Abstract
Growth differentiation factor 15 (GDF15), a transforming growth factor (TGF)‐β superfamily member, has been cloned from a placenta cDNA library as a gene product that has promoted activation of pro‐matrix metalloproteinase (MMP)2 mediated by membrane type (MT)1‐MMP. Expression of MT1‐MMP in HEK293T cells caused cleavage of the GDF15 mature form at N252–M253 to produce a 6‐kDa C‐terminal fragment. Treatment of MCF7 cells with GDF15 induced activation of p53 and enhanced expression of p21, which was abrogated by MT1‐MMP expression. GDF15 mRNA synthesis was also shown to be induced by treatment of cells with GDF15. Treatment of MCF7 cells with GDF15 caused suppression of cell proliferation. However, proliferation of MCF7 cells transfected with the MT1‐MMP gene was not affected by GDF15 treatment, but was suppressed in the presence of the MMP inhibitor BB94. HT1080 cells transfected with the GDF15 gene, which endogenously express MT1‐MMP, synthesize a high‐level GDF15 precursor form and a low‐level mature form, and treatment of cells with BB94 enhanced production of the GDF15 mature form. Consistent with GDF15 production, HT1080 cells transfected with the GDF15 gene proliferated almost equally with control cells, and addition of BB94 effectively suppressed growth of HT1080 cells transfected with the GDF15 gene concomitant with the accumulation of the GDF15 mature form, but not control cells. These results suggest that MT1‐MMP contributes to tumor cell proliferation through the cleavage of GDF15, which down‐regulates cell proliferation by inducing activation of p53 and p21 synthesis. (Cancer Sci 2007; 98: 1330–1335)
Abbreviations:
- APP
amyloid‐β precursor protein
- ATCC
American Type Culture collection
- ConA
Concanavalin A
- DMEM
Dulbecco's modified Eagle's medium
- ECM
extracellular matrix
- FCS
fetal calf serum
- GAPDH
glyceraldehyde‐3‐phosphate dehydrogenase
- GDF15
growth differentiation factor 15
- IL
interleukin
- MIC
macrophage inhibitory cytokine
- MMP
matrix metalloproteinase
- MT
membrane type
- NAG‐1
NSAID activated gene protein‐1
- PCR
polymerase chain reaction
prostate derived factor
- PLAB
placental bone morphogenetic protein
- PTGF‐β
placental transformation growth factor‐β
- PVDF
polyvinylidene fluoride
- SDS
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TCA
trichloroacetic acid
- TGF
transforming growth factor
- TNF
tumor necrosis factor
MMP is a family of Zn2+‐dependent enzymes that are essential for ECM turnover in normal and pathological conditions.( 1 , 2 , 3 ) To date, at least 28 mammalian MMP have been identified by cDNA cloning, and they can be subgrouped into soluble MMP and MT‐MMP.( 4 , 5 ) MMP are overexpressed in various human malignancies and have been considered to be particularly important in the malignant behavior of tumor cells.( 1 , 3 , 5 ) Thus, the level of MMP expression correlates with the invasiveness or malignancy of tumors.( 6 , 7 ) Among the MMP, MT1‐MMP, MMP‐2, MMP‐7 and MMP‐9 have been reported to be most closely associated with tumor invasion and metastasis. Although important in the degradation of the ECM, growing evidence has implicated MMP in specific processing resulting in activation or degradation of cell surface receptors and ligands.( 8 ) The Fas ligand,( 9 ) TNF‐α,( 10 ) the ectodomain of fibroblast growth factor receptor‐1,( 11 ) heparin‐binding epidermal growth factor,( 12 ) and IL‐8,( 13 ) have been reported to be released or activated by MMP. In contrast, MMP cleave and inactivate IL‐1β,( 14 ) the CC chemokine MCP‐3,( 15 ) the CXC chemokines stromal cell‐derived factor‐1α and β,( 16 , 17 ) and KiSS‐1/metastin.( 18 )
GDF15 is a distant member of the TGF‐β cytokine superfamily, along with MIC‐1, PTGF‐β, PLAB, NAG‐1, and PDF.( 19 ) GDF15 behaves as an anti‐tumorigenic and pro‐apoptotic protein in the early stage of tumors.( 20 )
In the present study, the authors demonstrated for the first time that MT1‐MMP cleaves GDF15 between N252–M253, which abrogates GDF15‐induced suppression of tumor cell growth.
Materials and Methods
Materials. DMEM was obtained from Sigma (St Louis, MO, USA). Primers were synthesized by Genest (Kyoto, Japan). A human placenta cDNA library constructed in a pEAK8 expression vector was obtained from EdgeBio System (Gaithersburg, MD, USA). Monoclonal antibodies against the FLAG epitope were purchased from Sigma. A monoclonal antibody against MT1‐MMP hemopexin domain (113‐5B7), recombinant TIMP‐1 and TIMP‐2 proteins were gifts from Daiichi Fine Chemical Co. Ltd. (Takaoka, Japan). A monoclonal biotinylated‐antibody against human GDF15 and recombinant human GDF protein were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Rabbit antibodies against p21 and phosphorylated p53 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Cell Signaling Technology (Denvers, MA, USA), respectively. Monoclonal antibodies against p53 and α‐tubulin were from Calbiochem/EMD Biosciences, Inc. (San Diego, CA, USA) and Sigma, respectively.
