Abstract
p120‐catenin, a member of the Armadillo gene family, has emerged as both a master regulator of cadherin stability and an important modulator of small GTPase activities. Therefore, it plays novel roles in tumor malignant phenotype, such as invasion and metastasis. We have reported previously that abnormal expression of p120‐catenin is associated with lymph node metastasis in lung squamous cell carcinomas (SCC) and adenocarcinomas. To investigate the role and possible mechanism of p120‐catenin in lung cancer, we knocked down p120‐catenin using small interfering RNA (siRNA). We found that ablation of p120‐catenin reduced the levels of E‐cadherin and β‐catenin proteins, as well as the mRNA of β‐catenin. Furthermore, p120‐catenin depletion inactivated RhoA, but increased the activity of Cdc42 and Rac1, and promoted proliferation and the invasive ability of lung cancer cells both in vitro and in vivo. Our data reveal that p120‐catenin gene knockdown enhances the metastasis of lung cancer cells, probably by either depressing cell–cell adhesion due to lower levels of E‐cadherin and β‐catenin, or altering the activity of small GTPase, such as inactivation of RhoA and activation of Cdc42/Rac1. (Cancer Sci 2009; 100: 441–448)
E‐cadherin is the main epithelial cell–cell adhesion molecule, which is frequently found to be functionally disrupted or aberrantly expressed during tumor progression.( 1 , 2 , 3 ) p120‐catenin (p120ctn), a member of the catenin family, can regulate E‐cadherin stability, and thus plays important roles in cell–cell adhesion, signal transduction and gene expression.( 4 , 5 , 6 , 7 ) However, the role of p120ctn in cancer is currently controversial since evidence suggests that p120ctn can both positively and negatively regulate adhesive ability of cancer cells. One reason for this contradiction may lay in the large number of p120ctn isoforms, which arise from alternative splicing events, expressed in different cells and tissues.( 6 , 8 , 9 )
p120ctn is also an important modulator of the small GTPases (e.g. RhoA, Rac, Cdc42), which mediate cytoskeletal dynamics and emerge as crucial regulators of cadherin‐dependent adhesion,( 10 , 11 , 12 , 13 ) perhaps by acting as a molecular switch to orchestrate the balance between cellular adhesion and migration, which may in part, explain its roles in regulating cadherin stability and/or cadherin clustering.( 14 , 15 , 16 , 17 ) Several lines of evidence elicited from overexpression of p120ctn in various cell lines suggest that p120ctn regulates the activity of small GTPase, but the results are controversial.( 12 , 14 , 18 )
Studies have demonstrated that the p120ctn proteins were reduced or even eliminated in many human cancers and have suggested a role for p120ctn in the development and metastasis of cancer.( 19 ) We have reported previously that abnormal expression of p120ctn is associated with lymph node metastasis in lung squamous cell carcinomas (SCC) and adenocarcinomas.( 20 ) However, it is currently unknown how p120ctn regulates the invasiveness and metastasis of cancer cells in lung cancer. To address this, we knocked down p120ctn with small interfering RNA (siRNA) in human lung cancer cell lines BE1, SPC and LTE, and explore the levels of E‐cadherin and β‐catenin expression and the activity of the small GTPases, as well as the invasiveness and metastatic ability of lung cancer cells both in vitro and in vivo.
Materials and Methods
Cell culture. BE1, SPC, LTE cell lines were cultured in RPMI 1640 tissue culture medium (Invitrogen, Carlsbad, CA, USA), containing 10% fetal calf serum (Invitrogen), 100 IU/mL penicillin (Sigma, St Louis, MO, USA), and 100 µg/mL streptomycin (Sigma).
p120ctn siRNA plasmids and transfection. For the production of the p120ctn‐siRNA (GenBank accession no. NM001331) plasmids used in the experiments, three double‐stranded (ds) RNAs were tested for their ability to down‐regulate endogenous p120ctn expression. The sequences of the three double‐stranded oligonucleotides were as follows:
-
1
A: 5′‐ggatcacagtcaccttcta‐3′; 5′‐tagaaggtgactgtgatcc‐3′
-
2
B: 5′‐gcacttgtattacagacaa‐3′; 5′‐ttgtctgtaatacaagtgc‐3′
-
3
C: 5′‐ggataacaagattgccata ‐3′; 5′‐tatggcaatcttgttatcc‐3′
The BE1, SPC, LTE cells were stably transfected with the p120ctn‐siRNA plasmids using Lipofectamine 2000 (Invitrogen), following the manufacturer's instructions. The empty plasmid was used as a negative control. Selection was accomplished with G418 (Sigma) at a concentration of 0.3 mg/mL. Drug‐resistant cells were tested for p120ctn expression by Western blot and reverse transcription polymerase chain reaction (RT‐PCR).
