Abstract
Dose‐dependent promotion effects of combined treatment with sodium nitrite (NaNO2) and ascorbic acid (AsA) on gastric carcinogenesis were examined in rats pretreated with N‐methyl‐N′‐nitro‐N‐nitrosoguanidine (MNNG). Groups of 15 6‐week‐old F344 male rats were given 0.01% MNNG in their drinking water for 10 weeks to initiate carcinogenesis in the glandular stomach and a single intragastric administration of 100 mg/kg/bodyweight of MNNG by stomach tube at week 9 to initiate carcinogenesis in the forestomach. From week 11, they received either drinking water containing 0.05, 0.1 or 0.2% NaNO2 and a diet supplemented with 0.1 or 0.2% AsA in combination, each individual chemical alone or a basal diet until the end of week 42. In the forestomach, the incidence of hyperplasia was increased dose dependently by the treatment with NaNO2 alone. Incidences of neoplastic lesions were dramatically increased by the combined treatment with NaNO2 and AsA in a dose‐dependent manner, but AsA itself had no effect. In the glandular stomach, only toxicity and regenerative changes were increased by the high‐dose combination. In a second short‐term experiment conducted for sequential observation, necrosis and strong inflammation were found in the forestomach epithelium shortly after commencing combined treatment with 1.0% AsA and 0.2% NaNO2, followed by hyperplasia, whereas there were no obvious effects in the glandular stomach. In addition, after a 4 h treatment with 1.0% AsA and 0.2% NaNO2, a slight increase in the 8‐hydroxy‐deoxyguanosine levels in the forestomach epithelium was observed by high‐performance liquid chromatography and an electrochemical detection system, albeit without statistical significance. In vitro, electron spin resonance demonstrated nitric oxide formation during incubation with NaNO2 and AsA under acidic conditions. Thus, NaNO2 was demonstrated to exert promoter action in the forestomach, with AsA acting as a strong copromoter through cytotoxicity and regenerative cell proliferation, possibly mediated by oxidative DNA damage, but the combined treatment with NaNO2 and AsA had little influence on glandular stomach carcinogenesis. (Cancer Sci 2006; 97: 175 –182)
Abbreviations:
- AsA
ascorbic acid
- BrdU
bromodeoxyuridine
- DCTS
N‐(dithiocarboxy)sarcosine
- ESR
electron spin resonance
- HPLC
high‐performance liquid chromatography
- MNNG
N‐methyl‐N′‐nitro‐N‐nitrosoguanidine
- NaNO2
sodium nitrite
- NO
nitric oxide
- 8‐OHdG
8‐hydroxy‐deoxyguanosine.
Sodium nitrite is used widely as a food additive to preserve and tinge color‐cured meat and fish. We intake nitrate from exogenous sources such as vegetables and water, and this is partly reduced to nitrite in vivo by microflora in the buccal cavity.( 1 , 2 , 3 ) It has been shown that sodium nitrite is a precursor of N‐nitroso compounds with strong genotoxic and carcinogenic potential,( 4 ) and an increased incidence of tumors, such as forestomach papillomas and lymphoreticular tumors, has been found in rat carcinogenicity studies of NaNO2.( 5 , 6 ) However, other studies failed to show any effects of nitrite administration on tumor incidence.( 7 , 8 , 9 ) Previously, we reported that when NaNO2 was administered simultaneously with phenolic antioxidants, such as catechol, 3‐methoxycatechol, hydroquinone, tert‐butylhydroquinone, gallic acid and pyrogallol, the NaNO2 induction of forestomach epithelial cell proliferation was augmented.( 10 , 11 , 12 , 13 ) After initiation with a carcinogen, NaNO2 weakly enhanced forestomach carcinogenesis, and this was further increased by coadministration of antioxidants such as catechol, 3‐methoxycatechol, tert‐butylhydroquinone, α‐tocopherol, propyl gallate and 1‐O‐hexyl‐2,3,5‐trimethylhydroquinone.( 11 , 12 , 13 , 14 , 15 ) Without carcinogen pretreatment, 0.2% NaNO2 and 0.8% catechol in combination for 51 weeks induced squamous cell carcinomas in the rat forestomach.( 12 )
Ascorbic acid is a well‐known vitamin with strong reducing as well as antioxidative activities, which inhibits formation of carcinogenic N‐nitroso compounds in vitro and in vivo by blocking the reaction of nitrite and secondary amines.( 16 , 17 ) In contrast, similar to phenolic antioxidants, simultaneous administration of 1.0% AsA and 0.3% NaNO2 has been reported to enhance forestomach epithelial cell proliferation in rats.( 13 ) Furthermore, combined treatment with 0.3% NaNO2 and 1.0% AsA has been shown to promote MNNG‐induced rat forestomach, but not glandular stomach, carcinogenesis, and the combination also induced forestomach papillomas without MNNG initiation.( 13 ) These results suggest that NaNO2 is a weak promoter and AsA is a strong copromoter of rat forestomach carcinogenesis, and that combined treatment has carcinogenic potential. Although the mechanisms of promotion or carcinogenicity by the combined treatment with NaNO2 and AsA are not well understood, the fact that AsA reduces nitrite into NO under acidic conditions( 17 , 18 , 19 , 20 ) indicates that NO in the stomach might be an important factor. Whereas at low levels NO acts as a key molecule in assisting in many physiological functions, data showing a contribution to carcinogenesis at high levels are accumulating.( 21 )
In our previous rat two‐stage carcinogenesis model, a single intragastric administration of MNNG was used for the initiation. However, this procedure did not induce appreciable yields of neoplastic lesions in the glandular stomach corresponding to human gastric cancers.( 14 ) Therefore, initiation against glandular stomach may not be sufficient to demonstrate the weak promotion effect by the combination of NaNO2 and AsA on glandular stomach. Generally, it requires at least 8–10 weeks of MNNG treatment in the drinking water to provide appropriate initiation of glandular stomach neoplasia.( 22 , 23 ) Therefore, we improved the initiation procedure by combining 100 mg/mL MNNG in the drinking water for 10 weeks with an intragastric administration of MNNG to investigate effects of NaNO2 with or without AsA on both glandular stomach and forestomach in rats. In addition, we used a short‐term study to examine whether oxidative stress might be involved in enhancement of carcinogenesis, and conducted an in vitro study to demonstrate NO formation.
