Abstract
A pilot study was performed to investigate the safety and feasibility of autologous formalin‐fixed tumor vaccines (AFTV) and the clinical responses to these vaccines by glioblastoma multiforme (GBM) patients. Twelve primary GBM patients were recruited. Eight had recurrent disease while four had been treated for primary disease but retained a visible tumor mass. AFTV were prepared from formalin‐fixed and/or paraffin‐embedded tumor tissue obtained upon surgery and premixed with original adjuvant materials. The patients were given three five‐site intradermal inoculations at weekly intervals. A delayed‐type hypersensitivity test was performed before and after each vaccination. In addition, the tumor tissues were subjected to immunohistochemical analysis to determine whether MIB‐1, p53, and major histocompatibility complex (MHC) class‐I complex expression could predict the response to the treatment. The treatment was well tolerated, with only local erythema, induration, and low‐grade fever being reported. Of the 12 patients, one showed a complete response, one showed a partial response, two showed minor responses, one had stable disease, and seven exhibited progressive disease. The median survival period was 10.7 months from the initiation of the AFTV treatment but three of the five responders survived for 20 months or more after AFTV inoculation. Low p53 and high MHC class‐I expression by the tumor may help predict the efficacy of this therapy. Thus, the AFTV is safe and feasible, and could significantly improve the outcome of GBM. Further clinical investigations to confirm this are highly desirable. (Cancer Sci 2007; 98: 1226–1233)
- Abbreviations: ACNU
nimustine hydrochloride
- AFTV
autologous formalin‐fixed tumor vaccine
- BCG
Bacille Calmette–Guerin
- CEA
carcinoembryonic antigen
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- DTH
delayed‐type hypersensitivity
- FPV
final packed volume
- GBM
glioblastoma multiforme
- Gd
gadolinium
- GM‐CSF
granulocyte macrophage–colony stimulating factor
- IL
interleukin
- IICP
increased intracranial pressure
- KPS
Karnofsky performance status
- MHC
major histocompatibility complex
- MR
minor response
- MRI
magnetic resonance imaging
- NC
no change
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- PD
progressive disease
- TAA
tumor‐associated antigen.
GBM is currently treated with a combination of surgical removal, external beam radiotherapy, and nitrosourea chemotherapy. However, it is not overstating the case to say that GBM patients are never completely cured because most quickly suffer relapses and more than 90% succumb within 5 years of diagnosis.( 1 ) Improving the outcome of GBM and extending the life span of GBM patients is thus a matter of great concern to clinicians. With regard to current GBM therapies, it has been suggested that cytoreductive surgery may be beneficial.( 2 , 3 ) However, other authors have failed to detect significant differences in the survival of cases who had been subjected to different surgical modalities.( 4 , 5 ) This difference has been attributed to the extreme diffuseness with which the tumor invades the brain parenchyma. In contrast, conventional X‐ray radiotherapy at a dose of approximately 60 Gy has been found to be significantly beneficial, as it extends the median survival time by 18 weeks( 6 ) or 5.6 months.( 7 ) In addition, a large‐scale meta‐analysis of the therapeutic effects of chemotherapy indicated that it extends survival by 2 months.( 8 ) Indeed, temozolomide chemotherapy was found to extend survival by 2.5 months.( 9 )
The current inability to cure GBM has led to the development of various novel GBM therapies. In particular, there is growing interest in treatments that involve tumor‐specific immune reactions, because such treatments potentially have a high benefit‐to‐risk ratio. Moreover, these treatments may be useful in preventing tumor recurrence after initial localized treatments that involve surgery and radiotherapy. Indeed, our preliminary clinical studies have revealed that immunotherapeutic treatment of recurrent malignant glioma patients with ex vivo‐expanded autologous tumor‐specific T lymphocytes yields favorable results.( 10 , 11 ) However, this therapeutic approach suffers from a serious limitation, namely, the successful expansion of autologous tumor‐specific T lymphocytes requires sufficient numbers of live tumor cells. This in turn necessitates the expansion of primary cultured tumor cells and the establishment of tumor cell lines.( 10 , 11 ) These processes are generally time‐consuming and tedious; moreover, tumor cells cannot always be successfully subcultured.( 12 )
An alternative immunotherapeutic approach is to use the formalin‐fixed paraffin‐embedded blocks of tumor tissues that are routinely prepared and stored after surgical removal. Because formalin fixation preserves the specific antigenicity of tumor cells,( 13 , 14 , 15 ) these preparations could serve as an alternative tumor antigen source for CTL induction. Indeed, Liu et al. have demonstrated that tumor‐specific autologous CTL can be generated by using several fixed sections; these cells have comparable activity and specificity to those induced by continuously cultured live tumor cells.( 14 , 15 ) Moreover, it has been shown that HLA‐A2402‐restricted CEA‐specific CTL can be induced by culturing human PBMC with formalin‐fixed (but not paraffin‐embedded) autologous adhesive PBMC loaded with CEA protein‐bound latex‐beads.( 16 ) Such CTL could also be generated by using formalin‐fixed adherent cells pulsed with 9‐ or 10‐mer CEA‐derived MHC‐class I‐presented TAA.( 16 ) The latter report further supports the notion that peptide TAA derived from fixed cells or proteins are formalin‐resistant. Thus, formalin‐fixed and/or paraffin‐embedded tumor cells/tissues may be useful TAA sources that can be used to generate effective antitumor immune cells, as the authors’ laboratory has shown a case whose CTL were induced ex vivo on the formalin‐fixed autologous GBM cells (see case no. 1 in Tsurushima et al. ( 11 )).
Based on these observations, the authors have previously constructed AFTV from surgically extirpated tumor tissues( 12 , 13 ) and showed that these vaccines had a positive immunotherapeutic effect when tested with an experimental rat brain tumor model.( 17 ) In addition, retrospective( 13 ) and prospective randomized clinical trials( 18 ) have revealed that AFTV are efficacious in patients with hepatocellular carcinoma. Here, the authors’ preliminary observations regarding the safety, feasibility and efficacy of AFTV‐based immunotherapy of GBM patients are reported.