Cell culture. Human embryonic kidney HEK293T cells and fibrosarcoma HT1080 cells were obtained from ATCC (Manassas, VA, USA) and cultured in DMEM supplemented with 5% FCS. Human breast cancer MCF‐7 cells were obtained from ATCC and cultured in DMEM supplemented with 10% FCS.
Preparation of MMP‐2 sample. HEK293T cells cultured in 10‐cm diameter dishes were transfected with 10 µg expression plasmid for pro‐MMP‐2 using a calcium‐phosphate method. Culture medium was replaced with serum‐free DMEM at 48 h after transfection. The culture supernatant was harvested after 24 h and used as a pro‐MMP‐2 sample.
Expression cloning. Expression cloning to identify candidate genes whose products interact with MT1‐MMP was carried out using the modified method of Miyamori et al.( 21 ) In brief, plasmid DNA from placenta cDNA library aliquots (100 ng) was co‐transfected with an MT1‐MMP plasmid (30 ng) into HEK293T cells cultured in a 96‐well microplate. At 48 h post‐transfection, cells were incubated with 10‐fold‐diluted pro‐MMP‐2 samples for 1 h, which were then subjected to gelatin zymography. A pool of cDNA, transfection of which promoted pro‐MMP2 processing, was used to transform Escherichia coli. A plasmid DNA from an individual bacterial colony, expression of which promoted activation of pro‐MMP2 by MT1‐MMP, was selected and the nucleotide sequence was determined as described previously.( 21 )
Construction of expression plasmids. The expression plasmid for MT1‐MMP tagged with the FLAG epitope adjacent to the furin cleavage site (MT1‐FLAG) was constructed as described previously.( 21 ) The expression plasmid for GDF15 tagged with the FLAG epitope at the C‐terminus (GDF15‐FLAG) was constructed as follows. A GDF15 cDNA fragment containing XbaI restriction sites in place of the stop codon was generated by PCR using GDF15 cDNA in a pEAK8 plasmid as a template and PCR primers of pEAK8 forward primer (TTCATTCTCAAGCCTCAGACAGTGG) and flanking reverse primer with an extra XbaI site (underlined) starting at nucleotide 949 (TTTCTAGATATGCAGTGGCAGTCTT) of the GDF15 gene (GenBank accession no. NM_004864.1). An amplified fragment was digested with EcoRI and XbaI, and inserted into the EcoRI and XbaI sites of the pEAK8‐FLAG vector as described previously.( 21 )
Western blotting. Cell lysates or proteins precipitated from conditioned medium with 10% TCA were analyzed using western blotting with the indicated antibodies. Goat anti‐mouse or anti‐rabbit IgG antibodies conjugated with Alexa Fluor 680 (Molecular Probes Inc., Eugene, OR, USA) were used as a second antibody. The signal was monitored using a LI‐COR Odyssey IR imaging system (Lincoln, NE, USA). To detect GDF15, conditioned medium samples were separated using 4%/10%/16% Tris‐Tricine SDS‐PAGE.( 22 )
Cell surface biotinylation. Expression plasmids for MT1‐FLAG and GDF15 were cotransfected into HEK293T cells. At 48 h post‐transfection, cell‐surface biotinylation and immunoprecipitation were performed as described previously.( 23 ) The immunoprecipitated materials were separated using 15% SDS‐PAGE, then blotted and detected with IRDyeTM800‐conjugated streptavidin.
Determination of the cleavage site of the GDF15 protein. Expression plasmids for GDF15‐FLAG (25 µg) and MT1‐MMP (75 µg) were cotransfected into HEK293T cells cultured in 10 dishes of 10‐cm diameter. Culture medium was replaced at 48 h after transfection with serum‐free Opti‐MEM (Invitrogen, Carlsbad, CA, USA), and cells were incubated for a further 24 h. Fragments of GDF15‐FLAG in the culture supernatant were purified using a FLAG‐M2 antibody bead column, separated by Tris‐Tricine SDS‐PAGE, and blotted onto PVDF membranes. The N‐terminal amino acid sequence of a 6‐kDa fragment was determined as described previously.( 8 )
Real‐time PCR assay. A total RNA was isolated as described previously.( 6 ) cDNA was synthesized using a QuantiTect Reverse Transfection Kit (Qiagen Inc., Valencia, CA, USA). Real‐time PCR analysis was performed using SYBR Green Realtime PCR master mix (Toyobo Co., Ltd, Osaka, Japan). The GDF15 mRNA level was examined using forward primer (TTAGCCAAAGACTGCCACTG) starting at nucleotide 926 and reverse primer (GAATCGGGTGTCTCAGGAAC) starting at nucleotide 1049 of the GDF15 gene (GenBank accession no. NM_004864.1). Hs_GAPDH_1_SG QuantiTect Primer (Qiagen) was used to monitor the amplification of GAPDH gene transcript as a control.