Materials and Methods
Animals and chemicals for the in vivo animal studies
Male 5‐week‐old F344 rats were obtained from Charles River Japan (Atsugi, Japan) and housed five animals per polycarbonate cage under standard laboratory conditions: room temperature, 23 ± 2°C; relative humidity, 60 ± 5%; and a 12:12 h L:D cycle. After a 1‐week acclimation period, the animals were used in the experiments. AsA and NaNO2 were purchased from Wako Pure Chemical Industries (Osaka, Japan) and MNNG from Tokyo Kasei Kogyo (Tokyo, Japan). AsA was mixed with Oriental CRF‐1 basal diet (Oriental Yeast, Tokyo, Japan) at various concentrations, replaced once a week, and stored at 4°C in the dark before use. NaNO2 was dissolved in distilled water at various concentrations and replaced twice a week. Food and tap water were available ad libitum. The protocols for these studies were approved by the Animal Care and Utilization Committee for the National Institute of Health Sciences, Japan.
Two‐stage carcinogenicity study
In the two‐stage carcinogenicity study, as shown in Fig. 1, a total of 180 6‐week‐old rats were given 0.01% MNNG in drinking water for 10 weeks for the initiation of carcinogenesis in the glandular stomach. At week 9, they were then given 100 mg/kg bodyweight MNNG in vehicle (DMSO:water, 1:1) by stomach tube after 16 h starvation for initiation of carcinogenesis in the forestomach. From week 11, they were divided into 12 groups consisting of 15 animals each and treated as follows: group 1, basal diet; groups 2 and 3, 0.1 and 0.2% AsA in the diet; groups 4–6, 0.05, 0.1 and 0.2% NaNO2, respectively, in the drinking water; groups 7–9, 0.1% AsA plus 0.05, 0.1 and 0.2% NaNO2, respectively; and groups 10–12, 1.0% AsA plus 0.05, 0.1 and 0.2% NaNO2, respectively. Bodyweight, food consumption and water intake were recorded at least once every 2–4 weeks and all survivors were killed under ether anesthesia at the end of week 42. The liver, kidneys, esophagus, stomach and intestines were excised, and the liver and kidneys were weighed and fixed in 10% buffered formalin solution. The esophagus, stomach and intestines were inflated with formalin solution and later opened. After fixation, the location and size of tumors were recorded, and six and three sections were cut from the forestomach and glandular stomach, respectively, and stained routinely with hematoxylin and eosin for histopathological assessment.
Figure 1.

Experimental design. F344 male rats were initiated with 0.01%N‐methyl‐N′‐nitro‐N‐nitrosoguanidine (MNNG) in the drinking water for 10 weeks for carcinogenesis of the glandular stomach, and 100 mg/kg bodyweight at week 9 for carcinogenesis of the forestomach. From week 11, they received either drinking water containing 0.05, 0.1 or 0.2% sodium nitrite (NaNO2) and diet supplemented with 0.1 or 0.2% ascorbic acid (AsA) in combination, each individual chemical alone or basal diet until the end of week 42.
Short‐term toxicity study
In the short‐term toxicity study, a total of 36 rats at age 6 weeks were divided into four groups of nine animals each and treated as follows without MNNG initiation: group 1, basal diet; group 2, 1.0% AsA in the diet; group 3, 0.2% NaNO2 in the drinking water; and group 4, 1.0% AsA plus 0.2% NaNO2. Sub‐groups of three animals each were killed after 1, 3 and 7 days of treatment. The stomachs were excised and treated in the same manner as described above.
