Abstract
Prognostic factors in patients with hepatocellular carcinoma (HCC) with tumor thrombosis are not well established, especially for those given external‐beam radiation therapy (EBRT). Patients (n = 136) with HCC who had portal vein (PV) or inferior vena cava (IVC) tumor thrombus received EBRT between January 1998 and October 2007. Demographic variables, laboratory values, tumor characteristics, and treatment modalities were determined at diagnosis and before EBRT. The total radiation dose ranged from 30 to 60 Gy (median, 50 Gy) and was focused on the tumor thrombi. Predictors of survival were identified using the univariate and multivariate analysis. Of the 136 patients, the tumor thrombus completely disappeared in 41 patients (30.1%), 36 patients (26.5%) had a partial response, 49 patients (36%) had stable disease, and 10 patients (7.4%) had progressive disease. On multivariate analysis, pretreatment unfavorable predictors were associated with lower albumin, higher γ‐glutamyltransferase and α‐fetoprotein levels, poorer Child–Pugh classification, intrahepatic multifocality, lymph node metastases, poorer response to EBRT, and 2‐dimension EBRT technique. Survival rates at 1, 2, and 3 years were 31.8%, 17.5%, and 8.8% for patients with PV tumor thrombi; 66.3%, 21.1%, and 15.8% for IVC tumor thrombi; and 25%, 8.3%, and 0% for PV plus IVC tumor thrombi, respectively. Overall median survival was 9.7 months. This study provides detailed information about the survival outcomes and prognostic factors of HCC with tumor thrombi in a relatively large cohort of patients treated with radiation, and the results will help in understanding the potential factors that influence survival for patients with HCC after EBRT. (Cancer Sci 2008; 99: 2510–2517)
Hepatocellular carcinoma (HCC) is one of the most common malignancies, with an increasing incidence in both Eastern and Western countries.( 1 , 2 ) The presence of portal vein (PV) or inferior vena cava (IVC) tumor thrombi is higher in patients with HCC and has been reported in as many as 44–84% of these patients in autopsy data( 3 ) and between 31.4% and 50%( 4 , 5 ) in clinical data. Despite the marked progress in diagnostic techniques and therapeutic procedures, the prognosis for these patients remains discouraging, especially for those with PV tumor thrombi. Most of these patients have poorer treatment outcomes, with a median survival of only 2.4–3.5 months without treatment.( 4 , 6 , 7 , 8 , 9 , 10 ) If patients with PV or IVC thrombi receive systemic chemotherapy, the median survival time ranges from 3.9 to 9.2 months.( 7 , 9 )
Transcatheter arterial chemoembolization (TACE) has also been attempted in patients with major PV invasion, and the median survival time in these patients is 10–12 months.( 10 ) Patients with PV tumor thrombosis were treated with hepatectomy and preoperative TACE likely increased survival.( 11 ) Some investigators have reported the median survival time was only 0.74 years for those with tumor thrombosis in the first branch or trunk of PV.( 12 ) However, neither TACE nor surgical resection is indicated for HCC with PV trunk occlusion by tumor thrombi because of a lack of efficacy and possible complications. The only choice is external‐beam radiation therapy (EBRT) in such cases. EBRT is effective not only for tumor responses but also for survival in patients with HCC with tumor thrombi and such treatment has been used frequently.( 13 , 14 , 15 , 16 ) The prognostic factors for patients with HCC with tumor thrombosis are not well established, especially for those receiving EBRT. To clarify prognostic factors involved in PV or IVC tumor thrombosis, we retrospectively studied the efficacy of EBRT and investigated the pretreatment predictive factors of demographics, laboratory values, tumor characteristics, and treatment modalities in a relative large number of patients with PV or IVC tumor thrombi.
Methods
Patients and diagnosis. Between January 1998 and October 2007, 136 patients with HCC with PV or IVC tumor thrombus received EBRT at Zhongshan Hospital, Fudan University (Shanghai, China). During this period, 5171 patients with HCC were hospitalized in the Surgical Department and 773 patients in the Internal Department of Liver Cancer Institute, Zhongshan Hospital. It is difficult to estimate the exact number of patients with PV or IVC tumor thrombi because these patients are rarely hospitalized for treatments; no treatment choices are readily available in such cases and most of the tumor thrombi appear during the follow‐up periods (not only at the initiation of therapy).
Among 136 patients who received treatments combined with EBRT, 32 underwent surgical resection with or without TACE for their intrahepatic primary tumors, 90 received TACE, and 14 received no additional treatment. Whether patients received EBRT was a matter of physician preference due to the extent of the tumor, at the discretion of the attending surgeon, and, ultimately, with the consent of the patients. It is difficult to define the indication for EBRT, but in patients with Child–Pugh C classification of liver function, EBRT is contraindicated.
Of the 136 patients who received EBRT, the diagnosis of HCC was confirmed by histologic testing (biopsy or surgical specimen) in 32 (23.5%) patients, by a typical clinical presentation and serum α‐fetoprotein (AFP) concentration >400 µg/L in 46 (33.8%) patients, and by unequivocal clinical and radiologic findings in 58 (42.6%) patients whose AFP level was ≤400 µg/L. A variety of the modalities available for imaging HCC,( 17 , 18 , 19 ) such as computed tomography (CT) scanning, magnetic resonance imaging (MRI), ultrasonography, and hepatic angiography, were used in this study.