GDF15‐stable transfectant. HT1080 cells were transfected with the expression plasmid for GDF15‐FLAG, selected under 0.5 µg/mL puromycin, and single clones were isolated as described previously.( 8 )
Cell proliferation assay. MCF7 cells or HT1080 cells (1.0 × 103 cell/well) in 200 µL of culture medium were plated into 96‐well plates. A GDF15 sample of 50 µL was added to the culture with or without BB94 at 24 h, and cells were incubated for a further 24 h. Cell proliferation was assayed using a Cell Counting Kit‐8 according to the manufacturer's instructions (Dojindo Laboratories, Kumamoto, Japan). Statistical analysis was carried out using a one‐tailed t‐test.
Results
Expression cloning. Aliquots of plasmid DNA from the human placenta cDNA library were cotransfected with MT1‐MMP cDNA into 293T cells, and cells were incubated with pro‐MMP‐2. Then, cell lysates were analyzed using gelatin zymography (Fig. 1). Transfection of one pool of cDNA partially stimulated processing of MMP‐2 to the 62‐kDa active form. One clone of 21 clones isolated from this cDNA pool by a second screening stimulated pro‐MMP‐2 activation when co‐transfected with MT1‐MMP cDNA. The size of the cDNA fragment in this plasmid was approximately 1.2 kbp. Homology search analysis of its nucleotide sequence revealed that this cDNA encodes GDF15 (GenBankTM accession no., BC008962.2), which has many synonyms, including MIC‐1, PTGF‐β, PLAB, NAG‐1, and PDF.( 20 )
Figure 1.
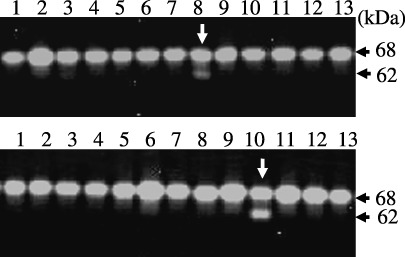
Expression cloning. In the first screening, plasmid DNA aliquots from the human placenta cDNA library were co‐transfected with membrane type (MT)1‐matrix metalloproteinase (MMP) into HEK293T cells cultured in 96‐well microplates. At 48 h post‐transfection, cells were incubated with a pro‐MMP‐2 sample for 1 h, and cell lysates from each well were subjected to gelatin zymography as described under Materials and Methods. Partial results of gelatin zymography are shown. Note that pro‐MMP‐2 activation to generate the 62‐kDa active form was stimulated in lane 8 (upper panel). In the second screening, single clones of plasmid DNA were examined as described above. Note that pro‐MMP‐2 activation was enhanced in lane 10 (lower panel).
GDF15 stabilizes cell‐surface MT1‐MMP. To explore the mechanism of GDF15‐induced stimulation of pro‐MMP‐2 activation by MT1‐MMP, the effect of GDF15 expression on the MT1‐MMP protein level was examined by comparing with that of TIMP‐2 (Fig. 2a). Co‐expression of TIMP‐2 enhanced the expression of the MT1‐MMP active form by 67‐fold, as detected using FLAG M1 antibody, which selectively detects the MT1‐MMP active form. TIMP‐2 expression dramatically reduced the level of the 43‐kDa fragment as detected using the anti‐MT1‐MMP antibody, which is the auto‐degradation product of MT1‐MMP. Co‐expression of GDF15 did not significantly affect the expression of the latent form and the 43‐kDa fragment of MT1‐MMP as detected using the anti‐MT1‐MMP and anti‐FLAG M2 antibodies; however, the level of the MT1‐MMP active form was enhanced by seven‐fold as detected by the FLAG M1 antibody. These results indicate that co‐expression of GDF15 did not affect significantly the expression of the overall MT1‐MMP protein level, but enhanced the level of the MT1‐MMP active form.
Figure 2.