Materials and Methods
Patients. Patients with pathologically verified primary GBM (WHO grade IV) and whose ages ranged from 17 to 70 years were included in this early clinical trial. Pathological diagnoses were made by two pathologists at the Department of Pathology, University Hospital of Tsukuba, Tsukuba, Ibaraki, Japan. Written informed consent was obtained from each patient before initiating any procedure related to this clinical study. The clinical study was approved by the Ethical Committee of University of Tsukuba on 23 October 2002. For all patients, the AFTV inoculations took place at least 4 weeks after prior antitumor treatments ceased. Patients with severe complications that could affect the survival time or patients in whom the expected survival period was less than 3 months were excluded from this trial.
To date, 12 primary GBM patients have been included in this pilot study (Table 1). Their average age was 50.5 years (range, 35–63 years) and the male : female ratio was 8:4. Of the 12 patients, eight had recurrent disease (#1–5, 7, 8, and 11) while four had been treated but retained a visible tumor mass (#6, 9, 10, and 12). The Karnofsky performance status (KPS) of the patients at the time of AFTV inoculation ranged from 50% to 90%; the average value was 70.8%. Three patients (#3, 5, and 9) presented with IICP at the time of treatment; of these, one patient (#9) was treated with oral steroids before, during and after the inoculations while the remaining two patients (#3 and 5) were given oral steroids several weeks after the inoculations. In addition, hemiparesis was observed in four patients (#1, 2, 4, and 6). Moreover, patients #6, 10, and 12 showed slight disorientation, while #11 had mild left limb ataxia plus dizziness due to the cerebellar lesion. One patient (#5) complained of lumbar pain due to dissemination of the tumor to the spinal canal.
Table 1.
Background of the 12 autologous formalin‐fixed tumor vaccine (AFTV)‐treated cases
| No. | Age/sex | Type of surgery | Total dose of RT (Gy) | Chemotherapy | Case type | KPS at inoculation (%) | FPV (%) |
|---|---|---|---|---|---|---|---|
| 1 | 55/M | Partial | 92.4 | ACNU × 2 | Rec. | 60 | 6.8 |
| 2 | 35/M | Subtotal | 60.0 | ACNU, IFN | Rec. | 70 | 5.8 |
| 3 | 39/M | Partial | 60.0 | ACNU, IFN | Rec. | 70 (IICP) | 13.3 |
| 4 | 59/F | Partial | 81.2 | ACNU × 2 | Rec. | 60 | 20.0 |
| 5 | 37/F | Partial | 92.4 | ACNU × 2, IFN | Rec. | 60 (IICP) | 20.0 |
| 6 | 59/M | Subtotal | 61.2 | ACNU × 2 | Init. | 50 | 20.0 |
| 7 | 43/M | Subtotal | 60.0 | ACNU × 3 | Rec. | 90 | 20.0 |
| 8 | 41/M | Partial | BNCT | ACNU × 2 | Rec. | 90 | 5.0 |
| 9 | 56/M | Partial | 92.4 | ACNU × 3, IFN | Init. | 70 (IICP) | 20.0 |
| 10 | 63/F | Subtotal | 92.4 | ACNU × 2 | Init. | 80 | 20.0 |
| 11 | 59/M | Subtotal | 61.2 | PAV × 1 | Rec. | 70 | 20.0 |
| 12 | 60/F | Subtotal | 61.2 | PAV × 1 | Init. | 80 | 20.0 |
ACNU, nimustine hydrochloride; BNCT, boron neutron capture therapy; F, female; FPV, final packed volume of the fixed tumor fragments in the AFTV; IICP, increased intracranial pressure; IFN, interferon‐β; Init., cases treated for the first time after diagnosis; KPS, Karnofsky performance status; M, male; Partial, partial removal (removal of <75% of the enhanced area); PAV, procarvasin + ACNU + vincristine; Rec., recurrent case; RT, radiation therapy; Subtotal, subtotal removal (removal of >75% of the enhanced area).
Preparation of AFTV and treatment schedule. AFTV were prepared from autologous formalin‐fixed and/or paraffin‐embedded tumor tissue and administered as described previously( 13 ) with slight modifications. The lesions used to prepare the vaccines were the same as those examined histologically, and histologically viable GBM tissues with minimal necrotic lesion and no (or minimal) edematic gliosis lesions were used for the preparation. As the adjuvant, tuberculin microparticles were used instead of the two immunoadjuvants (GM‐CSF microparticles and IL‐2 microparticles) used previously.( 13 , 18 ) This is because preliminary animal studies have revealed tuberculin microparticles have a stronger adjuvant effect (data not shown). The microparticles were prepared as described previously.( 13 ) The paraffin‐embedded tissue was deparaffinized prior to use by washing with sufficient amounts of xylene and alcohol. The AFTV for patients #2, 4, and 7 were prepared from autologous paraffin‐embedded tumor tissue, while the AFTV for patient #9 was generated from both paraffin‐embedded and formalin‐fixed tissues. The AFTV for the remaining seven patients were produced from formalin‐fixed autologous tumor tissue.
To prepare the AFTV, the fixed tissues were first thoroughly fragmented. The packed volume of the tissue was then determined after centrifugation at 11 100g for 5 min, 0.1 mL of alcohol extract prepared from 1.2 mg freeze‐dried BCG vaccine (Japan BCG Laboratory, Tokyo) was then added to 0.22 mL of the packed tumor tissue pellet. After washing the pellet with saline, the final concentration of the tissue fragments was adjusted to 20% (v/v; packed volume) suspended in 1 mL saline that also contained the tuberculin microparticles (250 ng tuberculin) and 250 ng soluble tuberculin (Japan BCG Laboratory). In four patients (#1, 2, 3, and 8), the final volume of packed tumor tissue was less than 20% of the preparation due to the small amount of tumor tissue available for vaccine preparation. The details are shown in Table 1.