The diagnosis of HCC was made based on the guidelines proposed by the Chinese Liver Cancer Association in 1999.( 20 ) Using these criteria, a patient is considered to have HCC if the AFP level is >400 µg/L. Active liver disease, embryonal malignant teratoblastomas of testes and ovary, or other malignant tumors metastasizing to the liver should be ruled out, and the tumor should have a characteristic appearance on one of the HCC imaging modalities listed above. If the AFP level is ≤400 µg/L, the characteristic intrahepatic lesion should be confirmed by two of the HCC imaging methods listed above. The status of carcinoembryonic antigen or carbohydrate antigen 19‐9 should be negative for patients with negative AFP levels to exclude those with metastatic tumors from the digestive system or intrahepatic cholangiocarcinoma. Depending on the results of CT scanning or MRI, the pattern of tumor thrombi was categorized as PV or IVC/atrium thrombosis. PV thrombosis was sub‐grouped as branches and main trunk according to the main PV bifurcation.( 13 ) The PV branches are composed of both left and right branches, which bifurcate into the right anterior and right posterior branches (secondary branches). If thrombi were located in both branches and the main trunk, we categorized the thrombi as main trunk because of the retrograde invasion of PV thrombosis. Synchronous tumor thrombi involvement was defined as a diagnostic interval between intrahepatic HCC and the presence of tumor thrombi of not longer than 2 weeks. A tumor thrombus presents as highly echoic on ultrasonography or as hypoattenuation on a CT scan. MRI was mandatory for patients whose thrombus pattern could not be identified. Generally, intravascular tumor of thrombi are slightly hyperintense on SE (spin‐echo sequence) T1‐weighted images compared with signal‐void perfused vessels, and are hyperintense or isointense on FSE (fast spin‐echo squence) T2. Gd‐DTPA‐enhanced T1‐weighted images (gradute recall echo sequence) in the arterial phase usually show moderate to marked enhancement. The Karnofsky performance status score was >90 in all patients.
Therapies. Each patient provided written or oral informed consent regarding the treatment course. Patients received limited‐field EBRT using a linear accelerator with 6‐ or 15‐MV photons, depending on tumor location and depth, in the Department of Radiation Oncology, Zhongshan Hospital, Fudan University. Of the 136 patients who received EBRT, 21 received EBRT as initial therapy, 32 had been treated with resection for their intrahepatic tumors before receiving EBRT, and 90 completed TACE before or after undergoing EBRT. EBRT did not always follow TACE. The combined treatment sequence between TACE and EBRT was based on the status of PV trunk occlusion. If the PV trunk was completely occluded, we treated the patients with EBRT before TACE. The median interval between the presence of tumor thrombi and EBRT was 0.7 months (range, 0–1.8 months).
For 69 patients before June 2005, a CT scan of the abdomen was used for traditional (two‐dimensional) designs and delivery of EBRT.( 13 ) After July 2005, three‐dimensional conformal radiation therapy was used in 67 patients. The principle of designing radiation therapy fields has been previously reported.( 21 ) For EBRT planning, the patients underwent CT in the supine position with both arms raised above the head. CT images were transferred to a three‐dimensional conformal radiation therapy planning system (Pinnacle 7.6C). The tumor thrombi, hepatic tumor, liver, kidneys, and spinal cord were contoured and reconstructed to form a three‐dimensional representation; a dose–volume histogram was also generated. The tumor thrombi were contoured as gross target volume during the portal vein phase. Clinical target volume requires gross target volume expansions of 5 mm. Planning target volume is defined as clinical target volume with a margin of 7 mm to account for daily setup errors and target motion. The entire main hepatic tumor was also included unless the percent volume of the whole liver receiving a dose of >30 Gy exceeded 30%, and we preserved a part of normal liver without any radiation. Using two opposed fields has been preferred because such treatment allows maximization of the non‐irradiated volume of normal liver. This treatment planning strategy is based on the notion that normal liver tissue tolerance is lower in patients with the background of liver cirrhosis.
The patients also received training in respiration to reduce amplitude, increase frequency, and minimize tumor movement before the initiation of EBRT. The respiratory tumor movement was estimated during simulation, and if it was >1.0 cm, pressure was applied to the patient's abdomen with the goal of minimizing tumor movement. The cephalic and caudal margins for 1.5 cm beyond the gross tumor were considered during the end of phase of exhalations and inhalations.( 22 ) Parallel‐opposed portals were frequently used, and combinations of three or more ports were applied, depending on tumor location. Wedges or compensation devices were used as needed. An isocentric technique was used, and the source‐to‐axial distance was 100 cm. The median total dose was 50 Gy (range, 30–60 Gy) in daily doses of 2 Gy/fraction, five times a week. Depending on the field size and anatomic location, all or part of the right kidney was in the radiation field. In such cases, an initial left renal evaluation with intravenous pyelography was performed to ensure that the left kidney had adequate function. When the duodenum was encompassed by the EBRT fields, the total EBRT dose was limited to ≤50 Gy. We scheduled the full radiation dosage up to 50 Gy, but factors that indicated the need for a reduced dose were considered, such as adverse effects and distant metastases during EBRT. A radiation dosage ≤40 Gy was delivered in 25 patients because of distant metastases or side effects during EBRT. The dose was 40–50 Gy in 16 patients, 50 Gy in 59 patients, and 52–60 Gy in 37 patients.
Assessment of response and toxicity. Pretreatment evaluation included a medical history and physical examination, complete blood cell count, serum chemistries, liver function tests, AFP, carbohydrate antigen 19.9 (CA19–9) and carcinoembryonic antigen (CEA), chest X‐ray, abdominal ultrasonography, and enhanced CT or MRI (or both). Clinical monitoring was performed once weekly. Hepatic angiography was obtained in patients who received TACE.
One and 1.5 months after the completion of EBRT, patients were monitored with abdominal enhanced CT or MRI, as in the first follow‐up. Patients were monitored every 3 months thereafter. Tumor thrombi response was compared with two investigations approximately 3 months apart, from before EBRT to the first follow‐up. A complete response (CR) was defined as the complete disappearance of all clinical and radiographic evidence of thrombi. A partial response (PR) required a >50% reduction in the sum of the products of the longest diameter and its perpendicular on the CT scan or MRI. Stable disease (SD) indicated a decrease <50% or an increase <25% in the product of the longest perpendicular diameters of measurable thrombi. Progressive disease (PD) was defined as an increase ≥25% in the sum of the products of the longest diameter and its perpendicular, compared with the lowest value recorded, or as death from HCC within 3 months. Objective response was calculated for CR and PR; no response was calculated for SD or PD.