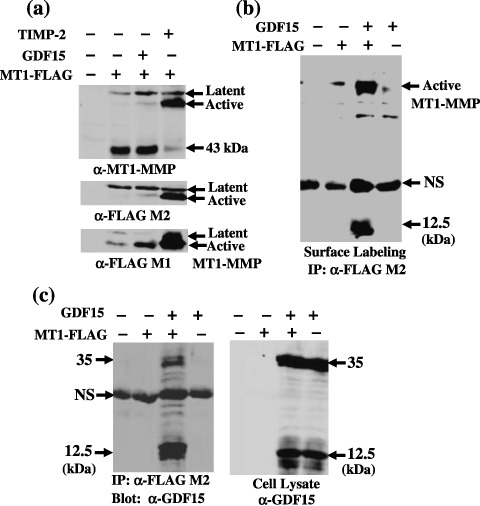
Stabilization of cell‐surface membrane type (MT)1‐matrix metalloproteinase (MMP) by growth differentiation factor 15 (GDF15). MT1‐FLAG or a control plasmid (200 ng) was co‐transfected into HEK293T cells cultured in 12‐well microplates with a control (–), GDF15 or TIMP‐2 (400 ng) plasmid, and cells were incubated for 48 h. (a) cell lysates were examined for total MT1‐FLAG expression using the anti‐MT1‐MMP monoclonal antibody 113‐5B7 (upper panel), FLAG M2 antibody (middle panel), and for the MT1‐FLAG active form using the FLAG M1 antibody (lower panel). (b) transfected cells were labeled with biotin, and immunoprecipitation was performed using anti‐FLAG M2 beads as described in Materials and Methods. Precipitated materials were blotted with IRDyeTM800‐conjugated streptavidin. Note that a 12.5‐kDa material was co‐precipitated with MT1‐FLAG from cells cotransfected with MT1‐FLAG and GDF15 plasmids. (c) MT1‐FLAG was immunoprecipitated from cells transfected with the indicated plasmid using anti‐FLAG M2 antibody beads, and co‐precipitated GDF15 was detected using western blotting with an anti‐GDF15 antibody (left panel). Aliquots of total lysates from transfected cells were examined for GDF15 expression using western blotting with the anti‐GDF15 antibody (right panel). Note that a 12.5‐kDa GDF15 mature form was predominantly co‐precipitated with MT1‐FLAG.
To confirm the enhanced expression of the MT1‐MMP active form by GDF15 that appears on the cell surface, the cell‐surface MT1‐MMP level was examined by cell‐surface biotinylation followed by immunoprecipitation using FLAG M2 antibody beads (Fig. 2b). Consistent with the results of western blotting, the cell‐surface MT1‐MMP active form level was elevated by GDF15 expression. Moreover, a 12.5‐kDa species was co‐precipitated with MT1‐MMP, which is expected to be a mature form of GDF15. Immunoprecipitation from the lysates of cells co‐transfected with MT1‐MMP and GDF15 demonstrated that the GDF15 mature form (12.5 kDa) was preferentially co‐precipitated with the active form of MT1‐MMP, and a minor part of the GDF15 precursor form (35 kDa) was also co‐precipitated (Fig. 2c). These results suggest that GDF15 stabilizes the MT1‐MMP active form by making a complex with it, which in turn enhances MT1‐MMP‐mediated pro‐MMP‐2 activation.
MT1‐MMP cleaves GDF15. Complex formation between the MT1‐MMP active form and the GDF15 mature form led us to examine whether GDF15 serves as a substrate for MT1‐MMP. An expression plasmid for GDF15 was co‐transfected with the MT1‐MMP plasmid, and GDF15 secreted into the culture medium was examined using western blotting (Fig. 3a). Transfection of the GDF15 plasmid alone produced a 35‐kDa precursor form and a 12.5‐kDa mature form. Co‐expression of MT1‐MMP with GDF15 caused a drastic loss in production of the mature form, and addition of BB94 recovered mature form production. These results suggest that the GDF15 mature form serves as a substrate for MT1‐MMP. To confirm the cleavage of GDF15 by MT1‐MMP, an expression plasmid for GDF15‐FLAG was co‐transfected with the MT1‐MMP plasmid. Secreted GDF15 was separated on Tris‐Tricine SDS‐PAGE to detect the small cleavage fragment, and analyzed using an anti‐FLAG M2 antibody (Fig. 3b). Co‐expression of MT1‐MMP with GDF15‐FLAG reduced production of the GDF15‐FLAG mature form, and a 6‐kDa fragment was detected. The addition of BB94 inhibited conversion of the GDF15 mature form to the 6‐kDa fragment. The addition of recombinant TIMP‐2 but not TIMP‐1 suppressed conversion from the 12.5‐kDa to the 6.0‐kDa fragment by the cells expressing MT1‐MMP. Selective inhibition by TIMP‐2 suggests that MT1‐MMP is involved in conversion from the GDF15 mature form to the 6.0‐kDa fragment. The cleavage product of GDF15‐FLAG collected on the FLAG M2 antibody beads column was tested with a peptide sequencer. The N‐terminal sequence of the 6‐kDa fragment was MHAQI, indicating that MT1‐MMP cleaves the N252–M253 peptide bond of GDF15.
Figure 3.
