gastric cancer development in Mongolian gerbils. In contrast, 0.25% NDGA significantly inhibited development of intestinal metaplasia both in the antrum and corpus, which has been extensively studied as a pre‐neoplastic lesion in the human stomach, and is strongly associated with gastric cancer development.( 38 ) The multistep morphogenesis from H. pylori infection to gastric cancer development includes sequential stages of chronic atrophic gastritis, intestinal metaplasia and focal dysplasia.( 39 , 40 ) Therefore, the present results indicate that the inhibitory mechanism of NDGA against gastric carcinogenesis in gerbils might be associated not only with antioxidative activity, but also with inhibitory effects on the progression of gastritis.
NDGA is a well‐known inhibitor of lipoxygenase that converts arachidonic acid and other polyunsaturated fatty acids into biologically active molecules, including leukotriene and hydroxylated arachidonic acid derivatives, associated with inflammatory responses and carcinogenesis. These arachidonic acid metabolites have been identified as mediators of tumor development and progression in various organs.( 8 ) Therefore, lipoxygenase has been proposed as a putative target for cancer chemoprevention.( 41 ) A previous study has suggested that H. pylori‐induced gastritis is associated with an increased capacity to generate leukotriene.( 42 ) In addition, Park et al. recently reported that H. pylori increased the biosynthesis of leukotriene by the 5‐lipoxygenase pathway in a gastric epithelial cell line, and that NDGA suppressed this H. pylori‐mediated 5‐lipoxygenase signaling.( 43 ) Thus, one of the underlying mechanisms of NDGA against gastric carcinogenesis might be considered to be an inhibitory effect on progression of H. pylori‐induced gastritis through the lipoxygenase signaling pathway. Further studies are required to clarify the association between progression of H. pylori‐induced gastritis and lipoxygenase‐mediated leukotriene synthesis.
The incidence of gastric cancer in women is approximately half that recorded in men. Recent epidemiological studies have showed that postmenopausal women are at increased risk of gastric cancer, suggesting an inverse association between estrogenic activity and stomach carcinogenesis,( 44 , 45 ) although this hypothesis is still controversial.( 46 ) Furukawa et al. reported that N‐methyl‐N′‐nitro‐N‐nitrosoguanidine‐induced gastric cancer in rats was also predominant in males, and that estrogens reduced the incidence.( 47 ) Plant lignans, generally not estrogenic themselves, are converted to mammalian lignans (enterodiol and enterolactone) that have weak estrogenic activities, through a series of metabolic reactions by the intestinal microflora.( 1 , 48 ) Furthermore, previous studies have demonstrated that NDGA itself can bind to the sex steroid binding protein, as well as estrogen receptors α and β.( 49 , 50 ) These observations indicate a possible hormonal effect of NDGA and/or its derivatives on gastric carcinogenesis, although this remains to be confirmed.
Arctigenin failed to reduce the incidence of gastric adenocarcinomas at the dose used in this study, despite the strong inhibitory effect of H. pylori proliferation in vitro. Serological examination showed that there were no significant differences in the titers of anti‐H. pylori antibodies among all H. pylori‐infected groups. Moreover, relative quantitative analysis for H. pylori using DNA specific for urease A in the gastric mucosa, known to be a highly sensitive and specific marker for the detection and quantification of H. pylori,( 51 , 52 ) also supported this observation. More continuous and/or highly concentrated exposure to NDGA and arctigenin might be necessary to inhibit H. pylori proliferation directly in vivo. It is well established that arctiin is rapidly transformed to arctigenin by intestinal microflora of rat and human, and the arctigenin is then also converted to enterolactone through a stepwise reaction.( 53 , 54 ) Thus, drug‐specific pharmacokinetic differences might account for the lack of effects and it is possible that a higher dietary dose of arctigenin might exhibit anti‐carcinogenic activity against H. pylori‐associated gastric cancer development.