p120ctn cDNA plasmids and transfection. Following Dr Reynolds’ method( 21 ) mouse p120ctn 1A and p120ctn 3A cDNA (a gift from Dr Reynolds, Vanderbilt University, Nashville, TE, USA) were transfected using Lipofectamine 2000 (Invitrogen) into p120ctn knockdown cells. Re‐expression of p120ctn was confirmed by Western blot.
RT‐PCR. Total RNA was extracted from cells with TRIzol Reagent (Invitrogen). RT‐PCR was performed with the RNA PCR Kit (AMV) Version 3.0 (TaKaRa Bio Inc., Dalian, Liaoning, China), according to the manufacturer's instructions. The primer sequences werer as follows:
-
1
p120ctn: 5′‐TGCCCTGCTGGATTTGTCTT‐3′; 5′‐CGAGTGGTCCCATCATCTG‐3′
-
2
β‐actin: 5′‐AGAGCTACGAGCTGCCTGAC‐3′; 5′‐AGTACTTGCGCTCAGGAGGA‐3′
-
3
E‐cadherin: 5′‐ACCAGAATAAAGACCAAGTGACCA‐3′; 5′‐TGCCCCATTCGTTCAAGTAGT‐3′
-
4
β‐catenin: 5′‐GCCAAGTGGGTGGTATAGAG‐3′; 5′‐GCTGGGTCCTGATGTGC‐3′
After electrophoresis on a 1.5% agarose gel, the PCR product bands were visualized with BioImaging Systems and quantitated with Labworks Image Acquisition and Analysis Software (UVP Inc., Upland, CA, USA). The relative mRNA levels were normalized to the relative amount of β‐actin mRNA.
Western blot. The cells were lysed in lysis buffer [50 mM Tris‐HCl (pH 8.0), 150 mM NaCl, 0.5% Nonidet P40, 0.5% sodium deoxycholate, and phenylmethylsulfonyl fluoride (PMSF; all from Sigma)]. Each sample (50 µg) was separated by 8% or 12% sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After blocking with 1% bovine serum albumen (BSA) in Tris‐buffered saline‐Tween (TBST; 20 mM Tris‐HCl, 500 mM NaCl, 0.05% Tween‐20), the membrane was incubated overnight at 4°C with either the mouse monoclonal antibody against p120ctn (1 : 400; BD Transduction Laboratories, Franklin Lakes, NJ, USA), E‐cadherin (1 : 200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), β‐catenin (1 : 200; Santa Cruz Biotechnology), Cdc42 (1 : 300; Santa Cruz Biotechnology), or Rac1 (1 : 500; Upstate Biotechnology Incorporated, Lake Placid, NY, USA). After incubation with peroxidase‐coupled antimouse immunoglobulin G (IgG) (SABC, Beijing, China) at 37°C for 2 h, the protein bands were visualized using ECL (Pierce, Rockford, IL, USA) and detected using the BioImaging Systems. The relative protein levels were calculated by comparison with the amount of β‐actin protein.
Rhoa, cdc42, and rac1 activity assay. RhoA activity was measured using colorimetric‐based RhoA G‐LISA™ activation assay (BK124, Cytoskeleton, Denver, CO, USA) according to the manufacturer's instructions. The resulting samples were analyzed by spectrophotometer (BD Transduction Laboratories). Cdc42 and Rac1 activity was measured using a pull‐down assay (EZ‐detect Cdc42/Rac1 activation kit, Pierce, Rockford, IL, USA). Cdc42‐GTP/Rac1‐GTP‐bound proteins were confirmed by Western blot.
Flow cytometry (FCM). After 48 h of culture, the cells from each experimental group were collected and resuspended with 50 µg/mL propidium iodide (Sigma) for 45 min in the dark before analysis. The percentages of cells in the different cell cycle phases were determined using a FACS Calibur Flow Cytometer with CellQuest 3.0 software (BD Biosciences, San Jose, CA, USA). Experiments were performed in triplicate.