Measurement of nuclear 8‐OHdG in the forestomach epithelium
For measurement of nuclear 8‐OHdG in the forestomach epithelium, a total of 60 6‐week‐old rats were divided into four groups of 15 animals each and treated as follows: group 1, basal diet; group 2, 1.0% AsA in the diet; group 3, 0.2% NaNO2 in the drinking water; and group 4, 1.0% AsA plus 0.2% NaNO2. They were fasted for 16 h before the chemical treatment and killed 4 h thereafter, when obvious histopathological changes were not observed in the forestomach epithelium. From excised stomachs, the forestomach epithelium was collected using a slide glass. Samples were frozen immediately in liquid nitrogen and stored at −80°C until measurement of 8‐OHdG in nuclear DNA, according to the method of Nakae et al.( 24 ) As the amount of forestomach tissue was small, forestomachs from three animals were combined as one sample. Nuclear DNA was extracted with a DNA Extracter WB Kit (Wako Pure Chemical Industries), digested to deoxynucleotides with nuclease P1 and alkaline phosphatase, and the levels of 8‐OHdG (8‐OHdG/105 deoxyguanosine) were assessed by HPLC with an electrochemical detection system (Coulochem II; ESA, Bedford, MA, USA).
Nitric oxide measurement after reaction of NaNO2 with AsA using ESR
Ascorbic acid, NaNO2, ferrous sulfate and hydrochloric acid were purchased from Wako Pure Chemical Industries, and DTCS‐Na was from Dojindo Laboratory (Kumamoto, Japan). Iron chelate Fe(DTCS)2 was prepared freshly by mixing a stock solution of ferrous sulfate and DTCS‐Na at a molar ratio of 1:5 in phosphate‐buffered saline (pH 7.4, 100 mM) prior to the experiment. Fe2+ complexed with DTCS‐Na was used as an NO‐trapping agent and NO formation was determined by detecting the characteristic triplet ESR signal (g = 2.04) resulting from the reaction of the Fe(DTCS)2 complex with NO, as reported previously.( 25 , 26 ) The ESR was measured with a JEOL JES‐FR30 spectrometer (JEOL, Tokyo, Japan) under the following conditions: magnetic field, 330.7 ± 5 mT; power, 9.0 mW; frequency, 16 GHz; modulation width, 0.063 mT; gain, 500; sweep time, 0.5 min; and time constant, 0.03 s. The reaction procedure was as follows: 100 µL of 1 mM Fe(DTCS)2 complex was added to pH 1.5 hydrochloric acid, followed by the addition of 100 µL of 50, 100 or 200 mM ascorbic acid, and/or 100 µL of 0.5 or 1 mM NaNO2, at intervals of 15 s. This mixture was transferred to a quartz flat cell of 200 µL for ESR measurement after mixing for 30 s.
Statistical analysis
The Fisher's extract probability test and Dunnett's t‐test were used for statistical analysis of the data.
Results
Two‐stage carcinogenicity study
Final body and organ weights The final bodyweights were decreased significantly in the 1.0% AsA plus 0.1 or 0.2% NaNO2 groups (groups 11 and 12) compared with the basal diet group (group 1). The relative kidney weights were generally increased in all groups and liver weights were slightly decreased in the 1.0% AsA plus 0.2% NaNO2 group (group 12) compared with the basal diet group (group 1) (Table 1).
Table 1.
Final body and organ weights
| Group | Treatment | No. rats | Final bodyweight (g) | Organ weight (g/100 g bodyweight) | ||
|---|---|---|---|---|---|---|
| MNNG | Chemical | Liver | Kidneys | |||
| 1 | + | Basal diet | 15 | 386 ± 22 | 2.88 ± 0.10 | 0.59 ± 0.04 |
| 2 | + | 0.1% AsA | 14 | 408 ± 20 † | 2.90 ± 0.09 | 0.64 ± 0.04 †† |
| 3 | + | 1.0% AsA | 15 | 389 ± 21 | 2.87 ± 0.11 | 0.62 ± 0.05 |
| 4 | + | 0.05% NaNO2 | 15 | 381 ± 21 | 2.73 ± 0.23 | 0.63 ± 0.05 † |
| 5 | + | 0.1% NaNO2 | 15 | 381 ± 20 | 2.80 ± 0.21 | 0.66 ± 0.05 †† |
| 6 | + | 0.2% NaNO2 | 13 | 372 ± 22 | 2.77 ± 0.11 | 0.63 ± 0.03 † |
| 7 | + | 0.1% AsA + 0.05% NaNO2 | 14 | 385 ± 22 ‡‡ | 2.89 ± 0.11§ | 0.64 ± 0.04 †† |
| 8 | + | 0.1% AsA + 0.1% NaNO2 | 15 | 389 ± 21 ‡ | 2.86 ± 0.11 | 0.65 ± 0.04 †† |
| 9 | + | 0.1% AsA + 0.2% NaNO2 | 15 | 372 ± 16 ‡‡ | 2.85 ± 0.10 | 0.66 ± 0.04 †† |
| 10 | + | 1.0% AsA + 0.05% NaNO2 | 15 | 376 ± 24 | 2.93 ± 0.09 §§ | 0.66 ± 0.03 †† , ‡ |
| 11 | + | 1.0% AsA + 0.1% NaNO2 | 15 | 365 ± 21 † , ‡‡ | 2.88 ± 0.10 | 0.66 ± 0.03 †† , ‡ |
| 12 | + | 1.0% AsA + 0.2% NaNO2 | 14 | 336 ± 18 †† , ‡‡ , §§ | 2.77 ± 0.15 † | 0.69 ± 0.06 †† , ‡‡ , §§ |
P < 0.05,
P < 0.01 compared to the basal diet group;
P < 0.05,
P < 0.01 compared to respective ascorbic acid (AsA) only groups;
P < 0.05,
P < 0.01 compared to respective sodium nitrite (NaNO2) only groups. MNNG, N‐methyl‐N′‐nitro‐N‐nitrosoguanidine.