Although NDGA has been used as a food and pharmaceutical preservative for its antioxidative effect, it has been banned in some countries because of reports of toxicity in the liver and kidney with high‐dose use.( 55 , 56 ) The reported LD50 (oral) of NDGA is 0.8–5.5 g/kg body weight in rodents.( 4 ) In the present study, the average body weights of 0.25% NDGA‐treated groups (groups B and G) were significantly lower than those of the control groups (groups E and H, respectively). However, the total food intake of group B (831.4 ± 21.8 g; means ± SD) was also significantly reduced compared with that of group E (1073.7 ± 35.2 g; P < 0.01). Similarly, the total food intake of group G (1029.2 ± 42.1 g) showed a decreasing tendency compared with that of group H (1133.5 ± 39.9 g; P = 0.064). In addition, histological examination for groups F–H revealed no pathological findings in the liver, spleen, kidney, heart, lung, pancreas, and testis, except for a microscopic renal hemangioma in group G, which has been reported as a spontaneous neoplasm in aging Mongolian gerbils.( 57 ) No macroscopic lesions in the internal organs, including kidneys, were observed in any other groups. Therefore, it was considered that NDGA toxicity was relatively low at the dose used in the present study. The body‐weight loss of group B was unlikely to influence the incidence of gastric tumors because previous epidemiological studies have demonstrated that body weight is not associated with risk of non‐cardiac gastric adenocarcinoma.( 58 , 59 )
In conclusion, the present study showed a chemopreventive effect of NDGA on MNU‐initiated and H. pylori‐promoted gastric carcinogenesis in Mongolian gerbils. While NDGA failed to reduce H. pylori proliferation in vivo, H. pylori‐associated intestinal metaplasia was suppressed by NDGA treatment. The results indicate that the anti‐carcinogenic effects of NDGA might be due to inhibition of the progression of gastritis and to antioxidative properties, rather than to direct antimicrobial activity.
Acknowledgments
This study was supported in part by a Grant‐in‐Aid for the Third‐term Comprehensive 10‐year Strategy for Cancer Control, a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare, Japan, and a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
- 1. Webb AL, McCullough ML. Dietary lignans: potential role in cancer prevention. Nutr Cancer 2005; 51: 117–31. [DOI] [PubMed] [Google Scholar]
- 2. Adlercreutz H. Phyto‐oestrogens and cancer. Lancet Oncol 2002; 3: 364–73. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki R, Kohno H, Yasui Y et al . Diet supplemented with citrus unshiu segment membrane suppresses chemically induced colonic preneoplastic lesions and fatty liver in male db/db mice. Int J Cancer 2007; 120: 252–8. [DOI] [PubMed] [Google Scholar]
- 4. Arteaga S, Andrade‐Cetto A, Cardenas R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US‐American deserts and its metabolite nordihydroguaiaretic acid. J Ethnopharmacol 2005; 98: 231–9. [DOI] [PubMed] [Google Scholar]
- 5. Wang ZY, Agarwal R, Zhou ZC, Bickers DR, Mukhtar H. Antimutagenic and antitumorigenic activities of nordihydroguaiaretic acid. Mutat Res 1991; 261: 153–62. [DOI] [PubMed] [Google Scholar]
- 6. Yu A, Hashimura T, Nishio Y, Kanamaru H, Fukuzawa S, Yoshida O. Anti‐promoting effect of nordihydroguaiaretic acid on N‐butyl‐N‐(4‐hydroxybutyl) nitrosamine and sodium saccharin‐induced rat urinary bladder carcinogenesis. Jpn J Cancer Res 1992; 83: 944–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hausott B, Greger H, Marian B. Naturally occurring lignans efficiently induce apoptosis in colorectal tumor cells. J Cancer Res Clin Oncol 2003; 129: 569–76. [DOI] [PubMed] [Google Scholar]
- 8. Ding XZ, Kuszynski CA, El‐Metwally TH, Adrian TE. Lipoxygenase inhibition induced apoptosis, morphological changes, and carbonic anhydrase expression in human pancreatic cancer cells. Biochem Biophys Res Commun 1999; 266: 392–9. [DOI] [PubMed] [Google Scholar]
- 9. Magari H, Shimizu Y, Inada K et al . Inhibitory effect of etodolac, a selective cyclooxygenase‐2 inhibitor, on stomach carcinogenesis in Helicobacter pylori‐infected Mongolian gerbils. Biochem Biophys Res Commun 2005; 334: 606–12. [DOI] [PubMed] [Google Scholar]
- 10. Hu PJ, Yu J, Zeng ZR et al . Chemoprevention of gastric cancer by celecoxib in rats. Gut 2004; 53: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takasaki M, Konoshima T, Komatsu K, Tokuda H, Nishino H. Anti‐tumor‐promoting activity of lignans from the aerial part of Saussurea medusa . Cancer Lett 2000; 158: 53–9. [DOI] [PubMed] [Google Scholar]