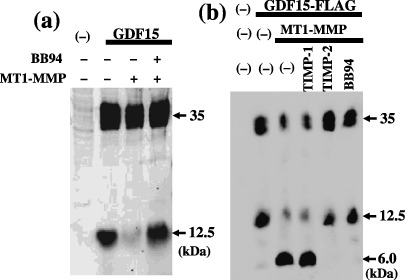
Cleavage of growth differentiation factor 15 (GDF15) by membrane type (MT)1‐matrix metalloproteinase (MMP). (a) expression plasmid for GDF15 (300 ng) was co‐transfected with control or MT1‐MMP plasmid (600 ng) into HEK293T cells plated in 12‐well microplates. At 24 h, the post‐transfection medium was replaced with serum free Opti‐MEM, and cells were incubated for a further 24 h. BB94 (1.0 × 10−7 M) was included in Opti‐MEM in the indicated culture. Conditioned medium proteins precipitated with 10% trichloroacetic acid were separated on 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) and blotted using anti‐GDF15 antibody as described in Materials and Methods. (b) Expression plasmid for GDF15‐FLAG was co‐transfected with control or the MT1‐MMP plasmid, and BB94 (1.0 × 10−7 M), recombinant TIMP‐1 or TIMP‐2 protein (2.0 µg/mL) was included in the indicated culture as described above. The conditioned medium proteins were separated on Tris‐Tricine SDS‐PAGE and blotted using the FLAG M2 antibody.
Effect of MT1‐MMP expression on GDF15‐induced p53 phosphorylation and p21 expression. GDF15 was reported to induce cell growth arrest in MCF‐7 breast cancer cells.( 24 ) GDF15 was added to the culture of MCF7 cells, and p53 phosphorylation at ser15 and p21 expression in these cells was examined (Fig. 4). The addition of recombinant GDF15 to the mock‐transfected cells induced p53 phosphorylation and p21 expression at 3 h, which continued until 9 h. GDF15 was equally effective to the cells transfected with the MT1‐MMP gene at 3 h after the addition of GDF15; however, the effect of GDF15 was lost in MT1‐MMP‐transfected cells at 9 h. Treatment of MT1‐MMP‐transfected cells with BB94 restored induction of p53 phosphorylation and p21 expression at 9 h after the addition of GDF15. The concentration of GDF15 added to the culture was not significantly altered by the incubation with MT1‐MMP‐expressing cells for 3 h, but was reduced by the incubation with MT1‐MMP‐expressing cells for 9 h. Addition of BB94 to the culture of MT1‐MMP‐expressing cells suppressed the reduction of GDF15 concentration. The expression level of MT1‐MMP was constant at 3 h and 9 h after addition of GDF15.
Figure 4.
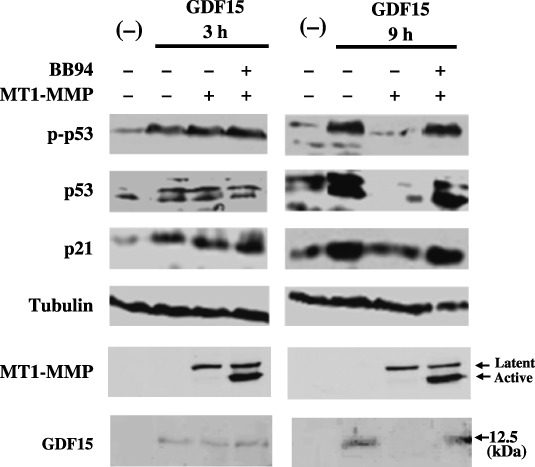
Effect of membrane type (MT)1‐matrix metalloproteinase (MMP) expression on growth differentiation factor 15 (GDF15)‐induced p53 phosphorylation and p21 synthesis. MCF‐7 cells in 12‐well plates were transfected with control or an MT1‐MMP plasmid (1 µg), and were incubated with a recombinant GDF15 sample (25 ng in 1 mL culture) for 3 h or 9 h in the presence or absence of BB94. Cell lysates were analyzed using western blotting for the levels of phosphorylated p53 (panel p‐p53), total p53 protein (panel p53), p21 protein (panel p21), tubulin protein (panel Tubulin) and MT1‐MMP protein (panel MT1‐MMP). GDF15 in the supernatant was also examined using western blotting (panel GDF15).
GDF15 induces GDF15 mRNA synthesis. Treatment of MCF7 cells with GDF15 induced activation of p53. The GDF15 gene has been reported to be one of the downstream target genes of p53,( 25 , 26 ) which led us to examine whether GDF15 mRNA expression is stimulated by treatment of MCF7 cells with GDF15 (Fig. 5). Real‐time PCR analysis showed that treatment of MCF7 cells with GDF15 induced expression of GDF15 mRNA, and induction of GDF15 mRNA expression by GDF15 was quite ineffective in MT1‐MMP‐expressing cells compared with that of control cells.
Figure 5.