Forestomach findings Grossly, a few small tumors were observed in the forestomach epithelium of animals treated with MNNG alone (group 1) and MNNG followed by 0.1 or 1.0% AsA (groups 2 and 3). However, the forestomachs of animals treated with MNNG followed by 0.1 or 1.0% AsA plus 0.2% NaNO2 (groups 9 and 12) and 1.0% AsA plus 0.1% NaNO2 (group 11) were thickened diffusely and filled with various‐sized masses. Microscopically, the lesions observed in the forestomach were characterized as hyperplasias, papillomas and squamous cell carcinomas. Hyperplasias were further classified into mild, moderate and severe types as described previously.( 11 ) The incidences of papillomas and carcinomas were increased dramatically in a dose‐dependent manner in the AsA plus NaNO2 groups, particularly with 1.0% AsA plus 0.1% NaNO2 (group 11) and 1.0% AsA plus 0.2% NaNO2 (group 12), compared with the basal diet (group 1), AsA (groups 2 and 3) and NaNO2 (groups 4–6) cases. In the 0.1 and 0.2% NaNO2 alone groups (groups 5 and 6), the grades of hyperplasias were much more severe than in the basal diet group (group 1). This was not the case with the 0.1 and 1.0% AsA alone groups (groups 2 and 3) (Table 2).
Table 2.
Incidence of neoplastic lesions in the forestomach
| Group | Treatment | No. rats | No. rats with (%) | |||||
|---|---|---|---|---|---|---|---|---|
| MNNG | Chemical | Hyperplasia | Papilloma | SCC | ||||
| Mild | Moderate | Severe | ||||||
| 1 | + | Basal diet | 15 | 15 (100) | 3 (20) | 0 (0) | 5 (33) | 4 (27) |
| 2 | + | 0.1% AsA | 14 | 14 (100) | 6 (43) | 0 (0) | 2 (14) | 2 (14) |
| 3 | + | 1.0% AsA | 15 | 15 (100) | 5 (33) | 1 (7) | 3 (20) | 3 (20) |
| 4 | + | 0.05% NaNO2 | 15 | 15 (100) | 7 (47) | 0 (0) | 5 (33) | 2 (13) |
| 5 | + | 0.1% NaNO2 | 15 | 15 (100) | 9 (60) † | 2 (13) | 8 (53) | 5 (33) |
| 6 | + | 0.2% NaNO2 | 13 | 13 (100) | 11 (85) †† | 3 (23) | 6 (46) | 3 (23) |
| 7 | + | 0.1% AsA + 0.05% NaNO2 | 14 | 14 (100) | 4 (29) | 0 (0) | 5 (36) | 4 (29) |
| 8 | + | 0.1% AsA + 0.1% NaNO2 | 15 | 15 (100) | 7 (47) | 0 (0) | 7 (47) | 2 (13) |
| 9 | + | 0.1% AsA + 0.2% NaNO2 | 15 | 15 (100) | 15 (100) †† , ‡‡ | 10 (67) †† , ‡‡ , § | 11 (73) † , ‡‡ | 7 (47) |
| 10 | + | 1.0% AsA + 0.05% NaNO2 | 15 | 15 (100) | 13 (87) †† , ‡‡ , § | 2 (13) | 13 (87) †† , ‡‡ , §§ | 8 (53) § |
| 11 | + | 1.0% AsA + 0.1% NaNO2 | 15 | 15 (100) | 15 (100) †† , ‡‡ , §§ | 14 (93) †† , ‡‡ , §§ | 14 (93) †† , ‡‡ , § | 9 (60) ‡ |
| 12 | + | 1.0% AsA + 0.2% NaNO2 | 14 | 14 (100) | 14 (100) †† , ‡‡ | 13 (93) †† , ‡‡ , §§ | 14 (100) †† , ‡‡ , §§ | 10 (71) † , ‡‡ , § |
P < 0.05,
P < 0.01 compared to the basal diet group;
P < 0.05,
P < 0.01 compared to respective ascorbic acid (AsA) only groups;
P < 0.05,
P < 0.01 compared to respective sodium nitrite (NaNO2) only groups. MNNG, N‐methyl‐N′‐nitro‐N‐nitrosoguanidine; SCC, squamous cell carcinoma.
Glandular stomach findings Grossly, only few animals had macroscopic tumors in the glandular stomach. Microscopically, the majority of the lesions observed in the glandular stomach were characterized as hyperplasias, adenomas, adenocarcinomas and others, with no significant differences in the incidences of these lesions among MNNG‐initiated groups (Table 3). In addition to the neoplastic lesions, erosion or regeneration were observed with high frequency especially with 1.0% AsA plus 0.05, 0.1 and 0.2% NaNO2 and 0.1% AsA plus 0.2% NaNO2 (Groups 9, 10, 11 and 12).