Survival in this study was defined by the interval from the date when EBRT was initial to the date of death or the last follow‐up appointment. Cumulative survival rates were analyzed by Kaplan–Meier plots.
Statistical analysis. Kaplan–Meier curves were generated for survival. The χ2‐test was used to compare the incidence of lung metastases. Differences between curves were assessed by the log‐rank test. For multivariate analysis, all variables were entered using the Backward:Wald method. P < 0.05 was considered statistically significant. All calculations were performed with SPSS 13.0 for Windows (SPSS, Chicago, IL, USA).
Results
Patient characteristics. The cohort included 129 men and seven women (ratio, 18.4:1), with a mean age of 50.4 ± 11.0 years (range, 10–81 years). Pretreatment variables that showed significant differences in survival in the univariate analysis are listed in Table 1, including demographics, clinical laboratory tests, tumor, and treatment information for all patients.
Table 1.
Univariate and multivariate analysis of baseline predictors of survival in 136 patients with hepatocellular carcinoma with tumor thrombi
| Variables | n | Survival status | P‐values | |||
|---|---|---|---|---|---|---|
| Average ± SD | Median ± SD | Univariate | Multivariate | |||
| Age (years) | ≤40 | 24 | 15.4 ± 16.2 | 8.6 ± 15.2 | 0.052 | 0.674 |
| 40–60 | 84 | 14.2 ± 15.6 | 8.5 ± 7.3 | |||
| ≥60 | 28 | 28.3 ± 33.9 | 17.4 ± 27.5 | |||
| Gender | Female | 7 | 36.6 ± 29.6 | 59.1 ± 0 | 0.125 | 0.499 |
| Male | 129 | 16.6 ± 21.6 | 9.0 ± 9.7 | |||
| HBsAg | Negative | 12 | 23.3 ± 23.9 | 16.6 ± 17.3 | 0.346 | 0.752 |
| Positive | 124 | 16.9 ± 22.3 | 9.0 ± 8.9 | |||
| Hemoglobin (g/L) | ≤110 | 26 | 8.4 ± 6.1 | 7.2 ± 6.1 | 0.002 | 0.665 |
| 110–140 | 97 | 20.3 ± 25.6 | 10.3 ± 14.8 | |||
| ≥140 | 13 | 16.1 ± 13.3 | 15.4 ± 25.2 | |||
| Platelet count (×109/L) | ≤100 | 64 | 20.9 ± 26.4 | 11.5 ± 15.2 | 0.005 | 0.659 |
| 100–200 | 54 | 15.3 ± 16.9 | 9.1 ± 7.3 | |||
| ≥200 | 18 | 8.4 ± 8.1 | 4.7 ± 2.5 | |||
| Total bilirubin (mmol/L) | <20 | 79 | 16.5 ± 17.8 | 10.2 ± 7.1 | 0.711 | 0.947 |
| ≥20 | 57 | 18.6 ± 26.4 | 8.0 ± 7.5 | |||
| Albumin (g/L) | ≤35 | 28 | 10.7 ± 16.4 | 7.2 ± 8.5 | 0.001 | 0.005 |
| 35–44 | 71 | 15.1 ± 16.9 | 8.6 ± 9.3 | |||
| ≥44 | 37 | 25.2 ± 26.8 | 15.4 ± 24.9 | |||
| ALT (IU/mL) | <75 | 111 | 16.8 ± 21.1 | 9.1 ± 9.5 | 0.611 | 0.337 |
| ≥75 | 25 | 21.1 ± 25.0 | 11.5 ± 16.0 | |||
| AST(IU/mL) | <75 | 98 | 19.7 ± 23.8 | 10.2 ± 14.6 | 0.006 | 0.718 |
| ≥ 75 | 38 | 9.8 ± 6.8 | 6.5 ± 9.6 | |||
| ALP(IU/mL) | <150 | 86 | 20.1 ± 23.8 | 11.5 ± 15.8 | 0.014 | 0.146 |
| ≥150 | 50 | 10.7 ± 8.2 | 7.5 ± 4.2 | |||
| γ‐GT (IU/L) | ≤75 | 25 | 27.6 ± 28.5 | 18.4 ± 22.2 | 0.028 | 0.018 |
| 75–150 | 39 | 15.5 ± 22.7 | 9.0 ± 10.1 | |||
| ≥150 | 72 | 13.6 ± 14.5 | 8.0 ± 7.5 | |||
| AFP status (µg/L) | ≤20 | 49 | 23.1 ± 24.2 | 13.0 ± 26.3 | 0.008 | 0.032 |
| 20–400 | 32 | 15.3 ± 18.1 | 9.0 ± 9.4 | |||
| ≥400 | 55 | 12.5 ± 16.1 | 7.5 ± 5.3 | |||
| Child–Pugh classification | A | 107 | 20.4 ± 24.8 | 10.7 ± 10.8 | <0.001 | 0.005 |
| B | 28 | 7.4 ± 4.4 | 5.4 ± 3.9 | |||
| C† | 1 | 3.3 ± 0 | 3.3 ± 0 | |||
| Size of intrahepatic tumors (cm) | ≤5 | 48 | 24.3 ± 27.8 | 13.2 ± 13.2 | 0.009 | 0.269 |
| 5–10 | 58 | 12.2 ± 11.1 | 7.4 ± 6.8 | |||
| ≥10 | 30 | 13.1 ± 12.1 | 7.5 ± 4.1 | |||
| Intrahepatic tumor number | Solitary | 68 | 22.3 ± 24.2 | 13.2 ± 22.6 | <0.001 | 0.008 |
| Multiple | 68 | 12.5 ± 18.2 | 7.0 ± 5.2 | |||
| Thrombus location | PV branches | 27 | 17.7 ± 18.7 | 10.1 ± 3.7 | 0.047 | 0.224 |
| PV trunk | 67 | 14.7 ± 25.9 | 7.4 ± 7.3 | |||
| IVC | 30 | 23.5 ± 23.6 | 18.4 ± 14.1 | |||
| IVC + PV | 12 | 12.5 ± 6.6 | 7.5 ± 4.5 | |||
| Presence of tumor thrombi | Synchronous | 71 | 12.6 ± 16.1 | 7.4 ± 6.4 | 0.002 | 0.382 |
| Asynchronous | 65 | 22.3 ± 25.5 | 12.2 ± 20.9 | |||
| LN metastases | Absent | 118 | 18.7 ± 23.1 | 10.2 ± 12.5 | 0.001 | <0.001 |
| Present | 18 | 7.8 ± 6.2 | 5.0 ± 2.2 | |||