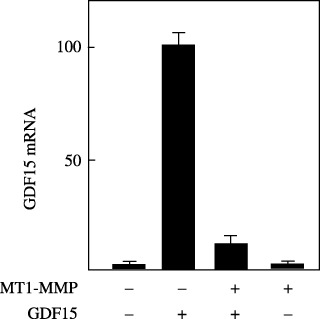
Growth differentiation factor 15 (GDF15)‐induced GDF15 mRNA synthesis. MCF‐7 cells in 35‐mm diameter dishes were transfected with control or an membrane type (MT)1‐matrix metalloproteinase (MMP) plasmid (2 g), and were incubated with or without a recombinant GDF15 protein (25 ng/mL) for 9 h. A total mRNA was extracted, and was subjected to real‐time polymerase chain reaction assay to compare the GDF15 mRNA level as described in Materials and Methods. The highest mRNA level was arbitrarily set to 100%, and the mRNA levels of other cells were adjusted accordingly.
MT1‐MMP abrogates GDF15‐induced cell‐growth suppression. The effect of GDF15 on the proliferation of MCF7 cells was examined (Fig. 6a). The addition of GDF15 to the culture induced growth retardation of MCF7 cells by 34% (P < 0.001). In contrast, the growth of cells transfected with the MT1‐MMP gene was not significantly affected by GDF15 treatment, but GDF15 suppressed proliferation of MT1‐MMP‐transfected cells by 43% (P < 0.001) in the presence of BB94. Next, the effect of GDF15 was examined using HT1080 cells that endogenously express MT1‐MMP. HT1080 cells stably transfected with the GDF15 gene produced a high level of the GDF15 precursor form, but the level of the mature form was low (Fig. 6b). ConA treatment, which enhances the level of MT1‐MMP, further reduced the level of the mature form, and produced a 6‐kDa cleavage fragment. The addition of BB94 to the culture of GDF15‐transfected cells induced accumulation of the GDF15 mature form. Transfection of the GDF15 gene did not significantly affect the growth of HT1080 cells (Fig. 6c). The addition of BB94 did not affect the proliferation of mock‐transfected cells, although it suppressed the growth of the GDF15‐expressing cells by 35% (P < 0.001) concomitant with accumulation of the GDF15 mature form.
Figure 6.
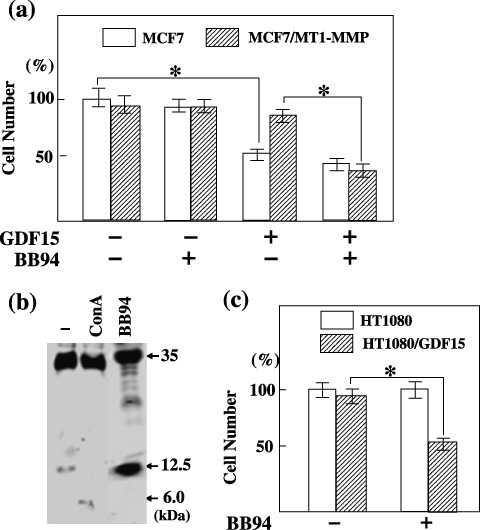
Effect of membrane type (MT)1‐matrix metalloproteinase (MMP) expression on growth differentiation factor 15 (GDF15)‐induced cell‐growth suppression. (a) control or a recombinant GDF15 (25 ng) was added to the 1‐mL culture of MCF7 cells transfected with a control plasmid or MT1‐MMP plasmid with or without BB94 and the cell number was compared after 24 h as described in Materials and Methods. The highest cell number was arbitrarily set to 100%, and the numbers of other cells were adjusted accordingly. Bars indicate mean value. *P < 0.001. (b) HT1080 cells stably transfected with the GDF15‐FLAG plasmid were incubated for 24 h in the presence or absence of BB94 or Concanavalin A (10 g/mL). Conditioned medium proteins were separated on Tris‐Tricine sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted using the FLAG M2 antibody. Note that the level of GDF15 mature form (12.5‐kDa) increased by BB94 treatment. (c) HT1080 cells stably transfected with control plasmid or GDF15‐FLAG plasmid were cultured in the presence or absence of BB94 for 24 h, and the cell number was compared as described above. *P < 0.001.
Discussion
Previously, the authors have identified KiSS‐1/metastin, syndecan‐1, lumican and APP using the expression cloning strategy as molecules that serve as substrates for MT1‐MMP.( 8 , 18 , 23 , 27 ) In the present study, it was found that expression of the GDF15 gene stimulated activation of pro‐MMP‐2 mediated by MT1‐MMP in HEK293T cells. MT1‐MMP was shown to form a stable complex with the GDF15 mature form, resulting in stabilization of MT1‐MMP on the cell surface. This may account for the stimulatory effect of GDF15 on pro‐MMP‐2 activation mediated by MT1‐MMP. Previously, the authors identified syndecan‐1 and APP as substrates, expression of which stimulated MT1‐MMP‐mediated pro‐MMP‐2 processing. Here, it was also shown that GDF15 is cleaved at the N252–M253 peptide bond by MT1‐MMP. Because auto‐degradation is one of the most common regulatory steps in MT1‐MMP activity, complex formation with a substrate may interfere with auto‐degradation and consequently augment the activity against favorable substrate pro‐MMP‐2.