Matrigel invasion assay. Following the manufacturer's instructions, in the upper chambers, 5 × 105 cells were grown in serum‐free medium on 8‐µm porous polycarbonate membranes (Corning, Acton, MA, USA), which were coated with Matrigel basement membrane matrix (BD Biosciences). The lower chambers were filled with RPMI 1640 medium containing 10% fetal calf serum. After incubation for 6, 16, or 24 h at 37°C in a humid atmosphere of 5% CO2 and 95% air, the cells that had migrated through the pores were fixed with methanol for 30 min and stained with hematoxylin (Sigma). Then the number of cells counted visually using Nikon E200 microscope in five different fields under 200× magnifications per filter. Each experiment was performed in triplicate.
Tumor cells transplanted into nude mice. All of the nude mice in this study were treated following the experimental animal ethics rules of China Medical University. Four‐week‐old male BALB/c nude mice were obtained from the animal facility approved by the China Medical University. Mice were kept in a laminar‐flow cabinet under specific pathogen‐free conditions for 2 weeks before use. Each mouse was inoculated subcutaneously in the back with 2.4 × 106 tumor cells (untransfected cells, empty‐vector‐transfected cells, or p120ctn‐siRNA‐transfected cells) in 0.3 mL sterile phosphate buffered saline (PBS). Six weeks after injection, the mice were sacrificed and one intact tumor and the whole heart, liver, lung, and the muscle of leg from each animal were carefully trimmed. Part of the tumor tissue and the organ was fixed in 4% paraformaldehyde (Sigma) in PBS, and then embedded in paraffin. Serial sections were cut at 6 µm thickness and stained with hematoxylin and eosin (H&E). Tumor cells in pleural fluid were stained by Papanicolaou staining. Samples were examined with an Olympus CH30 microscope, and images were obtained with a CoolPIX 5400 camera (Nikon, Tokyo, Japan).
H&E staining and Papanicolaou staining. The sections were stained by H&E (Sigma), and the pleural fluid sections were stained by Orange G6 (Sigma) and EA36 (Sigma).
Statistical analysis. All statistical calculations were performed by SPSS 11.5 for Windows software. The data from cells in different experimental groups was compared using the independent‐samples t‐test. P‐values less than 0.05 were considered statistically significant.
Results
Reduction in p120ctn expression after transfection with p120ctn‐siRNA. We used RNA interference (RNAi) to down‐regulate endogenous p120ctn expression. Of the three plasmids tested, we found one (sequence B) that reduced all endogenous isoforms of p120ctn expression to a level hardly detectable by Western blot and RT‐PCR. Sequence A and sequence C transductants showed inefficient for p120ctn depletion (data not shown). As described in Materials and Methods, we isolated nine clones from the p120ctn‐siRNA‐transfected BE1, SPC, and LTE cells, named BE1/SPC/LTE‐siRNA1, BE1/SPC/LTE‐siRNA2, and BE1/SPC/LTE‐siRNA3 separately, which displayed over 90% reduction of endogenous p120ctn. Empty vector was introduced into the three cell lines and clones were isolated as controls. We also re‐introduced mouse p120ctn 1A and p120ctn 3A to the clonal cell lines to confirm that sequence B was specific (Fig. 1).
Figure 1.

Expression of p120‐catenin (p120ctn) after transfection of p120ctn‐small invasive (si)RNA.Following transfection with siRNAs as described in the Materials and Methods section, p120ctn expression was analyzed by Western blot and reverse transcription – polymerase reaction (RT‐PCR). To test the specificity of our siRNAs, we re‐introduced p120ctn cDNA to three clonal cell lines. (A) Protein bands representing p120ctn isoforms were detected at approximately 120 kDa (isoform 1) and 100 kDa (isoform 3) in BE1 and control cells. The clonal cell lines designated siRNA1‐3 revealed more than 90% reduction in p120ctn. (B) RT‐PCR analysis indicates that mRNA expression of p120ctn produced several specific bands, in BE1 and control cells. After transfection with p120ctn‐siRNA, the BE1 cells showed a reduction or the complete absence of all p120ctn isoforms. (C) Lanes 1–3 were siRNA‐transfected cells transfected with mouse p120ctn 1A, and lanes 4–6 were the siRNA‐transfected cells transfected with p120ctn 3A. At approximately 120 kDa and 100 kDa, protein bands representing p120ctn isoform 1 or isoform 3, respectively, were detected. SPC (D–F) and LTE (G–I) cells showed similar results.