The therapeutic schedule employed is shown in Fig. 1. Each course comprised three five‐site intradermal injections of AFTV. Thus, 0.2 mL of vaccine per site was injected into the upper arm every week. DTH tests were performed 48 h before the first vaccination (DTH‐1) and 2 weeks after the third and final vaccination (DTH‐2) by intradermally injecting the forearm with fixed autologous tissue fragments (10% v/v suspended in 0.1 mL of saline in the absence of adjuvant). The degree of DTH was determined by using the following cutoff values: localized erythema (including induration) measuring ≥10 mm in diameter was classified as (+), an erythema measuring 5–9 mm in diameter was classified as (+/–), while an erythema measuring ≤4 mm in diameter was classified as (–).
Figure 1.
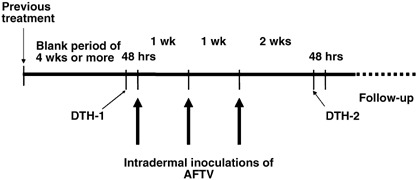
Schedule of autologous formalin‐fixed tumor vaccine (AFTV) treatment of glioblastoma multiforme (GBM) patients. Each treatment course consisted of three five‐site intradermal injections of AFTV into the upper arm every week. Delayed‐type hypersensitivity (DTH) tests were performed 48 h before the first vaccination (DTH‐1) and two weeks after the final vaccination (DTH‐2).
All patients except for #11 were followed without any additional treatment. In case #11, the patient was treated with oral administration of temozolomide (240 mg daily) without evidence of recurrence 3 months after AFFV therapy, because this newly available treatment was strongly insisted on by the patient himself with the reason that prognosis of patients with GBM of cerebellum is generally considered to be extremely poor. Therefore, the outcome for this patient was noted as 11.5+ and 3.0+ months after the operation and the vaccination, respectively, as shown in Table 2.
Table 2.
Clinical results of the autologous formalin‐fixed tumor vaccine (AFTV)‐treated glioblastoma multiforme (GBM) cases
| No. | DTH‐2 response | pre‐AFTV vol. (mL) | Response after AFTV (months) | Best response | Outcome‐op (months) | Outcome‐vac (months) | Outcome (KPS) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 (vol.) | 6 | 9 | 12 | 24 | |||||||
| 1 | + | 54.0 | NC | NC (67) | PD | PD | PD | – | NC | 31.7 | 20.0 | D |
| 2 | – | 1.8 | PD | PD (n.m.) | PD | PD | – | – | PD | 41.5 | 10.7 | D |
| 3 | + | 96.0 | PD | PD (139) | – | – | – | – | PD | 10.8 | 5.0 | D |
| 4 | + | 67.0 | NC | NC (42) | MR | PR (24) | PR | PD | PR | 31.8 | 20.3 | D |
| 5 | + | 13.0 | PD | PD (40) | – | – | – | – | PD | 9.1 | 4.5 | D |
| 6 | +/– | 32.0 | NC | NC (23) | PR | PR | PR | CR (0) | CR | 48.0+ | 44.3+ | A (60) |
| 7 | +/– | n.m. | PD | PD (n.m) | – | – | – | – | PD | 15.7 | 4.5 | D |
| 8 | – | n.m. | PD | PD (n.m) | – | – | – | – | PD | 10.8 | 3.6 | D |
| 9 † | –, – | 13.0 | PD + PD | PD (50) + PD | PD + PD | – | – | – | PD | 11.6 | 7.0 | D |
| 10 | + | 2.6 | PD | PD (3.5) | PD | PD | PD | – | PD | 24.0 | 17.0 | D |
| 11 | +/– | 10.0 | NC | MR (7) ‡ | (PR) ‡ | (PR) ‡ | (PD) ‡ | n.r. | MR ‡ | 11.5+ | 3.0+ | A (70) |
| 12 | +/– | 11.0 | NC | NC (12) | MR (6.3) | PD | n.r. | n.r. | MR | 12.7+ | 8.0+ | A (80) |
This patient was treated with two AFTV courses using initial and recurrent tumor tissue.
This patient showed 30% regression 3 months after the vaccination, and was then treated with temozolomide without evidence of recurrence because of the reason as described in Materials and Methods. Note that the best response was recorded as MR and his survival as 11.5+ and 3.0+ months after the first operation and the AFTV therapy, respectively. These values do not include the later period with the additional temozolomide treatment. (+) (+/–), and (–): diameter of the erythema is 10 mm or more, is from 9 to 5 mm, or is no more than 4 mm, respectively; A, alive; CR, complete response; D, dead; DTH‐2, delayed‐type hypersensitivity test 2 weeks after the last vaccination; KPS, Karnofsky performance status; MR, minor response; NC, no change; n.m., not measured using volumetry or not evaluated by MRI (magnetic resonance imaging); n.r., not reached; Outcome‐op, duration from first operation to outcome; Outcome‐vac, duration from vaccine inoculation to outcome; PD, progressive disease; PR, partial response; vol., volume.
Endpoint design and evaluation of the safety of the AFTV. The primary endpoints in this study were the safety and feasibility of ATFV immunotherapy in primary GBM patients. The secondary endpoint was the overall survival of the patients and the effect of the AFTV on the remaining tumor; the final response was determined to be the best response that was observed throughout the observation period. Because the cohort in this study was a mixture of eight patients with recurrent disease and three patients recruited after their first treatment for primary disease, survival time was calculated from the day of the first AFTV inoculation.
Extensive clinical and laboratory assessments were conducted before and after the vaccination, and adverse events and changes in laboratory values were graded according to National Cancer Institute Common Toxicity Criteria version 2.0 (CTC ver.2).
Evaluation of the effect of AFTV. The tumor regression rate was determined at approximately 1, 3, and 6 months after initiating the AFTV inoculations. After 6 months, follow‐up MRI was performed every 3 months if neurological deterioration was not observed. Tumor sizes were measured by volumetry of the Gd‐enhanced area on 3‐D MRI, as described previously.( 10 ) However, three patients (#2, 7, and 8) were evaluated to have PD by using the Response Evaluation Criteria in Solid Tumors( 19 ) criteria without volumetry; this was because these patients were followed by remote hospitals after vaccination and only limited information, including the maximal length of the tumor and the patient's condition, could be obtained for these patients.