| Distant metastases | Absent | 94 | 17.7 ± 22.9 | 8.2 ± 10.9 | 0.287 | 0.281 |
| Lung | 38 | 12.7 ± 9.3 | 9.5 ± 9.2 | |||
| Other sites | 4 | 37.2 ± 35.0 | 9.9 ± 54.1 | |||
| Previous treatment modalities for intrahepatic tumors | Without therapy | 14 | 17.7 ± 25.6 | 5.7 ± 3.1 | 0.427 | 0.408 |
| TACE | 90 | 16.5 ± 20.2 | 9.1 ± 9.9 | |||
| Resection ± TACE | 32 | 18.7 ± 21.7 | 12.0 ± 10.0 | |||
| EBRT dose (Gy) | 30–48 | 40 | 14.1 ± 18.0 | 8.0 ± 4.7 | 0.078 | 0.386 |
| 50 | 59 | 16.6 ± 22.8 | 9.7 ± 11.5 | |||
| 52–60 | 37 | 23.2 ± 23.5 | 12.5 ± 21.3 | |||
| Response to EBRT | CR | 41 | 25.4 ± 22.6 | 19.5 ± 16.0 | <0.001 | <0.001 |
| PR | 36 | 20.6 ± 30.8 | 10.2 ± 8.8 | |||
| SD | 49 | 11.1 ± 13.6 | 7.2 ± 3.9 | |||
| PD | 10 | 4.2 ± 1.8 | 3.5 ± 0.5 | |||
| Radiation technique | 2‐D | 69 | 16.4 ± 19.1 | 8.5 ± 12.5 | 0.393 | <0.001 |
| 3‐D | 67 | 14.4 ± 11.5 | 10.7 ± 18.3 | |||
2‐D, two‐dimensional conformal EBRT; 3‐D, three‐dimentional conformal EBRT; AFP, α‐fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, complete response; EBRT, external‐beam radiation therapy; HbsAg, hepatitis B surface antigen; LN, lymph node; PD, progressive disease; PR, partial response; SD, stable disease; SD, standard deviation; TACE, transcatheter arterial chemoembolization; WBC, white blood cell; γ‐GT, γ‐glutamyltransferase.
Child–Pugh C was excluded because of only one case; P‐value is the comparison between Child–Pugh A and B.
To identify the incidence of lung metastases, we categorized the patients with PV, IVC, or PV plus IVC tumor thrombi. The incidence of lung metastases between PV and IVC tumor thrombosis was significant (P < 0.001; Table 2). Fifty percent of the patients with IVC tumor thrombi had lung metastases during the entire period of treatment compared with only 18.1% of patients with PV tumor thrombi.
Table 2.
Incidence of lung metastases between portal vein (PV) and inferior vena cava (IVC) tumor thrombi
| n | Lung metastases | P‐values | ||
|---|---|---|---|---|
| Absent | Present | |||
| PV | 94 | 77 (81.9%) | 17 (18.1%) | <0.001 |
| IVC | 30 | 14 (46.7%) | 16 (53.3%) | |
| PV + IVC | 12 | 7 (58.3%) | 5 (41.7%) | |
Of 136 HCC patients with tumor thrombi, 71 were identified as having tumor thrombi at their initial treatments. Although the platelet count was lower than in healthy subjects, the incidence of tumor thrombi was also lower, as shown in Table 3. Higher platelet count is significantly related to a higher cumulative incidence of tumor thrombosis (P = 0.058) (Fig. 1a) and lower cumulative survival rate (P = 0.007; Fig. 1b).
Table 3.
Interval between presence of intrahepatic tumor and tumor thrombi
| Platelet count (×109/L) | n | Synchronous tumor thrombi | Mean interval between presence of intrahepatic tumor and thrombi ± SE (months) | P‐values |
|---|---|---|---|---|
| ≤100 | 64 | 23 (35.9%) | 14.9 ± 3.42 | 0.058 |
| 100–200 | 54 | 33 (61.1%) | 10.5 ± 4.07 | |
| ≥200 | 18 | 15 (83.3%) | 3.6 ± 2.25 |
Figure 1.
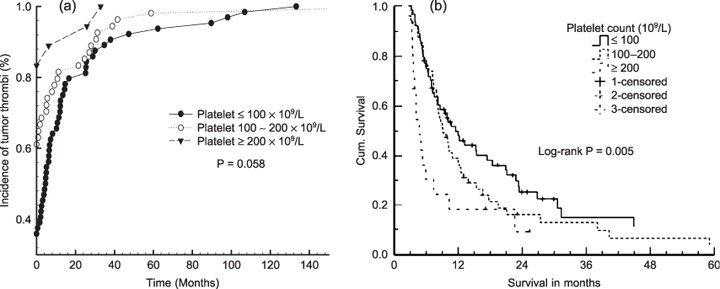
Platelet count is a useful predictor for the incidence of tumor thrombi (a) and cumulative survival (b). The higher the platelet count, the earlier tumor thrombi appear and the poorer survival outcomes.
Response. Of the 136 patients with tumor thrombi who received EBRT, the tumor thrombus completely disappeared (CR) in 41 patients (30.1%), 36 patients (26.5%) had a PR, 49 patients (36%) had SD, and 10 patients (7.4%) had PD. Because 12 patients had both PV and IVC tumor thrombi concurrence, we treated a total of 106 PV tumor thrombi and 42 IVC tumor thrombi. In comparison with PV and IVC tumor thrombi, the percentages of CR, PR, SD, and PD were 17.9% (19/106) versus 61.9% (26/42), 31.1% (33/106) versus 19% (8/42), 41.5% (44/106) versus 19.1% (8/42), and 9.5% (10/106) versus 0%, respectively. The objective response (CR + PR) rate was significantly higher in the patients with IVC tumor thrombi (P < 0.001). The responses were confirmed by CT or MRI during follow‐up 1.5–2 months after completion of EBRT.