Down‐regulation of endogenous p120ctn expression results in reduced E‐cadherin and β‐catenin expression. As shown in Fig. 2, we examined E‐cadherin expression using Western blot and RT‐PCR, and found that E‐cadherin protein levels were obviously reduced in p120ctn knocked down cells, with no significant change at the mRNA level. In addition, expression of β‐catenin, a major member of the cadherin/catenin complex, was reduced at both protein and mRNA levels. So, knockdown of endogenous p120ctn down‐regulates E‐cadherin only at the protein level, while reducing β‐catenin expression at mRNA level.
Figure 2.

Down‐regulation of endogenous p120‐catenin (p120ctn) expression reduces the expression of both E‐cadherin and β‐catenin. Expression of E‐cadherin and β‐catenin was analyzed by Western blot (A) and reverse transcription – polymerase reaction (RT‐PCR) (B). (A) E‐cadherin and β‐catenin protein levels were severely reduced in BE1/SPC/LTE‐p120ctn‐small invasive (si)RNA cells, compared with untransfected cells and control cells, which had specific bands at 120 kDa and 92 kDa, respectively. (B) There was no significant change in mRNA levels of E‐cadherin, and a specific band at 585 bp was detected in all cells. The expression of β‐catenin was reduced in the p120ctn knockdown cells. The band positions are indicated on the left with molecular weight markers.
p120ctn re‐introduction rescues expression of E‐cadherin and β‐catenin. After p120ctn sequence 1A or 3A were re‐introduced to the p120ctn‐siRNA‐transfected BE1, SPC, and LTE cell lines, we examined the expression of E‐cadherin and β‐catenin. Our results showed that after the transfection, E‐cadherin and β‐catenin re‐expressed in varying degrees (Fig. 3A–C).
Figure 3.
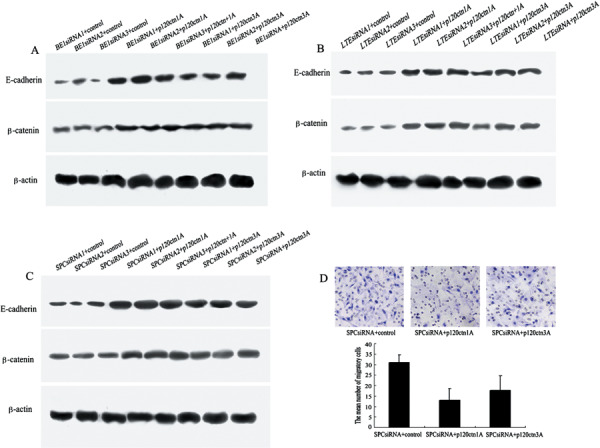
Restoration of p120‐catenin (p120ctn) rescued the expression of E‐cadherin and β‐catenin, and inhibited the invasion of SPC cell line.Re‐expression of E‐cadherin and β‐catenin was analyzed by Western blot (A–C). (A) E‐cadherin and β‐catenin protein levels were significantly increased in BE1 small invasive (si)RNA+ p120ctn isoform 1A cells or +p120ctn isoform 3A cells, compared with BE1siRNA+ control cells, which had specific bands at 120 kDa and 92 kDa, respectively. There was a similar change in LTE‐siRNA (B) and SPC‐siRNA (C) cell lines. (D) Serum‐stimulated Matrigel invasion assay of SPC cell line. The average number of migrating cells that had migrated through the pores was counted after 18 h. Statistical analysis showed that fewer of the SPC siRNA+ p120ctn‐1A‐expressing cells (13 ± 5.57) appeared to have migrated compared to SPC siRNA+ control (31 ± 3.61) and SPC siRNA+ 3A‐expressing cells (18 ± 7.02). The average number of cells that had migrated in each cell shown in bars was counted.
p120ctn regulates the activity of RhoA, Cdc42, and Rac1 in lung cancer cells. To explore p120ctn‐mediated changes in small GTPase activities, we examined the activities of RhoA, Cdc42, and Rac1 in p120ctn knocked down cells. Ablation of p120ctn decreased RhoA activity, while increasing Cdc42 and Rac1 activities (Fig. 4). So, the three Rho GTPases respond differently from p120ctn knockdown in lung cancer cells.