Immunohistologic analysis. Paraffin‐embedded specimens were stained with HE or immunostained using the indirect immunoperoxidase method (Dako, Glostrup, Denmark) according to the manufacturer's recommendations. We used monoclonal antibodies against MIB‐1 (Dako), p53 (Dako), and the MHC class‐I complex (H‐300; Santa Cruz Biotechnology, Santa Cruz, CA, USA). In MIB‐1 and p53 staining, positive cells were counted and the MIB‐1 or p53 positivity indices were determined by calculating the ratios (%) of positive cells per 100 cells. For MHC class‐I staining, three homogenous areas that showed high tumor cell density at a low magnification (×100) on HE staining were selected, and pictures of the corresponding areas after MHC class‐I staining were captured. The results were examined by two independent investigators according to the method reported by Al‐Batran et al.( 20 ) MHC class‐I expression was graded as 0, 1+, 2+, or 3+ (0%, 1–24%, 25–50%, or >50% positive tumor cells, respectively). Heterogeneous staining was graded based on the area with the highest level of antigen expression. The scores shared by two or more of the three areas photographed by the two investigators were used for the analysis.
Results
The results of the preliminary AFTV trial are summarized in Table 2. It was observed that DTH after vaccination was (+) in five patients (#1, 3, 4, 5, and 10), (+/–) in four patients (#6, 7, 11, and 12), and (–) in three patients (#2, 8, and 9). Of the three DTH‐negative patients, patient #9 had received steroids before and during the inoculations. The remaining two DTH‐negative patients (#2 and 8) had been inoculated with AFTV preparations that contained less than 6% (v/v) packed tumor tissue (Table 1).
The treatment‐related adverse effects consisted of erythema, induration and swelling of the inoculated sites but were well tolerated by all patients (CTC ver.2 grade 1). Low‐grade fever with mild fatigue was noticed in six patients a few days after AFTV inoculation (CTC ver.2 grade 1); however, these were transient and did not require any medical treatment. Neither allergic dermatitis nor anaphylaxis was observed.
MRI revealed seven patients showed increases in the enhanced volume 1 and 3 months after the AFTV inoculations (Table 2), while five patients (#1, 4, 6, 11, and 12) showed stable disease (NC) 1 month after the AFTV inoculations. Of these five patients, three (#4, 6, and 11) subsequently showed a reduction in the enhanced volume at 3 months, and these changes correlated well with their clinical symptoms. The maximum regression ratios in patients #4 and 6 as determined by volumetry, were 63%, and 100%, respectively.
Patient #11 showed 30% regression 3 months after the vaccination (defined as an MR), the patient was then treated with temozolomide without evidence of recurrence because of the reason as described in Materials and Methods. Although this patient was excluded from the cohorts after this particular additional treatment and his survival was noted as 3.0+ months after the AFTV therapy, the tumor volume regression of this patient reached to 55% and kept the regressed volume for 9 months after the AFTV therapy (Table 2). Of the seven patients that showed tumor progression, three failed to show DTH‐2 response. In contrast, the DTH‐2 was (+/–) or (+) in all five patients whose responses were greater than NC (Table 2) and their FPV of the fixed tumor fragments in the AFTV were all 20% (Table 1). The median survival of DTH‐2 (+) or (+/–) patients tended to be superior to that of DTH‐2 (–) patients, although statistical significance was not proved (17.0 vs 7.0 months, P = 0.12). None of the 12 patients showed positive DTH‐1 response.
A surgical specimen from 10 patients was available for immunohistochemical analysis; specimens were not available from patients #2 and 7 (Table 3). The MIB‐1 index ranged from 3.2 to 42.6, with an average value of 25.3. In patient #9, the MIB‐1 index of 3.2 was calculated by using the specimen obtained at the second surgery, which was performed after the patient showed a recurrence of the disease. A specimen taken during the first surgery was not available for this patient. The p53 staining index varied widely from 0 to 64.4, with an average value of 16.4. The MHC class‐I staining scores were determined by two investigators and did not vary widely; these scores ranged from 1 to 3 and the average value was 2.3.
Table 3.
Immunohistochemical analysis of tumor tissue taken from glioblastoma multiforme (GBM) cases prior to autologous formalin‐fixed tumor vaccine inoculation
| No. | MIB‐1 (%) | p53 (%) | MHC class‐I (score) |
|---|---|---|---|
| 1 | 23.0 | 3.0 | 2+ |
| 2 | n.a. | n.a. | n.a. |
| 3 | 25.9 | 64.4 | 2+ |
| 4 | 8.8 | 2.8 | 3+ |
| 5 | 25.0 | 10.5 | 2+ |
| 6 | 31.0 | 6.0 | 3+ |
| 7 | n.a. | n.a. | n.a. |
| 8 | 38.2 | 45.5 | 2+ |
| 9 † | 3.2 | 26.2 | 2+ |
| 10 | 26.2 | 3.0 | 1+ |
| 11 | 29.1 | 0.0 | 3+ |
| 12 | 42.6 | 2.5 | 3+ |
Tumor tissue used was taken during the second surgery (after the disease recurred). MHC, major histocompatibility complex; n.a., specimen was not available for immunohistochemistry.
The ability of the immunohistochemical data to predict responsiveness to AFTV treatment and the predictive value of various patient factors were analyzed. Thus, the mean values of these factors of the responders (#1, 4, 6, 11 and 12) were compared to those of the non‐responders who showed disease progression (Table 4). The responders were significantly older than the non‐responders (58.4 vs 44.9 years; P = 0.019) at the time of AFTV inoculation. Comparison of the tumor immunohistochemistry data of the five responders (#1, 4, 6, 11, and 12) with those of five non‐responders (#3, 5, 8, 9, and 10) revealed equivalent MIB‐1 indices (mean: 26.9 vs 23.7; range: 8.8–42.6 vs 3.2–38.2) but significantly lower p53 indices (mean: 2.9 vs 29.9; range: 0–6 vs 3.0–64.4; P = 0.021) and significantly higher MHC class‐I staining (mean: 2.8 vs 1.8; range: 2–3 vs 1–2; P = 0.015).