Table 3.
Incidence of neoplastic lesions in the glandular stomach
| Group | Treatment | No. rats | No. rats with (%) proliferative lesions | No. rats with (%) erosion or regeneration | ||||
|---|---|---|---|---|---|---|---|---|
| MNNG | Chemical | Hyperplasia | Adenoma | Adeno‐ carcinoma | Leiomyosarcoma | |||
| 1 | + | Basal diet | 15 | 0 (0) | 3 (20) | 0 (0) | 0 (0) | 1 (7) |
| 2 | + | 0.1% AsA | 14 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (14) |
| 3 | + | 1.0% AsA | 15 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (20) |
| 4 | + | 0.05% NaNO2 | 15 | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 4 (27) |
| 5 | + | 0.1% NaNO2 | 15 | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 4 (27) |
| 6 | + | 0.2% NaNO2 | 13 | 1 (8) | 0 (0) | 1 (8) | 0 (0) | 2 (15) |
| 7 | + | 0.1% AsA + 0.05% NaNO2 | 14 | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 3 (21) |
| 8 | + | 0.1% AsA + 0.1% NaNO2 | 15 | 0 (0) | 2 (13) | 0 (0) | 0 (0) | 2 (13) |
| 9 | + | 0.1% AsA + 0.2% NaNO2 | 15 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (47) † |
| 10 | + | 1.0% AsA + 0.05% NaNO2 | 15 | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 8 (53) †† |
| 11 | + | 1.0% AsA + 0.1% NaNO2 | 15 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (80) †† , ‡‡ , §§ |
| 12 | + | 1.0% AsA + 0.2% NaNO2 | 14 | 1 (7) | 0 (0) | 1 (7) | 0 (0) | 9 (64) †† , ‡ , § |
P < 0.05,
P < 0.01 compared to the basal diet group;
P < 0.05,
P < 0.01 compared to respective ascorbic acid (AsA) only groups;
P < 0.05,
P < 0.01 compared to respective sodium nitrite (NaNO2) only groups. MNNG, N‐methyl‐N′‐nitro‐N‐nitrosoguanidine.
Regarding other organs, only one animal given 0.2% NaNO2 plus 1.0% AsA had a duodenal tumor. No proliferative lesions were observed in the esophagus.
Short‐term toxicity study
Microscopically, the forestomachs of rats treated with 1.0% AsA plus 0.2% NaNO2 exhibited necrosis or erosion of the squamous epithelium accompanied by strong inflammatory changes with edema in the submucosa. These changes gradually became stronger from days 1–7 of treatment, and regenerative hyperplasia eventually developed on day 7 (4, 5). In the 1.0% AsA and 0.2% NaNO2 alone groups, there were no apparent changes. In the glandular stomach, toxicity as well as proliferative changes were not apparent in any of the groups.
Table 4.
Histopathological findings for the epithelium of the forestomach in the short‐term study
| Group | No. rats/day | Necrosis or erosion | Ulcer | Hyperplasia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | ||
| Basal diet | 3 | − | − | − | − | − | − | − | − | − |
| 1.0% AsA | 3 | − | − | − | − | − | − | − | − | − |
| 0.2% NaNO2 | 3 | − | − | − | − | − | − | − | − | − |
| 1.0% AsA + 0.2% NaNO2 | 3 | ++ | ++ | ++ | − | + | ++ | − | − | + |
+, moderate; ++, severe; AsA, ascorbic acid; NaNO2, sodium nitrite.
Table 5.
Histopathological findings for the submucosa of the forestomach in the short‐term study
| Group | No. rats/day | Edema | Inflamatory cell infiltration | Granulation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | Day 1 | Day 3 | Day 7 | ||
| Basal diet | 3 | − | − | − | − | − | − | − | − | − |
| 1.0% AsA | 3 | − | − | − | − | − | − | − | − | − |
| 0.2% NaNO2 | 3 | − | − | − | − | − | − | − | − | − |
| 1.0% AsA + 0.2% NaNO2 | 3 | + | ++ | ++ | ± | + | ++ | − | − | + |
±, mild; +, moderate; ++, severe; AsA, ascorbic acid; NaNO2, sodium nitrite.
Measurement of nuclear 8‐OHdG in the forestomach epithelium
Data for 8‐OHdG levels in the rat forestomach given 1.0% AsA and/or 0.2% NaNO2 for 4 h are summarized in Fig. 2. At this stage, no apparent gross or microscopic changes were observed in the forestomach epithelium. Values for two samples given AsA and NaNO2 simultaneously were higher than the range of control values, but the mean (mean ± SD, 0.89 ± 0.57/105 dG) was not significantly different from the control value (0.54 ± 0.17/105 dG). Values for the AsA (0.57 ± 0.23/105 dG) and NaNO2 (0.44 ± 0.07/105 dG) alone groups also were not significantly different from the control value.
Figure 2.

8‐Hydroxy‐deoxyguanosine (8‐OHdG) levels in the rat forestomach epithelium 4 h after administration of 1.0% ascorbic acid (AsA) and/or 0.2% sodium nitrite (NaNO2). Two samples given 1.0% AsA and 0.2% NaNO2 demonstrated levels higher than in the controls. However, overall values were without statistical significance. Levels of 8‐OHdG with AsA or NaNO2 alone were comparable to control values.