Figure 4.

Knockdown of p120‐catenin (p120ctn) decreased the activity of RhoA and increased the activity of Cdc42 and Rac1.Expression of total Cdc42, and Rac1 was analyzed by Western blot. Activity of these proteins was measured as described in the Materials and Methods section. (A) Cdc42 and Rac1 activities increased in the p120ctn‐knockdown BE1 cells, which were determined using pull‐down assay. Total Cdc42 and Rac1 protein did not change. (B) The activity of RhoA was obviously decreased in (si)RNA‐transfected BE1 cells. Results of RhoA G‐lisa in p120 knockdown cells and control cells. Columns with error bars indicate the activities of RhoA. SPC (C, D) and LTE (E, F) cells showed similar results.
p120ctn knockdown promotes lung cancer cell growth and migration. The cells in the G1 and S stages were assessed by staining the nuclei with propidium iodide to determine the amount of DNA present and the percentage of which were measured using FCM analysis. As shown in Fig. 5A and 5B the p120ctn knockdown groups showed a significant reduction in the percentage of G1 cells and a significant increase in the percentage of S phase cells in comparison to the untransfected cells and the negative control. Therefore, p120ctn participated in the regulation of cell cycle, and p120ctn depletion promoted tumor cell mitosis and growth.
Figure 5.
p120‐catenin (p120ctn) knockdown promotes lung cancer cell growth and migration.We added the result of BE1, which has representative results, to Fig. 5. (A, B) FCM analysis indicated that cells transfected with small invasive (si)RNA showed a significant increase in number at the S stage and a significant reduction in number at the G1 stage, compared with the untransfected cells and control group. (C, D) The number of cells that migrated through the pores was counted after 16 h and 24 h. No migrated cells were detected in the untransfected and control groups, while migratory p120ctn knockdown cells were detected after 16 h of culture. Although there were migratory cells in untransfected cells and control cell groups after 24 h, more migratory cells were detected in the p120ctn‐siRNA cell culture.
Since both E‐cadherin and β‐catenin play important roles in cell migration, and knockdown of p120ctn down‐regulates both of these proteins, we examined the invasive ability of p120ctn knockdown cells by performing serum‐stimulated Matrigel invasion assay. Six hours after seeding, no cells were detected on the bottom of Transwell in the parental cultures, control transfectant cultures and p120ctn‐siRNA‐transfected cultures (data not shown). Sixteen hours after plating, we found invaded cells in the p120ctn‐siRNA cultures, whereas no invasiveness was present in the untransfected and control cell cultures. After 24 h, while there were invaded cells in the untransfected and control cell cultures, significantly more in the p120ctn‐siRNA culture were observed (Fig. 5C,D). In addition, restoration of p120ctn elicited decreased migration ability in SPC cells when the selected SPC‐p120ctn‐siRNA cells were transfected with p120ctn 1A and 3A (Fig. 3D). These data indicated that p120ctn knockdown significantly increased invasiveness of lung cancer cells.
p120ctn knockdown enhances invasive and metastatic ability of lung cancer cells in nude mice. We injected tumor cells (BE1 cells, control transfectant cells, or BE1‐p120ctn(–) cells) into nude mice, and examine the effect of p120ctn knockdown on tumor formation in vivo. Tumors developed in all groups 6 weeks after the subcutaneous injection (Fig. 6A–C). However, the mean tumor weight in the BE1‐p120ctn(–) group (7.84 ± 1.50) was significantly higher than in either the BE1 (2.85 ± 0.60) or control transfectant groups (2.55 ± 0.56) (Fig. 6D). Mice injected with p120ctn knockdown BE1 cells developed more metastases (lung 5/6 and liver 6/6, respectively) than animals injected with BE1 cells (lung 3/6 and liver 2/6, respectively), and animals injected with control cells (lung 2/6 and liver 2/6, respectively). H&E staining showed that the tumors developed in BE1 or control groups had clear boundaries with less invasiveness (Fig. 6E for developed tumor on back; Fig. 6G for liver metastatic tumor). In contrast, tumors raised from p120ctn knockdown cells displayed characteristics of invasion (Fig. 6F for developed tumor on back; Fig. 6H for liver metastatic tumor). In addition, nude mice injected with BE1‐p120ctn(–) cells also developed lower extremities (1/6) (Fig. 6I) and heart (3/6) (Fig. 6J) metastases, and showed pleural fluid formation by Papanicolaou staining (3/6) (Fig. 6K), both of which were not found in the mice injected with BE1 cells or control cells. The results indicating that knockdown of p120ctn in lung cancer cells up‐regulated invasive and metastatic ability in vivo.