Table 4.
Ability of various factors to predict the efficacy of autologous formalin‐fixed tumor vaccine (AFTV) treatment
| Cases | Responders (n = 5) | Non‐responders (n = 7) | P‐value | |||
|---|---|---|---|---|---|---|
| Age (years) | 58.4 | ±1.9 | 44.9 | ±10.5 | 0.019 | # |
| Total dose of RT † (Gy) | 71.4 | ±14.6 | 76.2 | ±17.7 | n.s. | # |
| Recurrent cases (n) | 3 | 5 | n.s. | ## | ||
| KPS at inoculation (%) | 64.0 | ±11.4 | 75.7 | ±11.3 | n.s. | ### |
| IICP (n) | 0 | 3 | n.s. | ## | ||
| FPV (%) | 17.3 | ±5.9 | 14.8 | ±6.9 | n.s. | ### |
| DTH‐2 ‡ (score) | 1.4 | ±0.3 | 1.0 | ±1.0 | n.s. | ### |
| MIB‐1 § (%) | 26.9 | ±12.4 | 23.7 | ±12.7 | n.s. | ### |
| p53 § (%) | 2.9 | ±2.1 | 29.9 | ±25.2 | 0.021 | ### |
| MHC class‐I § (score) | 2.8 | ±0.4 | 1.8 | ±0.4 | 0.015 | ### |
| Outcome‐op. (months) | 31.8 ¶ | ±0.1 | 11.6 ¶ | ±3.2 | 0.049 | #### |
| Outcome‐vac. (months) | 20.3 ¶ | ±0.2 | 5.0 ¶ | ±1.6 | 0.004 | #### |
One (#8) of the non‐responder cases was excluded from analysis because of boron neutron capture therapy.
+, +/–, and –: 2, 1, and 0 points, respectively.
Two non‐responders (#2 and #7) were excluded from analysis because their specimens were not available for immunohistochemistry.
¶ Median values.
Student's t‐test,
## Fischer's direct method,
Mann–Whitney's U‐test,
Log‐rank test. DTH‐2, delayed‐type hypersensitivity test 2 weeks after the last vaccination; FPV, final packed volume of the fixed tumor fragments in the AFTV; IICP, increased intracranial pressure; KPS, Karnofsky performance status; MHC, major histocompatibility complex; n.s., not significant; Outcome‐op, duration from first operation to outcome; Outcome‐vac, duration from vaccine inoculation to outcome.
At the time of writing, nine cases had died due to tumor progression, and three patients were alive and one of them had not demonstrated a recurrence of disease (#6). Fig. 2 shows the survival curve of all patients. The median and mean survival times of the patients from the day of AFTV inoculation were 10.7 and 11.9 months, respectively, while the median and mean survival times from the day of the first operation were 24.0 and 24.0 months, respectively. The average survival times from the day of AFTV inoculation for the responders and non‐responders were 20.3 and 5.0 months, respectively (n = 5 and 7, respectively). This difference was statistically significant (Table 4 and Fig. 2b, P = 0.004, log‐rank test).
Figure 2.
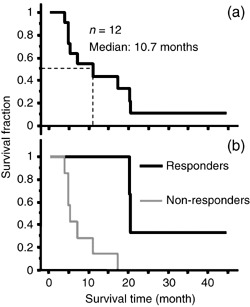
Survival curve of autologous formalin‐fixed tumor vaccine (AFTV)‐treated glioblastoma multiforme (GBM) cases. The survival curve of the 12 cases was calculated using the Kaplan–Meier method. Survival times were calculated from the day AFTV therapy was initiated. (a) Three patients survived 20 months or more. The median survival time is 10.7 months. (b) Survival curve of responders (#1, 4, 6, 11, and 12) and non‐responders (#2, 3, 5, 7–10). The median survival time is 20.3 and 5.0 months.
Representative cases.
Case #4. A 59‐year‐old woman presented with recurrent GBM in the right frontotemporal lobe after surgery. She received 81.2 Gy (61.2 Gy with conventional X‐ray and 20 Gy with proton boost) locally along with two ACNU (100 mg/m2) chemotherapeutic treatments. The recurrence was found 9 months after the previous treatment. The second surgery was performed to prove the diagnosis of the recurrence but not radiation necrosis and to obtain viable tissue. Her consciousness was clear and she consented to AFTV treatment. The tumor volume after the second operation was increased compared with before the operation because of drastic growth of the tumor. However, surprisingly, her enhanced volume gradually decreased up to 9 months after AFTV inoculation as demonstrated in Fig. 3; at that time, she was determined to be a partial responder. The tumor size was stable during the subsequent 8 months. However, 11 months after AFTV inoculation, tumor regrowth was noticed on MRI, and she died 9 months later due to tumor recurrence. The time from AFTV inoculation to death was 20.6 months.
Figure 3.
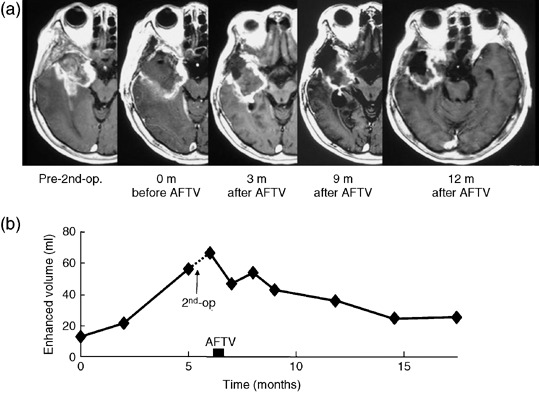
Case #4. This 59‐year‐old woman showed regrowth of a right temporal glioblastoma. After the second surgery, she was inoculated with autologous formalin‐fixed tumor vaccine (AFTV). (a) Serial T1‐weighted MRI with Gadolinium enhancement demonstrate a gradual decrease of the enhanced area with a resolution of mass effect. (b) The maximum reduction was 63%, as shown by plotting the volumetry results against time.