Nitric oxide measurement by the reaction of NaNO2 and AsA using ESR
Figure 3a illustrates a typical ESR signal indicating NO formation. ESR signals were not observed after treatment with 0.05 or 0.1 mM NaNO2, or with 5, 10 or 20 mM AsA alone under acidic conditions at pH 1.5 (Fig. 3b,c). However, formation of NO was clear after adding 5–20 mM AsA to 0.1 mM NaNO2, the signal height being dependent on the AsA concentration (Fig. 3d). As shown in Fig. 4, the amount of NO generated also depended on the AsA and NaNO2 doses.
Figure 3.

Electron spin resonance (ESR) spectra for nitric oxide (NO)‐Fe (N‐[dithiocarboxy]sarcosine)2 (DTCS). (a) NO‐Fe (DTCS)2 standard, (b) 20 mM ascorbic acid (AsA) + HCl (pH 1.5), (c) 0.1 mM sodium nitrite (NaNO2) + HCl (pH 1.5), and (d) 5,10 and 20 mM AsA + 0.1 mM NaNO2 + HCl (pH 1.5). ESR signals were not observed during treatment with NaNO2 or AsA alone under acidic conditions at pH 1.5, but formation of NO was apparent upon the addition AsA to NaNO2, the signal height being dependent on the AsA concentration.
Figure 4.
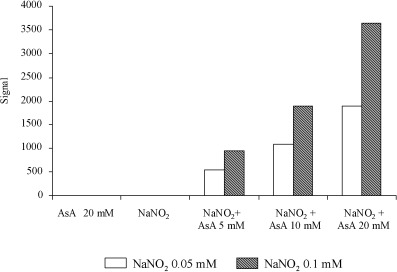
Nitric oxide (NO) formation by the reaction of sodium nitrite (NaNO2) with ascorbic acid (AsA) in HCl (pH 1.5). The amount of NO generated increased depending on both the AsA and NaNO2 doses. NO was not generated by treatment with NaNO2 or AsA alone under the same conditions.
Discussion
It has been reported that oral administration of 0.3% NaNO2 in the drinking water over 1 year causes a significant increase in the incidence of forestomach papillomas in Wistar rats.( 6 ) We also reported that treatment with 0.3% NaNO2 in drinking water for 51 weeks induces hyperplasia with a slight increase in the BrdU labeling index in the forestomach epithelium of F344 rats.( 13 ) Moreover, when 0.3% NaNO2 was administered in drinking water after MNNG initiation, forestomach carcinogenesis was clearly enhanced in F344 rats.( 13 ) Our present data, showing that treatment with 0.1 or 0.2% NaNO2 (but not 0.05% NaNO2) increases hyperplastic lesions, are in agreement with the previous results. Therefore, it is suggested that NaNO2 enhances forestomach tumorigenesis by increasing epithelial cell proliferation, depending on the dose. With combinations of NaNO2 and 0.1 or 1.0% AsA, proliferative changes were further enhanced, again with a clear dose dependence. As AsA itself did not induce any proliferative lesions or enhance forestomach carcinogenesis after initiation,( 13 ) it can be concluded that NaNO2 is a weak promoter of forestomach carcinogenesis and that AsA is a strong copromoter of forestomach carcinogenesis in the presence of NaNO2. Combined treatment with 0.3% NaNO2 in the drinking water and 1.0% AsA or sodium AsA in the diet for 51 weeks was earlier shown to induce forestomach papillomas in 20 and 53%, respectively, of animals without prior initiation treatment (accompanied by an increase in the BrdU labeling index), indicating carcinogenic potential with these combinations.( 13 )
Regarding the mechanisms underlying strong promotion of rat forestomach carcinogenesis by the combined treatment with NaNO2 and AsA, erosion and/or necrosis of the forestomach squamous epithelium and strong inflammatory changes in the submucosa followed by regenerative proliferation of the epithelium might be crucial factors. It is well known that promotion activity and cell proliferation activity, as a result of early stage cytotoxicity, are well correlated with the enhancement of forestomach carcinogenesis, as are several compounds such as butylated hydroxyanisole, catechol and caffeic acid.( 11 , 27 ) The observed cytotoxicity followed by cell proliferation in the present experiment thus provides an explanation for the initial step of the strong promotion of carcinogenesis by the combination of NaNO2 and AsA. The etiology of the cytotoxicity remains uncertain, but the fact that AsA has the potential to reduce nitrite into NO under acidic conditions,( 17 , 18 , 19 , 20 ) and the present in vitro finding of NO generation by the reaction of NaNO2 and AsA at pH 1.5, point to a role for oxidative stress. It is generally accepted that NO reacts with superoxide to form peroxynitrite, which can oxidize a variety of biomolecules either by its direct reactions or via secondary radicals such as nitrogen dioxide and carbonate or hydroxyl radicals.( 28 , 29 , 30 ) Considering that NO and peroxinitrite promote tissue damage by free radical‐mediated cytotoxicity,( 31 ) the production of NO resulting from the mixture of NaNO2 with AsA might play a key role in cell injury. Furthermore, we speculate that chronic stimulation consequently causes activation of a variety of inflammatory cells, which induce various oxidant‐generating enzymes such as NADPH oxidase and inducible NO synthase, subsequently generating high concentrations of diverse free radicals and oxidants in the forestomach.