Figure 6.

The decreased expression of p120‐catenin (p120ctn) promotes BE1 cell growth, and tumors lacking p120ctn have malignant features in vivo.The tumors on the back of the mice, which were injected with untransfected cells (A) or control cells (B), were smaller than the tumors in the mice with transfected cells (C). The mean tumor weights in the BE1‐p120ctn(–) group was significantly higher than in either the BE1 or control groups (D). Hematoxylin and eosin staining reveals that tumors in the BE1 group and control groups had clear boundaries (E). The p120ctn knockdown tumors showed characteristics of invasion (F). Similar observations were seen in the metastatic tumors. Liver metastatic tumor in BE1 cell‐injected mice had clear boundaries with noninvasive growth (G). Tumors in mice injected with BE1‐p120ctn(–) cells showed characteristics of invasion (H), and nude mice injected with BE1‐p120ctn(–) cells also developed leg (I) and heart (J) metastases, and pleural fluid formation (K), in which we can see the tumor cells by Papanicolaou staining (arrow). (E, F × 100 magnification, G–J × 200, and K ×400).
Discussion
In the present study, we addressed the functions of p120ctn in lung cancer. Our results revealed that p120ctn knockdown reduced the levels of E‐cadherin and β‐catenin proteins, as well as the mRNA level of β‐catenin in several lung cancer cells. Moreover, knockdown of p120ctn inactivated RhoA, but increased the activity of Cdc42 and Rac1, and promoted proliferation, invasive and metastatic ability of lung cancer cells both in vitro and in vivo.
Previous studies have shown that p120ctn interaction with E‐cadherin is sufficient to physically stabilize the E‐cadherin complex.( 21 , 22 , 23 , 24 , 25 , 26 ) In the present study, we knocked down the endogenous p120ctn in lung cancer cells and found that decreased expression of p120ctn resulted in reduced expression of E‐cadherin at post‐transcriptional levels, confirming that p120ctn has an impact on E‐cadherin complex, which supports previous reports. β‐catenin stability is regulated by cadherin binding and its fate is thought to be ultimately tied to cadherin level, which is clearly controlled by p120ctn( 21 ) this can explain the findings of the current study that p120ctn knowdown led to the reduced expression of β‐catenin at the protein level. More interestingly, our data showed that knockdown of p120ctn also reduced β‐catenin expression at the mRNA level. To the best of our knowledge, this is the first report concerning the down‐regulation of β‐catenin transcription induced by p120ctn knockdown, which raises the possibility that reduction of β‐catenin after p120ctn knockdown is actually E‐cadherin‐independent. How is p120ctn involved in regulating β‐catenin translationally? Is p120ctn involved in a signaling pathway that regulates β‐catenin gene expression, perhaps via the p120‐binding partner and transcription factor Kaiso? Is p120ctn impacting β‐catenin protein levels directly or indirectly through the canonical Wnt signaling pathway and the proteosomal degradation pathway? Many questions need to be investigated further. However, it is clear that E‐cadherin and catenins regulate dynamic cell–cell adhesion and cell morphology( 5 , 27 , 28 ) and their reduced expression may destabilize these connections, and increase the likelihood of metastasis, as observed in our results.