Case #6. A 59‐year‐old man with a right frontal GBM presented with left hemiparesis and mild disorientation. Because the posterior part of the tumor was located in the motor area, subtotal tumor removal was performed. Following surgery, the patient underwent conventional radiotherapy; in total, 61.2 Gy was administered to the field encompassing almost the entire right frontal and parietal lobes. The patient also had two sessions of ACNU (100 mg/m2) chemotherapy. After these initial treatments, the patient's family expressed the desire for maintenance immunotherapy. After obtaining consent, the patient was inoculated with AFTV 5 weeks after the completion of radiotherapy. At this time, tumor recurrence was not noticed on MRI. Serial MRI (Fig. 4) demonstrated a gradual decrease in the irregular enhanced area, despite the fact that the patient had not received any other treatment except AFTV inoculation. After 6 months, the patient was determined to be a partial responder. At 20 months after the AFTV inoculation, the patient was determined to be a complete responder. T1WI after Gd administration on MRI at 20 months demonstrated an increase in the volume of the resection bed with some areas of bright signal intensity; these changes are believed to be due to scarring because the lesions remained hyper‐intense before Gd administration and the brain tissue containing the bright signal intensity lesions was not edematous. At the time of writing, 44 months after AFTV inoculation, a recurrence has not been detected. The histopathology data of the tumor tissue of this patient are shown in Fig. 5. HE staining (×200) demonstrated high cellularity with various sized nuclei associated with multiple necrosis foci, which is typical of GBM. Immunohistochemical analyses of the same section demonstrated that MIB‐1 positivity was 31%, p53 positivity was 6%, and MHC class‐I staining was very high (a score of 3+).
Figure 4.
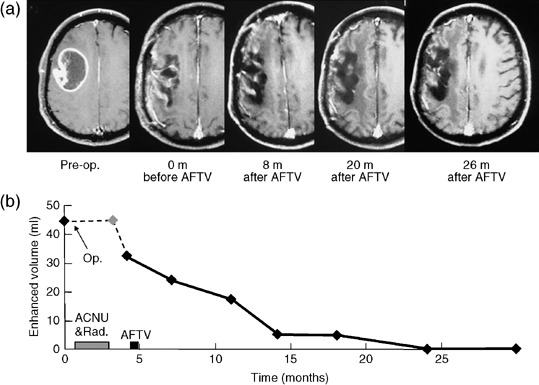
Case #6. This 59‐year‐old man with a right frontal glioblastoma was given the standard initial treatment and then inoculated with autologous formalin‐fixed tumor vaccine (AFTV). (a) Serial T1‐weighted MRI with Gadolinium enhancement demonstrate the irregular enhanced area gradually decreased, eventually disappearing altogether at 20 months after AFTV. (b) Plot of the volumetry results against time. This patient was evaluated to be in complete remission.
Figure 5.
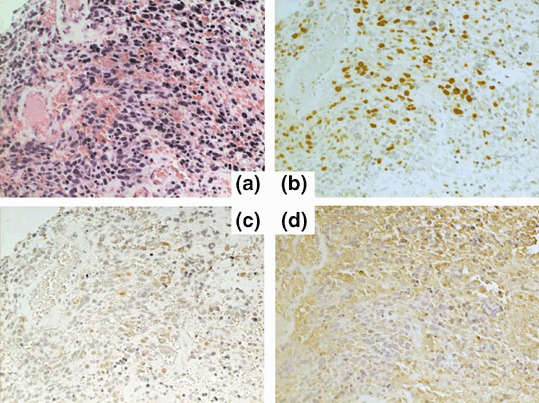
HE and immunohistochemical staining of the tumor tissue taken from patient #6. (a) HE staining (×200) shows the typical appearance of glioblastoma multiforme with (b) a high MIB‐1 index of 31%. (c) In the same area, p53 positivity is 6% and (d) there is strong immunoreactivity to an anti‐major histocompatibility complex class‐1 antibody.
Discussion
Concept and major features of AFTV treatment for GBM. The authors have previously showed that not only cultured tumor cells but also formalin‐fixed paraffin‐embedded tumor tissues can be used to expand autologous tumor‐specific CTL in vitro. ( 14 , 15 ) In addition, when such ex vivo‐expanded autologous tumor‐induced T lymphocytes (a mixture of CD8+ and CD4+ T cells) are returned to patients with recurring GBM, they efficiently kill malignant glioma cells without causing significant complications.( 10 , 11 ) These results indicate that glioblastoma cells present immunologically recognizable TAA and that it may be possible to induce immunological reactions against glioblastomas in vivo. Indeed, the authors have shown in a rat intracranial tumor model that immunization of the rats with AFTV prevents the expansion of tumor cells injected after AFTV inoculation.( 17 ) Moreover, AFTV were shown to act therapeutically in the same rat brain tumor model when delivered along with adoptively transferred autologous NK cells.( 17 )
Different types of antitumor vaccines have been proposed, including sensitization with purified TAA. However, the effectiveness of such peptide vaccines remains limited at present. Indeed, a recent report suggests that TAA peptide vaccines induce effective antitumor effects in only less than 3% of vaccinees.( 21 ) TAA peptide vaccines are also limited by the extreme difficulty in not only identifying TAA but also obtaining TAA in sufficient quantities. Nevertheless, promising results have been reported in a pilot clinical study on 25 patients with malignant glioma who were treated with personalized peptide vaccination.( 22 ) Of the 15 GBM cases who were submitted for clinical evaluation, although seven had progressive disease, three showed partial responses and five had stable disease. All three partial response patients had received more than 18 vaccinations.