- 12. Hirose M, Yamaguchi T, Lin C et al . Effects of arctiin on PhIP‐induced mammary, colon and pancreatic carcinogenesis in female Sprague‐Dawley rats and MeIQx‐induced hepatocarcinogenesis in male F344 rats. Cancer Lett 2000; 155: 79–88. [DOI] [PubMed] [Google Scholar]
- 13. Awale S, Lu J, Kalauni SK et al . Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res 2006; 66: 1751–7. [DOI] [PubMed] [Google Scholar]
- 14. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984; 1: 1311–15. [DOI] [PubMed] [Google Scholar]
- 15. Uemura N, Okamoto S, Yamamoto S et al . Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–9. [DOI] [PubMed] [Google Scholar]
- 16. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Infection with Helicobacter pylori . In: Schistosomes, Liver Flukes and Helicobacter pylori, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 61. Lyon: World Health Organization / International Agency for Research on Cancer, 1994; 177–241. [Google Scholar]
- 17. Malfertheiner P, Megraud F, O'Morain C et al . Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56: 772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerrits MM, Van Vliet AH, Kuipers EJ, Kusters JG. Helicobacter pylori and antimicrobial resistance: molecular mechanisms and clinical implications. Lancet Infect Dis 2006; 6: 699–709. [DOI] [PubMed] [Google Scholar]
- 19. Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori‐induced inflammation and oxidative stress. Free Radic Biol Med 2002; 33: 323–36. [DOI] [PubMed] [Google Scholar]
- 20. Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol 1996; 31 (Suppl 9): 24–8. [PubMed] [Google Scholar]
- 21. Sugiyama A, Maruta F, Ikeno T et al . Helicobacter pylori infection enhances N‐methyl‐N‐nitrosourea‐induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res 1998; 58: 2067–9. [PubMed] [Google Scholar]
- 22. Otsuka T, Tsukamoto T, Tanaka H et al . Suppressive effects of fruit‐juice concentrate of Prunus mume Sieb. et Zucc. (Japanese apricot, Ume) on Helicobacter pylori‐induced glandular stomach lesions in Mongolian gerbils. Asian Pac J Cancer Prev 2005; 6: 337–41. [PubMed] [Google Scholar]
- 23. Matsubara S, Shibata H, Ishikawa F et al . Suppression of Helicobacter pylori‐induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun 2003; 310: 715–19. [DOI] [PubMed] [Google Scholar]
- 24. Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res 2002; 22: 4179–81. [PubMed] [Google Scholar]
- 25. Cellini L, Di Campli E, Masulli M, Di Bartolomeo S, Allocati N. Inhibition of Helicobacter pylori by garlic extract (Allium sativum ). FEMS Immunol Med Microbiol 1996; 13: 273–7. [DOI] [PubMed] [Google Scholar]
- 26. Shimizu N, Inada KI, Tsukamoto T et al . New animal model of glandular stomach carcinogenesis in Mongolian gerbils infected with Helicobacter pylori and treated with a chemical carcinogen. J Gastroenterol 1999; 34 (Suppl 11): 61–6. [PubMed] [Google Scholar]
- 27. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–81. [DOI] [PubMed] [Google Scholar]
- 28. Nozaki K, Shimizu N, Ikehara Y et al . Effect of early eradication on Helicobacter pylori‐related gastric carcinogenesis in Mongolian gerbils. Cancer Sci 2003; 94: 235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsukamoto T, Fukami H, Yamanaka S et al . Hexosaminidase‐altered aberrant crypts, carrying decreased hexosaminidase alpha and beta subunit mRNAs, in colon of 1,2‐dimethylhydrazine‐treated rats. Jpn J Cancer Res 2001; 92: 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishihara K, Yamagishi N, Saito Y, Takasaki M, Konoshima T, Hatayama T. Arctigenin from Fructus Arctii is a novel suppressor of heat shock response in mammalian cells. Cell Stress Chaperones 2006; 11: 154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cranston EM, Jensen MJ, Moren A, Bray T, Bell ET, Breter RN. The acute and chronic toxicity of nordihydroguaiaretic acid. Fed Proc 1947; 6: 318–9. [PubMed] [Google Scholar]
- 32. Waring AJ, Drake IM, Schorah CJ et al . Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: effects of gastritis and oral supplementation. Gut 1996; 38: 171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Correa P, Malcom G, Schmidt B et al . Review article: Antioxidant micronutrients and gastric cancer. Aliment Pharmacol Ther 1998; 12 (Suppl 1): 73–82. [DOI] [PubMed] [Google Scholar]
- 34. Floriano‐Sanchez E, Villanueva C, Medina‐Campos ON et al . Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone‐induced tyrosine nitration in lungs. Free Radic Res 2006; 40: 523–33. [DOI] [PubMed] [Google Scholar]