Pretreatment variables, and results of univariate and multivariate analysis. According to pretreatment variables, the Kaplan–Meier survival curves on univariate analysis showed that hemoglobin values≤ 110 g/L, higher platelet count, lower albumin, higher liver enzymes (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, γ‐glutamyltransferase), higher AFP level, Child–Pugh B liver function, tumor diameter >5 cm, multiple intrahepatic lesions, tumor thrombi located in PV, synchronous tumor thrombi, positive lymph node metastases, and incomplete response to EBRT were related to unfavorable overall survival (Table 1 and Fig. 2). The survival rates at 1, 2, and 3 years were 37.4%, 14%, and 9.4% for patients with PV branch tumor thrombi; 29.7%, 17.8%, and 8.9% for PV trunk tumor thrombi; 66.3%, 31.6%, and 15.8% for IVC tumor thrombi; and 25%, 9.1%, and 0% for PV plus IVC tumor thrombi concurrence, respectively. The median survival was 10.1 months, 7.4 months, 18.4 months, and 7.5 months for patients with PV branch, PV trunk, IVC, and PV plus IVC tumor thrombosis, respectively.
Figure 2.
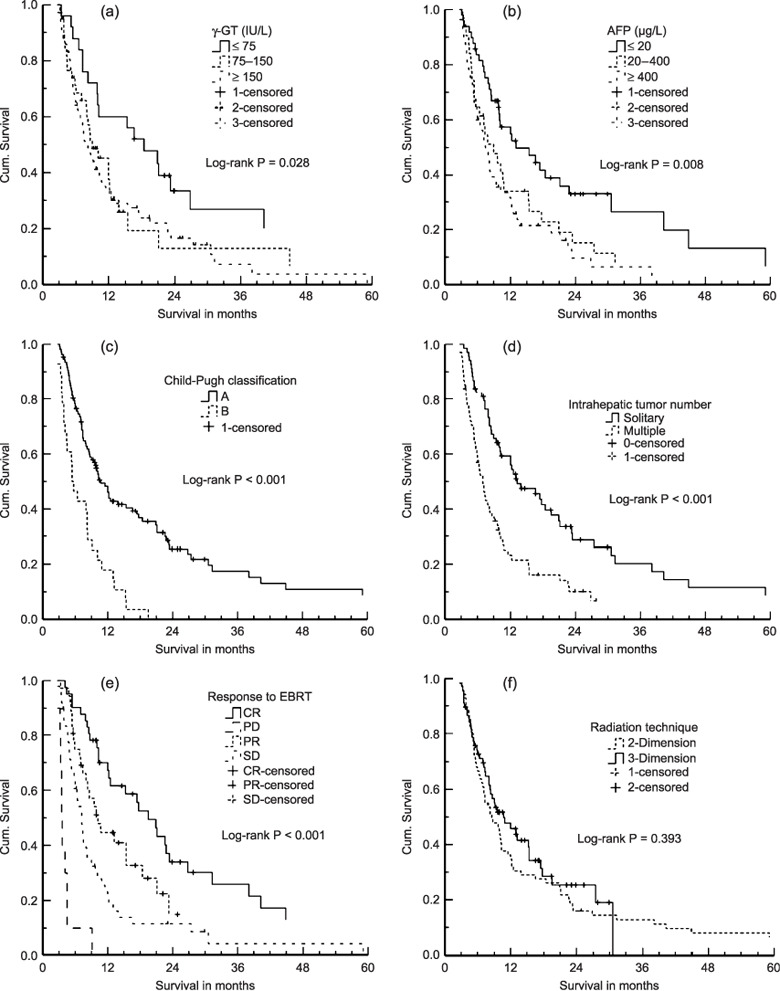
Cumulative (Cum.) survival curves on univariate analysis according to (a) γ‐glutamyltransferase, (b) α‐fetoprotein, (c) Child–Pugh classification, (d) intrahepatic tumor number, (e) response to external‐beam radiation therapy (EBRT), and (f) radiation therapy technique.
On multivariate analysis, pretreatment unfavorable predictors were associated with lower albumin (P = 0.005), higher γ‐glutamyltransferase (P = 0.018), higher AFP (P = 0.032), poorer Child–Pugh liver function (P = 0.005), intrahepatic multiple focals (P = 0.008), lymph node metastases (P < 0.001) and poorer response to EBRT (P < 0.001); however, platelet count (Fig. 3a) was not associated with survival (P = 0.659), as shown in Table 1, multivariate analysis column. It is very interesting to note that the radiation therapy technique is not associated with survival on the univariate analysis, but is significantly related to prognostic factors on multivariate analysis (Fig. 3b).
Figure 3.
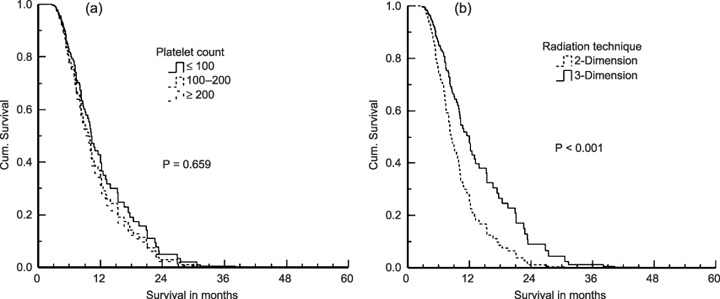
Survival curves on multivariate analysis. (a) Platelet count; (b) radiation therapy technique. Cum., cumulative.