Previously studies have demonstrated that p120ctn knockdown increased the RhoA activity and decreased the activity of Rac1.( 26 , 29 , 30 ) However, in the present study, we detected that ablation of p120ctn decreased RhoA activity, while increasing Cdc42 and Rac1 activity, an apparently opposite effect. However, it is notable that a previous study showed the p120ctn functions on small GTPases at E‐cadherin depletion, which may due to the results in our study. The underlying mechanism is unknown, but the results were highly reproducible and appear to reflect a cell‐ or tissue‐specific manner on p120ctn interaction with various small GTPases.( 31 ) These results lead to interesting questions about the mechanism by which p120ctn regulates the activity of RhoA, Cdc42, and Rac1. When RhoA is inactive, cells lose stress fibers and focal adhesions, which inhibit cell adhesion, and since active Cdc42 and Rac1 enhance extension of the membrane, the net effect will be to elevate the migratory activity of the cells.( 10 , 32 , 33 , 34 ) Thus, either elevated Cdc42/Rac1 activity or decreased RhoA activity can promote tumor cell migration and invasiveness.
Recent studies indicate that p120ctn could regulate cell proliferation and contact inhibition.( 11 , 35 ) These reports prompt a new function for p120ctn in the regulation of cell cycle progression. In the present study, we also found that p120ctn participated in the regulation of the cell cycle. As we know, cyclin E plays an essential role in fundamental biological processes, such as cell cycle control, DNA replication, apoptosis, and DNA repair, thus its expression must be finely regulated throughout the cell cycle.( 36 , 37 ) Chartier et al. found an impaired cyclin E down‐regulation and accumulation in centrosomes in cells overexpressing p120ctn, suggesting that p120ctn participated in regulation of the cell cycle by cyclin E.( 35 ) However, in the cells with p120ctn‐siRNA, this supposition needs to be investigated further.
Despite extensive literature on the pathology of p120ctn down‐regulation in tumors, there is no consensus on whether p120ctn down‐regulation promotes tumor progression or metastasis.( 9 ) In summary, our present study strongly suggests that p120ctn down‐regulation may promote tumor progression and metastasis by reducing E‐cadherin and β‐catenin expression, and altering the activities of RhoA, Cdc42 and Rac1. In addition, we have transfected isoforms 1A and 3A into the p120ctn‐siRNA transfected cell lines, and found that the expression of E‐cadherin and β‐catenin, and the invasion of lung cancer cells were inhibited at different levels. Based on our results, we postulate that p120ctn suppresses the metastatic ability of lung cancer cells.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China [No. 30470764, No. 30670917, and No. 30870977 to En‐Hua Wang, No. 30670888 to Chen Zhao], and none of the authors of this manuscript have a financial interest related to this work. We sincerely thank Dr Albert B. Reynolds, Department of Cancer Biology, Vanderbilt University for his constructive suggestions and useful comments to this work, and his generous gifts of p120ctn 1A and p120ctn 3A plasmids.
References
- 1. Berx G, Van Roy F. The E‐cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 2001; 3: 289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conacci‐Sorrell M, Zhurinsky J, Ben‐Ze’ev A. The cadherin‐catenin adhesion system in signaling and cancer. J Clin Invest 2002; 109: 987–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bremnes RM, Veve R, Gabrielson E et al . High‐throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E‐cadherin pathway in non‐small‐cell lung cancer. J Clin Oncol 2002; 20: 2417–28. [DOI] [PubMed] [Google Scholar]
- 4. Ireton RC, Davis MA, Van Hengel J et al . A novel role for p120 catenin in E‐cadherin function. J Cell Biol 2002; 159: 465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson WJ, Nusse R. Convergence of Wnt, beta‐catenin, and cadherin pathways. Science 2004; 303: 1483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci 2000; 113: 1319–34. [DOI] [PubMed] [Google Scholar]
- 7. Thoreson MA, Anastasiadis PZ, Daniel JM et al . Selective uncoupling of p120 (ctn) from E‐cadherin disrupts strong adhesion. J Cell Biol 2000; 148: 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montonen O, Aho M, Uitto J, Aho S. Tissue distribution and cell type‐specific expression of p120ctn isoforms. J Histochem Cytochem 2001; 49: 1487–96. [DOI] [PubMed] [Google Scholar]
- 9. Van Hengel J, Van Roy F. Diverse functions of p120ctn in tumors. Biochim Biophys Acta 2007; 1773: 78–88. [DOI] [PubMed] [Google Scholar]