GDF15 is a secretory protein sharing 25% overall sequence identity with TGF‐β superfamily members, but has their main characters as a signal peptide, a consensus RXXRA/S cleavage signal for processing to mature form, and seven conserved cysteine residues in the carboxyl terminal.( 28 )
GDF15 has been reported to induce cell growth arrest or apoptosis in MCF‐7 breast cancer cells,( 24 ) PC‐3 human prostate carcinoma and xenografted tumors,( 29 ) as well as Du‐145 prostate carcinoma through the TGF‐β signaling pathway.( 28 , 30 ) It has also mediated TPA‐induced apoptosis in LNCaP prostate cancer cells.( 31 ) Treatment of HEK293 cells with TGF‐α1 was reported to activate p53 by stimulating its phosphorylation at Ser15, which leads to apoptosis.( 32 ) In the present study, the authors demonstrated that treatment of MCF7 cells with GDF15 induced phosphorylation of p53 and expression of p21 concomitant with cell growth retardation, which are abrogated by MT1‐MMP expression. The biological functions of p53 depend on its downstream target genes, such as those for growth arrest that are mediated by its transactivation of p21 for G1 arrest,( 33 ) and which may be induced in the absence of Smad signaling on treatment with GDF15.( 26 ) Furthermore, GDF15 is the downstream target gene of p53,( 25 , 26 ) so GDF15 may induce further GDF15 mRNA synthesis. Indeed, treatment of MCF7 cells with GDF15 has been shown to induce GDF15 mRNA synthesis concomitant with p53 phosphorylation. Here, it was demonstrated that GDF15 induces phosphorylation of p53 and increases p21 as markers of cell growth arrest, and also that cleavage of GDF15 by MT1‐MMP abrogates the GDF15‐mediated growth arrest of these cells. Thus, HT1080 cells, which endogenously express MT1‐MMP, are not sensitive to GDF15, while the addition of BB94 with GDF15 induces effective growth suppression of HT1080 cells. The authors propose that combined use of an MMP inhibitor would enhance the potent cytostatic effect of GDF15.
Acknowledgments
This work was supported in part by a Grant in Aid for Scientific Research on Priority Areas (18013023) from the Ministry of Education, Culture, Sports, Sciences and Technology.
References
- 1. Woessner JFJ. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991; 5: 2145–54. [PubMed] [Google Scholar]
- 2. Birkedal HH, Moore WG, Bodden MK et al . Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993; 4: 197–250. [DOI] [PubMed] [Google Scholar]
- 3. Stetler‐Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993; 9: 541–73. [DOI] [PubMed] [Google Scholar]
- 4. Nagase H, Woessner JFJ. Matrix metalloproteinases. J Biol Chem 1999; 274: 21 491–4. [DOI] [PubMed] [Google Scholar]
- 5. Seiki M. Membrane‐type matrix metalloproteinases. APMIS 1999; 107: 137–43. [DOI] [PubMed] [Google Scholar]
- 6. Nomura H, Sato H, Seiki M et al . Expression of membrane‐type matrix metalloproteinase in human gastric carcinomas. Cancer Res 1995; 55: 3263–6. [PubMed] [Google Scholar]
- 7. Ueno H, Nakamura H, Inoue M et al . Expression and tissue localization of membrane‐type 1, 2 and 3 matrix metalloproteinases in human invasive breast carcinoma. Cancer Res 1997; 57: 2055–60. [PubMed] [Google Scholar]
- 8. Li Y, Aoki T, Mori Y et al . Cleavage of lumican by membrane‐type matrix metalloproteinase‐1 abrogates this proteoglycan‐mediated suppression of tumor cell colony formation in soft agar. Cancer Res 2004; 64: 7058–64. [DOI] [PubMed] [Google Scholar]
- 9. Powell WC, Fingleton B, Wilson CL et al . The metalloproteinase matrilysin proteotically generates active soluble form Fas ligand and potentiates epithelial cell apoptosis. Curr Biol 1999; 9: 1441–7. [DOI] [PubMed] [Google Scholar]
- 10. Gearing AJ, Beckett P, Christodoulou M et al . Processing of tumor necrosis factor‐alpha precursor by metalloproteinases. Nature (Lond) 1994; 370: 555–7. [DOI] [PubMed] [Google Scholar]
- 11. Levi E, Fridman R, Miao HQ, Ma YS et al . Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA 1996; 93: 7069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki M, Raab G, Moses MA et al . Matrix metalloproteinase‐3 releases active heparin‐binding EGF‐like growth factor by cleavage at specific juxtramembrane site. J Biol Chem 1997; 272: 31 730–7. [DOI] [PubMed] [Google Scholar]