Failure patterns. At the end of this study, 28 patients (20.6%) were alive, and 108 patients (79.4%) died. The causes of death were liver failure in 84 patients (77.8%) due to hepatic decompensation or tumor progression (or both), brain metastases in eight, lung metastases in six, and lymph node metastasis in five, which were all induced by tumor progression. Other causes of death perhaps related to tumor progression included pulmonary infarction induced by dislodged thrombi in one patient, hemolytic anemia in one patient, gastrointestinal bleeding in one patient, systemic debility in one patient, and heart attack in one patient. Of the 38 patients with lung metastases in this study group, only six patients (15.8%) died from pulmonary failure.
Toxicity. Table 4 lists the sequelae of EBRT according to Radiation Therapy Oncology Group criteria. The patients had significantly more moderate to acute gastrointestinal and hepatic toxicity. Loss of appetite and nausea were frequent; however, patients did not need fluid infusion. None of these adverse effects affected the timing and delivery of EBRT. There was no deterioration of liver function during EBRT. Increasing liver enzymes levels were more common, but generally showed less than a five‐fold elevation of the upper normal limit. Slight elevation of total bilirubin was also common in the patients with a history of liver cirrhosis. A decrease in platelet and white blood cell counts may reflect portal hypertension and associated hypersplenism, so the reduction of white blood cell and platelet counts may not have been completely related to EBRT. Even though increasing concentration of liver enzymes is more common, we did not observe any radiation‐induced hepatitis. The toxicities were evaluated at the first follow‐up, about 1.5 months after completion of EBRT.
Table 4.
Sequelae of external‐beam radiation therapy (EBRT) according to RTOG criteria
| n | RTOG grade, n (%) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| GI | |||||
| Anorexia | 136 | 37 (27.2%) | 10 (7.4%) | 3 (2.2%) | 0 |
| Nausea/vomiting | 136 | 16 (11.8%) | 4 (2.9%) | 2 (1.5%) | 0 |
| Diarrhea | 136 | 2 (1.5%) | 1 (0.7%) | 0 | 0 |
| Gastroduodenal ulcer | 136 | – | 10 (7.4%) | 1 (0.7%) | 0 |
| Liver | |||||
| Total bilirubin | 136 | 15 (11%) | 12 (8.8%) | 0 | 0 |
| ALT | 136 | 26 (19.1%) | 1 (0.7%) | 5 (3.7%) | 0 |
| AST | 136 | 35 (25.7%) | 15 (11%) | 6 (4.4%) | 0 |
| ALP | 136 | 47 (34.6%) | 8 (5.9%) | 1 (0.7%) | 0 |
| Marrow | |||||
| Hb | 136 | 58 (42.6%) | 21 (15.4%) | 0 | 0 |
| WBC | 136 | 35 (25.7%) | 28 (20.6%) | 7 (5.1%) | 1 (0.7%) |
| Platelets | 136 | 22 (16.2%) | 33 (24.3%) | 34 (25%) | 0 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GI, gastrointestinal; Hb, hemoglobin; RTOG, Radiation Therapy Oncology Group; WBC, white blood cells.
Discussion
None of prognostic systems has really been applied to identifying patients with unresectable tumors who received medical treatments including EBRT. However, HCC comprises a variety of subsets of patients, often with quite different prognoses. This is reflected in different survival data published for either resection or non‐resection from various centers as well as the wide range of survivals using the same treatment modality even within any individual large center. Even among the same subsets of patients who undergo the same treatment modality, the different outcomes may be due to different prognostic factors considered. The median survival time ranged from 5.3 to 10.7 months for the patients with PV tumor thrombi treated with EBRT (Table 5). Currently, we lack the information to identify HCC patients with tumor thrombi with potentially better or worse outcomes after receiving EBRT. The reason for this situation is the shorter survival and lack of good treatment choice for such patients.
Table 5.
Treatment outcomes of radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma
| Author | Year | n | Dose (Gy) | Combined treatment | Response rate | MST (months) |
|---|---|---|---|---|---|---|
| Tazawa et al.( 23 ) | 2001 | 24 | 50 | TACE | 50% | 3.8–9.7 |
| Ishikura et al.( 24 ) | 2002 | 20 | 50 | TACE | 50% | 5.3 |
| Yamada et al.( 25 ) | 2003 | 19 | 46–60 | TACE | 57.9% | 7.0 |
| Kim et al.( 14 ) | 2005 | 59 | 30–54 | Unknown | 45.8% | 5.3–10.7 |
| Zeng et al.( 13 ) | 2005 | 44 | 36–50 | TACE ± resection | 45.5% | 8.0 |
| Lin et al.( 16 ) | 2006 | 43 | 45 | None | 75% | 6.0–6.7 |
| Toya et al.( 15 ) | 2007 | 38 | 23–59 | None | 44.7% | 9.6 |
| This study† | 2007 | 94 | 30–60 | TACE ± resection | 41.3% | 9.7 |
MST, median survival time; TACE, transcatheter arterial chemoembolization.
This study overlaps and contains the 44 patients treated with EBRT shown above for reference 13.
In this study, many prognostic factors could complicate the situation; however, we categorized them into three groups. The first was tumor‐related factors, including γ‐glutamyltransferase, AFP, tumor size, intrahepatic tumor number, tumor thrombi status, lymph node involvement, and distant metastases. The second was liver function‐related factors, including total bilirubin, albumin, liver enzymes, and Child–Pugh classification. The third was treatment‐related factors, such as previous therapy modalities, radiation dose, radiation technique, and response to EBRT. Routine blood evaluation is associated with both tumor and liver function status. A decrease in hemoglobin and white blood cell and platelet counts may reflect portal hypertension and associated hypersplenism, which are strongly related to a history of liver cirrhosis. However, protal hypertension frequently is present in patients with PV tumor thrombi. Thrombocytosis sometimes is a paraneoplastic syndrome of patients with HCC due to the overproduction of serum thrombopoietin. It is frequently associated with a large tumor volume and high serum AFP concentration.( 26 ) Decline of the hemoglobin level is a condition associated with tumor growth, which induces anemia away from the primary tumor or its metastasis.