Another interesting antitumor immunotherapeutic approach is to inject patients with DC pulsed with tumor lysate or TAA. This approach requires the ex vivo culture of DC in the presence of cytokines and thus is an adoptive immunotherapeutic strategy. Recently, Yamanaka et al. reported the preliminary results of 24 malignant gliomas cases who were given DC pulsed with autologous tumor lysate. Image analyses of the 19 GBM patients revealed that although eight had progressive disease, one showed a partial response, three had a minor response, and seven showed stable disease.( 23 )
Of the 12 AFTV‐treated GBM patients who were evaluated in this report, although seven had progressive disease, one had a complete response, one had a partial response, two had minor responses, and one showed stable disease. Thus, AFTV appears to be as good, if not better, at slowing or halting disease in malignant glioma patients as personalized peptide vaccines( 22 ) or DC pulsed with autologous tumor lysate.( 23 ) Moreover, the AFTV approach has a number of additional advantages. First, AFTV are considerably easier and cheaper to prepare and handle. Indeed, patients could be treated with AFTV on an outpatient basis, which is not possible with adoptive immunotherapy using CTL. However, AFTV immunotherapy also suffers from a serious limitation, namely, it requires sufficient amounts of pure autologous tumor tissue. To be most effective, the AFTV source should ideally comprise only tumor cells and should be as representative of the entire tumor as possible. It was found in this study that in some cases it was not possible to obtain sufficient amounts of usable quality material from the paraffin‐embedded tissues: indeed, four patients received vaccines that consisted of less than 20% packed tumor tissues. Of these patients, three did not respond to the therapy. Thus, the efficacy of AFTV is likely to depend on the amount and purity of the tumor tissues used to prepare the vaccines.
Evaluation of the AFTV treatment results. The main purpose of the present clinical study on the efficacy of AFTV in GBM patients was to test its safety and ability to suppress tumor growth after the patients had been treated with surgery and radiotherapy. Possible acute adverse effects associated with this immunotherapy include induration at the inoculated sites, fever, fatigue, and anaphylactic shock. In addition, late adverse effects may be the development of autoimmune disorders, including leukoencephalopathy, due to contamination of the vaccine with normal brain tissues. In the study reported here, however, no severe adverse effects were observed. Erythema/indurations at the inoculation sites and mild fevers were reported but did not require additional medical treatment. While the natural course of glioblastoma may mask the appearance of the late adverse effects in many PD patients, it seems that the AFTV did not generate significant side‐effects and that this treatment was well tolerated by the patients.
The small sample size employed in our study makes it difficult to determine the true efficacy of AFTV treatment but it appears that at least five patients (#1, 4, 6, 11 and 12) benefited from the treatment. In particular, patient #6 showed a complete response and was disease‐free at the time of writing. Considering the time course of clinical responses in primary GBM cases, the initial antitumor effect in case #6 may have been caused by irradiation and ACNU rather than AFTV treatment, because 61.2 Gy of irradiation had been administered before AFTV inoculation. However, over the authors’ clinical experience of more than 20 years, few cases with visible GBM remaining after primary resection have resulted in CR and survival for more than 40 months after the standard irradiation and ACNU treatment. So, it is highly probable that the initial treatment and following AFTV treatment affected the CR and the patient's long survival synergistically.
In contrast, the other seven patients failed to show significant slowing of the disease course. Thus, AFTV treatment seems to generate an all‐or‐nothing response. Why a particular subset of patients responded to this particular type of therapy while others did not is not clear at present. The presence of large intracranial mass lesions and treatment with steroids may limit the efficacy of AFTV treatment. Given these encouraging results, AFTV should be investigated further to establish its true efficacy.
Moreover, it would be valuable to determine the factors that can predict its efficacy so that the patients who could benefit from this therapy can be identified. At present, it is likely that the treatment will not be effective in patients with rapidly progressive neurological symptoms who suffer from IICP (e.g. patients #3, #5 and #9). Concerning the DTH‐2 responses, although a significant statistical difference was not detected between responders and non‐responders, all patients with clinical response showed (+) or (+/–). All patients that showed negative DTH‐2 response exhibited no clinical response. So, the authors speculate that, because all DTH‐1 responses were (–), in patients with DTH‐2 (+/–) as well as those with DTH‐2 (+), glioblastoma‐specific CTL might have been induced in vivo. The reason why the older and the lower KPS patients were included in the responder group is unclear, although in general older age and lower KPS are considered to be adverse factors.
The immunohistochemical analysis of the resected tumors obtained from the patients suggests that the clinical response is poor in patients #3, 5, 8, and 9, whose tumors were highly p53 positive. Notably, a previous report has suggested that low levels of p53 protein are a fairly reliable indicator of the presence of the wild‐type gene.( 24 ) However, because some tumors show p53 immunopositivity in the absence of a detectable mutation, it is not necessarily true that high p53 protein levels indicate the presence of a p53 gene mutation. Nevertheless, it is currently accepted that the p53 gene status correlates highly with the p53 expression status,( 24 ) which suggests that tumors bearing a p53 mutation may be less sensitive to AFTV therapy. This is in accord with the recent reports by Thiery et al. who found that restoration of wild‐type p53 expression and function significantly potentiates target tumor susceptibility to CTL‐mediated lysis.( 25 , 26 ) In addition, the authors’ previous in vitro study indicated that the Fas–FasL‐mediated CTL lysis of tumor cells depends on the p53 status of the tumor.( 27 )
It was also found that AFTV prepared from tumors that showed strong MHC class‐I staining (Table 3) tended to induce an effective antitumor response (Table 2, patients #4, 6, and 11). This is supported by the study of Al‐Batran et al., who reported a significant positive correlation between MHC class‐I expression by melanoma and the presence of CD4+ and CD8+ T cells in the melanoma tissue.( 20 ) Thus, it is possible that intratumoral infiltration of T cells and tumor cell killing may depend on the MHC class‐I expression of the tumor. Future studies employing more patients will be needed to confirm whether p53 and MHC class‐I expression by GBM tumors can indeed be used to predict the efficacy of AFTV therapy.
Here, the authors report a pilot study analyzing the efficacy of treating patients with primary GBM with AFTV. The AFTV therapy was safe and did not induce any severe adverse effects. Of the 12 patients examined, four responded to this therapy: one showed a complete response, one showed a partial response, two showed minor responses, and one had stabilization of disease. Indeed, three of these patients survived for 20 months or more after AFTV inoculation. Immunohistologic analysis of p53 and MHC class‐I expression by the tumor may help predict the efficacy of this therapy. In addition, the best therapeutic outcome requires that, ideally, 20% of the AFTV should be composed of the original tumor tissue.