- 13. Van den Steen PE, Proost P, Wuyrs A et al . Neutrophil gelatinase B potentiatesinterleukin‐8 tenfold by aminoterminal processing, whereas it degrades CTAP‐III, PF‐4, and GRO‐ alpha and leaves RANTES and MCP‐2 inactive. Blood 2000; 96: 2673–81. [PubMed] [Google Scholar]
- 14. Ito A, Mukaiyama A, Itoh Y, Nagase H et al . Degradation of interleukin 1 beta by matrix metalloproteinases. J Biol Chem 1996; 271: 14 657–60. [DOI] [PubMed] [Google Scholar]
- 15. McQuibban GA, Gong JH, Tam E et al . Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein‐3. Science (Wash DC) 2000; 289: 1202–6. [DOI] [PubMed] [Google Scholar]
- 16. McQuibban GA, Butler GS, Gong JH et al . Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell‐derived factor‐1. J Biol Chem 2001; 276: 43 503–8. [DOI] [PubMed] [Google Scholar]
- 17. McQuibban GA, Gong JH, Wong JP et al . Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti‐inflammatory properties in vivo. Blood 2002; 100: 1160–7. [PubMed] [Google Scholar]
- 18. Takino T, Koshikawa N, Miyamori H et al . Cleavage of metastasis suppressor gene product KiSS‐1 protein/metastin by matrix metalloproteinases. Oncogene 2003; 22: 4617–26. [DOI] [PubMed] [Google Scholar]
- 19. Eling TE, Baek SJ, Shim M et al . NSAID activated gene (NAG‐1), a modulator of tumorigenesis. J Biochem Mol Biol 2006; 39: 649–55. [DOI] [PubMed] [Google Scholar]
- 20. Bauskin AR, Brown DA, Kuffner T et al . Role of macrophage inhibitory cytokine‐1 in tumorigenesis and diagnosis of cancer. Cancer Res 2006; 66: 4983–6. [DOI] [PubMed] [Google Scholar]
- 21. Miyamori H, Takino T, Kobayashi Y et al . Claudin promotes activation of pro‐matrix metalloproteinase‐2 mediated by membrane type matrix metalloproteinases. J Biol Chem 2001; 276: 28 204–11. [DOI] [PubMed] [Google Scholar]
- 22. Schagger H, Von Jagow G. Tricine‐sodium dodecyl sulfate‐polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 1987; 166: 368–79. [DOI] [PubMed] [Google Scholar]
- 23. Ahmad M, Takino T, Miyamori H et al . Cleavage of amyloid‐β precursor protein (APP) by membrane‐type matrix metalloproteinases. J Biochem 2006; 139: 517–26. [DOI] [PubMed] [Google Scholar]
- 24. Li P, Wong J, Ayed A et al . Placental transforming growth factor‐β is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem 2000; 275: 20 127–35. [DOI] [PubMed] [Google Scholar]
- 25. Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non‐steroidal anti‐inflammatory drug activated gene (NAG‐1) by increasing the expression of p53. Carcinogenesis 2002; 23: 425–32. [DOI] [PubMed] [Google Scholar]
- 26. Osada M, Park HL, Park MJ et al . A p53‐type response element in GDF15 promoter confers high specificity for p53 activation. Biochem Biophysic Res Commun 2007; 354: 913–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Endo K, Takino T, Miyamori H et al . Cleavage of syndecan‐1 by membrane type matrix metalloproteinase‐1 stimulates cell migration. J Biol Chem 2003; 278: 40 764–70. [DOI] [PubMed] [Google Scholar]
- 28. Tan M, Wang Y, Guan K et al . PTGF‐β, a type β transforming growth factor (TGF‐β) superfamily member, is a p53 target gene that inhibit tumor cell growth via TGF‐β signaling pathway. Proc Natl Acad Sci USA 2000; 97: 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambert JR, Kelly JA, Shim M et al . Prostate derived factor in human prostate cancer cells: gene induction by vitamin D via a p53‐dependent mechanism and inhibition of prostate cancer cell growth. J Cell Physiol 2006; 208: 566–74. [DOI] [PubMed] [Google Scholar]
- 30. Liu T, Bauskin AR, Zaunders J et al . Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res 2003; 63: 5034–40. [PubMed] [Google Scholar]
- 31. Shim M, Eling TE. Protein kinase C‐dependent regulation of NAG‐1/placental bone morphogenic protein/MIC‐1 expression in LNCap prostate carcinoma cells. J Biol Chem 2005; 280: 18 636–42. [DOI] [PubMed] [Google Scholar]
- 32. Zhang S, Ekman M, Thakur N et al . TGFα1‐induced activation of ATM and p53 mediates apoptosis in a Smad7‐dependent manner. Cell Cycle 2006; 5: 2787–95. [DOI] [PubMed] [Google Scholar]
- 33. El‐Deiry WS, Tokino T, Velculescu VE et al . WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817–25. [DOI] [PubMed] [Google Scholar]