Patients with tumor thrombosis are difficult to treat with surgical resection. Not only is the surgical technique a problem, but these patients also have had larger tumors, multiple lesions in the liver, or extrahepatic metastases. PV thrombosis has previously been thought to be a contraindication for hepatic artery chemoembolization, because if the PV is completely blocked by a tumor and the hepatic artery is embolized for therapeutic purposes, then that lobe of the liver is thought to undergo necrosis, with resultant liver failure. So the tumor status is a critical factor. However, distant metastases did not affect the survival in this study because the lung is the most common site for metastasis. Even though the patients with lung metastasis from HCC did not receive any treatment, the median survival was as long as 8.8 months,( 27 ) which is mostly equal to those who had tumor thrombosis and received EBRT.
To date, standard treatment regimens have not been established for patients with HCC with PV or IVC tumor thrombi. Experience with EBRT is still limited, but more data are available regarding the efficacy of EBRT for such patients. EBRT has become an acceptable treatment modality for them (Table 5). We favored treating the patients with resection with or without TACE and EBRT. TACE can debulk the intrahepatic tumor owing to ischemic necrosis of the tumor. For irradiation to be used as an adjunct to TACE, it must be delivered safely and in adequate doses to sterilize the remaining viable tumor cells in the vascular bed. Based on this study, the ability of irradiation to control the tumor is related to the total dose of irradiation that can be delivered. We defined CR and PR as an objective response and SD and PD as no response. The response rate is between 40% and 50% in most reports (Table 5). SD might be considered to be venous thrombosis and fibrous or estimated too early. The responses were confirmed by CT or MRI during follow‐up 1.5–2 months after completion of EBRT. It seems to be too early, leading to possible underestimation of response. However, we could not wait too long, because these patients had shorter survival expectancies. TACE could not be followed up owing to the occlusion of all the PV branches and trunk. Patients with thrombus located at the PV had a poorer survival rate than those with the thrombus located at the IVC. This result reflects the fact that the patients with IVC tumor thrombi who did not have a completely obstructed PV, which is the obstacle to receiving TACE, had good treatment results.
It is interesting to note that the platelet count is a simple and important prognostic factor on univariate analysis. In this study, we realized that the higher the platelet count, the earlier tumor thrombi appear and the poorer the survival. This result also was reported by Hagiwara and his colleagues, who followed 312 HCC patients without PV tumor thrombi at the initial diagnosis; 25 patients presented PV tumor thrombi after diagnosis. The incidence of PV tumor thrombi was 31.1% in the group with platelet count ≥130 × 109/L, and 7.7% in those group with <130 × 109/L within 5 years.( 28 ) The platelet count has been reported as a prognostic factor in patients with inoperable HCC.( 29 , 30 , 31 ) Platelets act as transporters of tumor‐originated vascular endothelial growth factor, contributing to tumor angiogenesis and progression.( 32 ) Several previous studies reported correlations between platelet count and serum vascular endothelial growth factor level in patients with cancer. Also the correlation between the platelet count and the TNM (tumor, node, metastasis) stage was reported, and the TNM stage is advanced in patients with a higher platelet count. Cancer may readily infiltrate the portal vein via tumor vascular endothelial growth factor in patients who maintain a high platelet count, leading to an advanced TNM stage.( 33 ) Furthermore, platelets contain platelet‐derived growth factor and hepatocyte growth factor, which are known to play important roles in liver regeneration. Higher platelet counts in HCC patients are due to the overproduction of thrombopoietin by the tumor, mostly in patients with a large tumor burden. The mechanisms of thrombocytosis in HCC patients are similar to those for other paraneoplastic manifestations in some cases.( 26 ) However, platelet is not associated with survival on multivariate analysis. This is because the lower platelet count is associated with the presence of hypersplenism and poorer liver function which is a negative prognostic factor in this study.
Most patients with HCC have had a history of liver cirrhosis. Before EBRT, those patients had abnormal values on laboratory tests, such as liver function and routine blood examinations. Abnormal liver enzymes occur in about 20–30%, thrombocytopenia in 47%, leukopenia in 32%, and anemia in 19% before EBRT. The serum albumin is <30 g/L in 20%. So it is no surprise that grade 1 and 2 toxicity is most frequent (Table 4). However, no patient was diagnosed as having radiation‐induced liver injury in this study. Hence, there was no advantage to using the Radiation Therapy Oncology Group toxicity criteria to evaluate the side effects in the EBRT group.
It is very interesting to note that the radiation therapy technique (two‐dimension versus three‐dimension) was not associated with survival on the univariate analysis, but that it was significantly related to prognostic factors on multivariate analysis. We strongly believe that the use of three‐dimensional conformal therapy has had a major impact on the practice of radiation therapy. Patients identified to benefit most from three‐dimensional conformal EBRT are those with tumors in sites with complex anatomy, irregularly shaped tumor volumes, tumors adjacent to radiation‐sensitive normal structures, and small‐volume or high‐dose treatments. Conformal EBRT is the best choice for HCC patients. But as with any major technical advance in radiation oncology, its use must be supported with enhanced quality assurance from all members of the treatment team. We have treated HCC patients using conformal EBRT in the past 3 years. The longer survival in those patients treated with conformal EBRT is under follow‐up as shown in Fig. 2f. This may be useful for explaining the difference between the univariate and multivariate analysis results.
In summary, this study provides detailed information about the survival outcomes and prognostic factors of HCC with tumor thrombi in a relatively large cohort of patients treated with radiation, and the results will help in understanding the potential factors that influence survival for patients with HCC after EBRT. In our experience, EBRT is a useful palliative treatment for patients with HCC with PV or IVC tumor thrombi.