E. Ishikawa and K. Tsuboi contributed equally to this work.
References
- 1. The Committee of Brain Tumor Registry of Japan . Report of Brain Tumor Registry of Japan (1969–96), 11th edn. Neurolgia Medico‐Chirurugica, 43 (Suppl.). Tokyo: The Japan Neurological Society, 2003. [Google Scholar]
- 2. Hulshof MC, Koot RW, Schimmel EC et al . Prognostic factors in glioblastoma multiforme. 10 years experience of a single institution. Strahlenther Onkol 2001; 177: 283–90. [DOI] [PubMed] [Google Scholar]
- 3. Lacroix M, Abi‐Said D, Fourney DR et al . A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001; 95: 190–8. [DOI] [PubMed] [Google Scholar]
- 4. Prados MD, Larson DA, Lamborn K et al . Radiation therapy and hydroxyurea followed by the combination of 6‐thioguanine and BCNU for the treatment of primary malignant brain tumors. Int J Radiat Oncol Biol Phys 1998; 40: 57–63. [DOI] [PubMed] [Google Scholar]
- 5. Shrieve DC, Alexander E III, Black PM et al . Treatment of patients with primary glioblastoma multiforme with standard postoperative radiotherapy and radiosurgical boost: prognostic factors and long‐term outcome. J Neurosurg 1999; 90: 72–7. [DOI] [PubMed] [Google Scholar]
- 6. Walker MD, Green SB, Byar DP et al . Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 1980; 303: 1323–9. [DOI] [PubMed] [Google Scholar]
- 7. Kristiansen K, Hagen S, Kollevold T et al . Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer 1981; 47: 649–52. [DOI] [PubMed] [Google Scholar]
- 8. Glioma Meta‐analysis Trialists (GMT ) Group (Stewart LA ). Chemotherapy in adult high‐grade glioma: a systematic review and meta‐analysis of individual patient data from 12 randomised trials. Lancet 2002; 359: 1011–18. [DOI] [PubMed] [Google Scholar]
- 9. Stupp R, Mason WP, Van Den Bent MJ et al . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–96. [DOI] [PubMed] [Google Scholar]
- 10. Tsuboi K, Saijo K, Ishikawa E et al . Effects of local injection of ex vivo expanded autologous tumor‐specific T lymphocytes in cases with recurrent malignant gliomas. Clin Cancer Res 2003; 9: 3294–302. [PubMed] [Google Scholar]
- 11. Tsurushima H, Liu SQ, Tsuboi K et al . Reduction of end‐stage malignant glioma by injection with autologous cytotoxic T lymphocytes. Jpn J Cancer Res 1999; 90: 536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohno T. Autologous cancer vaccine: a novel formulation. Microbiol Immunol 2003; 47: 255–63. [DOI] [PubMed] [Google Scholar]
- 13. Peng BG, Liu SQ, Kuang M et al . Autologous fixed tumor vaccine: a formulation with cytokine‐microparticles for protective immunity against recurrence of human hepatocellular carcinoma. Jpn J Cancer Res 2002; 93: 363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu SQ, Saijo K, Todoroki T et al . Induction of human autologous cytotoxic T lymphocytes on formalin‐fixed and paraffin‐embedded tumour sections. Nat Med 1995; 1: 267–71. [DOI] [PubMed] [Google Scholar]
- 15. Liu SQ, Shiraiwa H, Kawai K et al . Tumor‐specific autologous cytotoxic T lymphocytes from tissue sections. Nat Med 1996; 2: 1283. [DOI] [PubMed] [Google Scholar]
- 16. Kim C, Matsumura M, Saijo K et al . In vitro induction of HLA‐A2402‐restricted and carcinoembryonic‐antigen‐specific cytotoxic T lymphocytes on fixed autologous peripheral blood cells. Cancer Immunol Immunother 1998; 47: 90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishikawa E, Tsuboi K, Takano S et al . Intratumoral injection of IL‐2‐activated NK cells enhances the antitumor effect of an intradermally injected paraformaldehyde‐fixed tumor vaccine in a rat intracranial brain tumor model. Cancer Sci 2004; 95: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuang M, Peng BG, Lu MD et al . Phase II randomized trial of autologous formalin‐fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res 2004; 10: 1574–9. [DOI] [PubMed] [Google Scholar]
- 19. Therasse P, Arbuck SG, Eisenhauer EA et al . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 20. Al‐Batran SE, Rafiyan MR, Atmaca A et al . Intratumoral T‐cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res 2005; 65: 3937–41. [DOI] [PubMed] [Google Scholar]
- 21. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10: 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yajima N, Yamanaka R, Mine T et al . Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res 2005; 11: 5900–11. [DOI] [PubMed] [Google Scholar]
- 23. Yamanaka R, Homma J, Yajima N et al . Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 2005; 11: 4160–7. [DOI] [PubMed] [Google Scholar]
- 24. Simmons ML, Lamborn KR, Takahashi M et al . Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res 2001; 61: 1122–8. [PubMed] [Google Scholar]
- 25. Thiery J, Dorothee G, Haddada H et al . Potentiation of a tumor cell susceptibility to autologous CTL killing by restoration of wild‐type p53 function. J Immunol 2003; 170: 5919–26. [DOI] [PubMed] [Google Scholar]
- 26. Thiery J, Abouzahr S, Dorothee G et al . p53 potentiation of tumor cell susceptibility to CTL involves Fas and mitochondrial pathways. J Immunol 2005; 174: 871–8. [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa E, Tsuboi K, Saijo K et al . X‐irradiation to human malignant glioma cells enhances the cytotoxicity of autologous killer lymphocytes under specific conditions. Int J Radiat Oncol Biol Phys 2004; 59: 1505–12. [DOI] [PubMed] [Google Scholar]