- 35. Sun YQ, Girgensone I, Leanderson P, Petersson F, Borch K. Effects of antioxidant vitamin supplements on Helicobacter pylori‐induced gastritis in Mongolian gerbils. Helicobacter 2005; 10: 33–42. [DOI] [PubMed] [Google Scholar]
- 36. Correa P, Fontham ET, Bravo JC et al . Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti‐Helicobacter pylori therapy. J Natl Cancer Inst 2000; 92: 1881–8. [DOI] [PubMed] [Google Scholar]
- 37. Ansar S, Iqbal M, Athar M. Nordihydroguairetic acid is a potent inhibitor of ferric‐nitrilotriacetate‐mediated hepatic and renal toxicity, and renal tumour promotion, in mice. Carcinogenesis 1999; 20: 599–606. [DOI] [PubMed] [Google Scholar]
- 38. Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut 1991; 32: 1110–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vauhkonen M, Vauhkonen H, Sipponen P. Pathology and molecular biology of gastric cancer. Best Pract Res Clin Gastroenterol 2006; 20: 651–74. [DOI] [PubMed] [Google Scholar]
- 40. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process – First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52: 6735–40. [PubMed] [Google Scholar]
- 41. Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res 2001; 61: 6307–12. [PubMed] [Google Scholar]
- 42. Ahmed A, Holton J, Vaira D, Smith SK, Hoult JR. Eicosanoid synthesis and Helicobacter pylori associated gastritis: increase in leukotriene C4 generation associated with H. pylori colonization. Prostaglandins 1992; 44: 75–86. [DOI] [PubMed] [Google Scholar]
- 43. Park S, Han SU, Lee KM, Park KH, Cho SW, Hahm KB. 5‐LOX inhibitor modulates the inflammatory responses provoked by Helicobacter pylori infection. Helicobacter 2007; 12: 49–58. [DOI] [PubMed] [Google Scholar]
- 44. La Vecchia C, D’Avanzo B, Franceschi S, Negri E, Parazzini F, Decarli A. Menstrual and reproductive factors and gastric‐cancer risk in women. Int J Cancer 1994; 59: 761–4. [DOI] [PubMed] [Google Scholar]
- 45. Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer 2002; 5: 213–19. [DOI] [PubMed] [Google Scholar]
- 46. Inoue M, Ito LS, Tajima K et al . Height, weight, menstrual and reproductive factors and risk of gastric cancer among Japanese postmenopausal women: analysis by subsite and histologic subtype. Int J Cancer 2002; 97: 833–8. [DOI] [PubMed] [Google Scholar]
- 47. Furukawa H, Iwanaga T, Koyama H, Taniguchi H. Effect of sex hormones on carcinogenesis in the stomachs of rats. Cancer Res 1982; 42: 5181–2. [PubMed] [Google Scholar]
- 48. Kilkkinen A, Pietinen P, Klaukka T, Virtamo J, Korhonen P, Adlercreutz H. Use of oral antimicrobials decreases serum enterolactone concentration. Am J Epidemiol 2002; 155: 472–7. [DOI] [PubMed] [Google Scholar]
- 49. Fujimoto N, Kohta R, Kitamura S, Honda H. Estrogenic activity of an antioxidant, nordihydroguaiaretic acid (NDGA). Life Sci 2004; 74: 1417–25. [DOI] [PubMed] [Google Scholar]
- 50. Martin ME, Haourigui M, Pelissero C, Benassayag C, Nunez EA. Interactions between phytoestrogens and human sex steroid binding protein. Life Sci 1996; 58: 429–36. [DOI] [PubMed] [Google Scholar]
- 51. Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol 1992; 30: 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rokbi B, Seguin D, Guy B et al . Assessment of Helicobacter pylori gene expression within mouse and human gastric mucosae by real‐time reverse transcriptase PCR. Infect Immun 2001; 69: 4759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nose M, Fujimoto T, Takeda T, Nishibe S, Ogihara Y. Structural transformation of lignan compounds in rat gastrointestinal tract. Planta Med 1992; 58: 520–3. [DOI] [PubMed] [Google Scholar]
- 54. Xie LH, Ahn EM, Akao T, Abdel‐Hafez AA, Nakamura N, Hattori M. Transformation of arctiin to estrogenic and antiestrogenic substances by human intestinal bacteria. Chem Pharm Bull (Tokyo) 2003; 51: 378–84. [DOI] [PubMed] [Google Scholar]
- 55. Lambert JD, Zhao D, Meyers RO, Kuester RK, Timmermann BN, Dorr RT. Nordihydroguaiaretic acid: hepatotoxicity and detoxification in the mouse. Toxicon 2002; 40: 1701–8. [DOI] [PubMed] [Google Scholar]
- 56. Evan AP, Gardner KD Jr. Nephron obstruction in nordihydroguaiaretic acid‐induced renal cystic disease. Kidney Int 1979; 15: 7–19. [DOI] [PubMed] [Google Scholar]
- 57. Vincent AL, Ash LR. Further observations on spontaneous neoplasms in the Mongolian gerbil, Meriones unguiculatus. Lab Anim Sci 1978; 28: 297–300. [PubMed] [Google Scholar]
- 58. MacInnis RJ, English DR, Hopper JL, Giles GG. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer 2006; 118: 2628–31. [DOI] [PubMed] [Google Scholar]
- 59. Chow WH, Blot WJ, Vaughan TL et al . Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst 1998; 90: 150–5. [DOI] [PubMed] [Google Scholar]