References
- 1. El‐Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999; 340: 745–50. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Clegg LX, Ward E et al . Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. Cancer 2004; 101: 3–27. [DOI] [PubMed] [Google Scholar]
- 3. Pirisi M, Avellini C, Fabris C et al . Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol 1998; 124: 397–400. [DOI] [PubMed] [Google Scholar]
- 4. Park KW, Park JW, Choi JI et al . Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus‐endemic area. J Gastroenterol Hepatol 2007; doi. DOI: 10.1111/j.1440-1746.200705112.x. [DOI] [PubMed] [Google Scholar]
- 5. Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western Center. Ann Surg 1999; 229: 790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheung TK, Lai CL, Wong CY, Fung J, Yuen MF. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther 2006; 24: 573–83. [DOI] [PubMed] [Google Scholar]
- 7. Takizawa D, Kakizaki S, Sohara N et al . Hepatocellular carcinoma with portal vein tumor thrombosis: clinical characteristics, prognosis, and patient survival analysis. Dig Dis Sci 2007; 52: 3290–5. [DOI] [PubMed] [Google Scholar]
- 8. Liovet JM, Bustamante J, Castells A et al . Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology 1999; 29: 62–7. [DOI] [PubMed] [Google Scholar]
- 9. Cheong JY, Lee KM, Cho SW et al . Survival benefits to intra‐arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Hepatol Res 2005; 32: 127–33. [DOI] [PubMed] [Google Scholar]
- 10. Jang JW, Bae SH, Choi JY et al . A combination therapy with transarterial chemo‐lipoidolization and systemic chemo‐infusion for large extensive hepatocellular carcinoma invading portal vein in comparison with conservative management. Cancer Chemother Pharmacol 2007; 59: 9–15. [DOI] [PubMed] [Google Scholar]
- 11. Minagawa M, Makuuchi M, Takayama T et al . Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg 2001; 233: 379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikai I, Hatano E, Hasegawa S et al . Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg 2006; 202: 431–8. [DOI] [PubMed] [Google Scholar]
- 13. Zeng ZC, Fan J, Tang ZY et al . A comparison of treatment combinations with and without radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys 2005; 61: 432–43. [DOI] [PubMed] [Google Scholar]
- 14. Kim DY, Park W, Lim DH et al . Three‐dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer 2005; 103: 2419–26. [DOI] [PubMed] [Google Scholar]
- 15. Toya R, Murakami R, Baba Y et al . Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol 2007; 84: 266–71. [DOI] [PubMed] [Google Scholar]
- 16. Lin CS, Jen YM, Chiu SY et al . Treatment of portal vein tumor thrombosis of hepatoma patients with either stereotactic radiotherapy or three‐dimensional conformal radiotherapy. Jpn J Clin Oncol 2006; 36: 212–17. [DOI] [PubMed] [Google Scholar]
- 17. Frazer C. Imaging of hepatocellular carcinoma. J Gastroenterol Hepatol 1999; 14: 750–6. [DOI] [PubMed] [Google Scholar]
- 18. Loyer EM, Chin H, DuBrow RA et al . Hepatocellular carcinoma and intrahepatic peripheral cholangiocarcinoma: enhancement patterns with quadruple phase helical CT – a comparative study. Radiology 1999; 212: 866–75. [DOI] [PubMed] [Google Scholar]
- 19. Blacher A, Federle MP, Ferris JV et al . Radiologists’ performance in the diagnosis of liver tumors with central scars by using specific CT criteria. Radiology 2002; 223: 532–9. [DOI] [PubMed] [Google Scholar]
- 20. Chinese Liver Cancer Association . The diagnostic criteria and stages of primary hepatoma in the 4th national conference of liver cancer. Problems and discussion. Chin J Gen Surg 2000; 15: 238–9. [Google Scholar]
- 21. Zeng ZC. Conformal radiation therapy for patients with unresectable primary liver cancerCRT is now a realistic option. Austral-Asian J Cancer 2008; 7 (2): 65–75. [Google Scholar]
- 22. Du SS, Zeng ZC, Wu Z et al . Clinical value of active breathing coordinator during three dimension conformal radiotheapy for patients with intrahepatic tumor. Austral-Asian J Cancer 2008; 7: 15–23. [Google Scholar]
- 23. Tazawa J, Maeda M, Sakai Y et al . Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol 2001; 16: 660–5. [DOI] [PubMed] [Google Scholar]
- 24. Ishikura S, Ogino T, Furuse J et al . Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol 2002; 25: 189–93. [DOI] [PubMed] [Google Scholar]
- 25. Yamada K, Izaki K, Sugimoto K et al . Prospective trial of combined transcatheter arterial chemoembolization and three‐dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2003; 57: 113–19. [DOI] [PubMed] [Google Scholar]
- 26. Hwang SJ, Luo JC, Li CP et al . Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol 2004; 10: 2472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang SM, Zeng ZC, Tang ZY et al . Prognostic analysis of pulmonary metastases from hepatocellular carcinoma. Hepatol Int 2008; 2: 237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hagiwara S, Kudo M, Kawasaki T et al . Prognostic factors for portal venous invasion in patients with hepatocellular carcinoma. J Gastroenterol 2006; 41: 1214–19. [DOI] [PubMed] [Google Scholar]
- 29. Sakar B, Ustuner Z, Karagol H, Aksu G, Camlica H, Aykan NF. Prognostic features and survival of inoperable hepatocellular carcinoma in Turkish patients with cirrhosis. Am J Clin Oncol 2004; 27: 489–93. [DOI] [PubMed] [Google Scholar]
- 30. Reichman TW, Bahramipour P, Barone A et al . Hepatitis status, Child‐Pugh classification, and serum AFP levels predict survival in patients treated with transarterial embolization for unresectable HCC. J Gastrointest Surg 2005; 9: 638–45. [DOI] [PubMed] [Google Scholar]
- 31. O'Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5‐year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg 2003; 90: 325–31. [DOI] [PubMed] [Google Scholar]
- 32. Webb NJA, Bottomley MJ, Watson CJ, Brenchley PEC. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci 1998; 94: 395–404. [DOI] [PubMed] [Google Scholar]
- 33. Poon RT, Lau CP, Cheung S, Yu WC, Fan S. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res 2003; 63: 3121–6. [PubMed] [Google Scholar]