( 32 ) Our present data, indicating a slight increase of 8‐OHdG (albeit without statistical significance) in DNA of the forestomach of rats 4 h after cotreatment with NaNO2 and AsA, suggest sustained oxidative stress even in the long term. Formation of oxidized bases also implies alteration of the intranuclear redox status, leading to the induction of cell cycle‐dependent transcriptional events.( 33 , 34 ) Therefore, it is highly probable that the enhancement of tumor promotion observed in rats treated with the combination of NaNO2 and AsA involves oxidative stress attributable to NO formation. However, we could not demonstrate immunohistochemical positivity for nitrotyrosine in the forestomach epithelium of these rats, probably due to a technical problem. We are now developing a new method for detecting nitroguanine using HPLC. In the glandular stomach, although ulceration and regenerative hyperplasia were not observed, erosion or regeneration was more frequent in animals given both NaNO2 and AsA than with either chemical alone. We can speculate that the same mechanisms are operating as in the forestomach. However, we could not detect any promotion effects of NaNO2, with or without AsA, on glandular stomach tumor development under the present experimental conditions, despite effective initiation. The difference in the extent of injury between forestomach and glandular stomach may be due to differences in protective factors such as mucus formation, epithelial thickness and the concentrations of antioxidant enzymes (such as glutathione S‐transferase and quinone reductase), which are active against external stress.( 35 , 36 ) In a rat multiorgan carcinogenesis model, simultaneous administration of 0.05% NaNO2 and 0.25% AsA in the promotion stage demonstrated no apparent enhancement of carcinogenesis in any organs.( 15 ) Therefore, it can be concluded that the glandular stomach, which resembles the human stomach both morphologically and functionally, and other major organs, are not the targets of combined treatment with NaNO2 and AsA, particularly at low dose levels.
Humans consume large amounts of AsA in foods such as vegetables and fruits, and daily intake is estimated to be approximately 50–200 mg per person.( 37 , 38 ) Some ascorbic acid derivatives, such as ascorbyl palmitate and ascorbyl stearate, have furthermore found application as food additives, and AsA and sodium AsA have been used for medical use and dietary supplements.( 39 , 40 , 41 ) AsA was shown to inhibit formation of endogenous N‐nitroso compounds, which contribute to the etiology of human cancers.( 16 , 17 ) Treatment with 0.25% AsA inhibited combined 0.05% aminopyrene and 0.05% NaNO2‐induced rat hepatocarcinogenesis, but this dose level of AsA and NaNO2 did not induce any forestomach lesions.( 15 ) However, combinations of higher doses of AsA and NaNO2 were shown to promote forestomach carcinogenesis and induce forestomach tumors,( 13 ) as in the present study. NaNO2 is also widely applied as a food additive and can be generated by a reduction in oral microflora of the entero‐salivary recirculated dietary nitrate from meats, vegetables and tap water.( 1 , 2 , 3 ) The estimated daily intakes of NaNO2 and sodium nitrate are approximately 0.1–10 and 30–400 mg per person, respectively.( 2 ) Furthermore, vegetarian intake of nitrite is several times higher than in non‐vegetarians. However, the intakes of AsA and nitrite in the present experiment are estimated to be approximately 20–200( 39 , 40 , 41 ) and 5–30 times greater, respectively, than those in the human situation. Therefore, combined exposure of nitrite and AsA may not be a risk factor, at least in healthy people. Recently, a possible contribution of NO to the development of human adenocarcinomas in the gastro‐esophageal junction in patients with reflux esophagitis has been suggested. A benchtop model indicated that when nitrite was added to the acidic luminal compartment containing AsA, a high concentration of NO was generated, and it rapidly diffused into the epithelial compartment. It could be that the combined exposure to low doses of AsA and NaNO2 might enhance tumor induction in the junction through enhancement of NO production, and experiments to assess this possibility are now in progress in our laboratory.
In conclusion, the present study demonstrates that combined treatment with NaNO2 and AsA causes initial toxicities, such as necrosis and inflammation, followed by regenerative hyperplasia, and such events enhance carcinogenesis in the rat forestomach, but not the glandular stomach. NO‐associated oxidative stress may contribute to the observed toxicity and enhancement of forestomach carcinogenesis.
Acknowledgments
This study was supported by a Grant‐in‐Aid from the Ministry of Health, Labour and Welfare of Japan.