- 10. Castano J, Solanas G, Casagolda D et al . Specific phosphorylation of p120‐catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol 2007; 27: 1745–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wildenberg GA, Dohn MR, Carnahan RH et al . p120‐catenin and p190RhoGAP regulate cell‐cell adhesion by coordinating antagonism between Rac and Rho. Cell 2006; 127: 1027–39. [DOI] [PubMed] [Google Scholar]
- 12. Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120‐catenin. Curr Opin Cell Biol 2001; 13: 604–10. [DOI] [PubMed] [Google Scholar]
- 13. Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 2000; 150: 567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho‐family GTPases: a link between cell‐cell contact formation and regulation of cell locomotion. J Cell Sci 2001; 114: 695–707. [DOI] [PubMed] [Google Scholar]
- 15. Anastasiadis PZ, Moon SY, Thoreson MA et al . Inhibition of RhoA by p120 catenin. Nat Cell Biol 2000; 2: 637–44. [DOI] [PubMed] [Google Scholar]
- 16. Magie CR, Pinto‐Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha‐catenin, and regulates cadherin‐based adherens junction components in Drosophila. Development 2002; 129: 3771–82. [DOI] [PubMed] [Google Scholar]
- 17. Davis MA, Reynolds AB. Blocked acinar development, E‐cadherin reduction, and intraepithelial neoplasia upon ablation of p120‐catenin in the mouse salivary gland. Dev Cell 2006; 10: 21–31. [DOI] [PubMed] [Google Scholar]
- 18. Cozzolino M, Stagni V, Spinardi L et al . p120 Catenin is required for growth factor‐dependent cell motility and scattering in epithelial cells. Mol Biol Cell 2003; 14: 1964–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation 2002; 70: 583–9. [DOI] [PubMed] [Google Scholar]
- 20. Wang EH, Liu Y, Xu HT et al . Abnormal expression and clinicopathologic significance of p120‐catenin in lung cancer. Histol Histopathol 2006; 21: 841–7. [DOI] [PubMed] [Google Scholar]
- 21. Davis MA, Ireton RC, Reynolds AB. A core function for p120‐catenin in cadherin turnover. J Cell Biol 2003; 163: 525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao K, Allison DF, Buckley KM et al . Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol 2003; 163: 535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reynolds AB, Carnahan RH. Regulation of cadherin stability and turnover by p120ctn. implications in disease and cancer. Semin Cell Dev Biol 2004; 15: 657–63. [DOI] [PubMed] [Google Scholar]
- 24. Reynolds AB, Roczniak‐Ferguson A. Emerging roles for p120‐catenin in cell adhesion and cancer. Oncogene 2004; 23: 7947–56. [DOI] [PubMed] [Google Scholar]
- 25. Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120‐catenin in cadherin trafficking. Biochim Biophys Acta 2007; 1773: 8–16. [DOI] [PubMed] [Google Scholar]
- 26. Yanagisawa M, Anastasiadis PZ. p120 catenin is essential for mesenchymal cadherin‐mediated regulation of cell motility and invasiveness. J Cell Biol 2006; 174: 1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pacquelet A, Rorth P. Regulatory mechanisms required for DE‐cadherin function in cell migration and other types of adhesion. J Cell Biol 2005; 170: 803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobielak A, Fuchs E. Alpha‐catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol 2004; 5: 614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res 2005; 65: 10938–45. [DOI] [PubMed] [Google Scholar]
- 30. Perez‐Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120‐catenin mediates inflammatory responses in the skin. Cell 2006; 124: 631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds AB. p120‐catenin: past and present. Biochim Biophys Acta 2007; 1773: 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 1999; 68: 459–86. [DOI] [PubMed] [Google Scholar]
- 33. Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin‐mediated cell‐cell adhesion by the Rho family GTPases. Curr Opin Cell Biol 1999; 11: 591–6. [DOI] [PubMed] [Google Scholar]
- 34. Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol 1999; 144: 1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chartier NT, Oddou CI, Laine MG et al . Cyclin‐dependent kinase 2/cyclin E complex is involved in p120 catenin (p120ctn)‐dependent cell growth control: a new role for p120ctn in cancer. Cancer Res 2007; 67: 9781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mazumder S, DuPree EL, Almasan A. A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy. Curr Cancer Drug Targets 2004; 4: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ekholm SV, Zickert P, Reed SI, Zetterberg A. Accumulation of cyclin E is not a prerequisite for passage through the restriction point. Mol Cell Biol 2001; 21: 3256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]