References
- 1. White JW. Relative significance of dietary sources of nitrate and nitrite. J Agric Food Chem 1976; 24: 202. [DOI] [PubMed] [Google Scholar]
- 2. Walker R. Nitrates, nitrites and N‐nitrosocompounds: a review of the occurrence in food and diet and the toxicological implications. Food Addit Contam 1990; 7: 717–68. [DOI] [PubMed] [Google Scholar]
- 3. Phizackerley PJ, Al‐Dabbagh SA. The estimation of nitrate and nitrite in saliva and urine. Anal Biochem 1983; 131: 242–5. [DOI] [PubMed] [Google Scholar]
- 4. Mirvish SS. Formation of N‐nitroso compounds: chemistry, kinetics, and in vivo occurrence. Toxicol Appl Pharmacol 1975; 31: 325–51. [DOI] [PubMed] [Google Scholar]
- 5. Newberne PM. Nitrite promotes lymphoma incidence in rats. Science 1979; 204: 1079–81. [DOI] [PubMed] [Google Scholar]
- 6. Mirvish SS, Bulay O, Runge RG, Patil K. Study of the carcinogenicity of large doses of dimethylnitrosamine, N‐nitroso‐l‐proline, and sodium nitrite administered in drinking water to rats. J Natl Cancer Inst 1980; 64: 1435–42. [DOI] [PubMed] [Google Scholar]
- 7. Inai K, Aoki Y, Tokuoka S. Chronic toxicity of sodium nitrite in mice, with reference to its tumorigenicity. Gann 1979; 70: 203–8. [PubMed] [Google Scholar]
- 8. Maekawa A, Ogiu T, Onodera H et al. Carcinogenicity studies of sodium nitrite and sodium nitrate in F344 rats. Food Chem Toxicol 1982; 20: 25–33. [DOI] [PubMed] [Google Scholar]
- 9. Grant D, Butler WH. Chronic toxicity of sodium nitrite in the male F344 rat. Food Chem Toxicol 1989; 27: 565–71. [DOI] [PubMed] [Google Scholar]
- 10. Hirose M, Fukushima S, Hasegawa R, Kato T, Tanaka H, Ito N. Effects of sodium nitrite and catechol or 3‐methoxycatechol in combination on rat stomach epithelium. Jpn J Cancer Res 1990; 81: 857–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirose M, Tanaka H, Takahashi S, Futakuchi M, Fukushima S, Ito N. Effects of sodium nitrite and catechol, 3‐methoxycatechol, or butylated hydroxyanisole in combination in a rat multiorgan carcinogenesis model. Cancer Res 1993; 53: 32–7. [PubMed] [Google Scholar]
- 12. Kawabe M, Takaba K, Yoshida Y, Hirose M. Effects of combined treatment with phenolic compounds and sodium nitrite on two‐stage carcinogenesis and cell proliferation in rat stomach. Jpn J Cancer Res 1994; 85: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida Y, Hirose M, Takaba K, Kimura J, Ito N. Induction and promotion of forestomach tumors by sodium nitrite in combination with ascorbic acid or sodium ascorbate in rats with or without N‐methyl‐N′‐nitro‐N‐nitrosoguanidine pre‐treatment. Int J Cancer 1994; 56: 124–8. [DOI] [PubMed] [Google Scholar]
- 14. Miyauchi M, Nakamura H, Furukawa F, Son H‐Y, Nishikawa A, Hirose M. Promoting effects of combined antioxidant and sodium nitrite treatment on forestomach carcinogenesis in rats after initiation with N‐methyl‐N′‐nitro‐N‐nitrosoguanidine. Cancer Lett 2002; 178: 19–24. [DOI] [PubMed] [Google Scholar]
- 15. Yada H, Hirose M, Tamano S et al. Effects of antioxidant 1‐O‐hexyl‐2,3,5‐trimethylhydroquinone or ascorbic acid on carcinogenesis induced by administration of aminopyrine and sodium nitrite in a rat multi‐organ carcinogenesis model. Jpn J Cancer Res 2002; 93: 1299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mirvish SS, Wallcave L, Eagen M, Shubik P. Ascorbate‐nitrite reaction: possible means of blocking the formation of carcinogenic N‐nitroso compounds. Science 1972; 177: 65–8. [DOI] [PubMed] [Google Scholar]
- 17. Mirvish SS. Experimental evidence for inhibition of N‐nitroso compound formation as a factor in the negative correlation between vitamin C consumption and the incidence of certain cancers. Cancer Res (Suppl) 1994; 54: 1948–51. [PubMed] [Google Scholar]
- 18. McKnight GM, Smith LM, Drummond RS, Duncan CW, Golden M, Benjamin N. Chemical synthesis of nitric oxide in the stomach from dietary nitrite in humans. Gut 1997; 40: 211–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut 1994; 35: 1543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iijima K, Grant J, McElroy K, Fyfe V, Preston TEL, McColl K. Novel mechanism of nitrosative stress from dietary nitrate with relevance to gastro‐oesophageal junction cancers. Carcinogenesis 2003; 24: 1951–60. [DOI] [PubMed] [Google Scholar]
- 21. Hofseth LJ, Hussain SP, Wogan GN, Harris CC. Nitric oxide in cancer and chemoprevention. Free Radic Biol Med 2003; 34: 955–68. [DOI] [PubMed] [Google Scholar]
- 22. Nishikawa A, Tanakamaru Z, Furukawa F et al. Chemopreventive activity of oltipraz against induction of glandular stomach carcinogenesis in rats by N‐methyl‐N′‐nitro‐N‐nitrosoguanidine. Carcinogenesis 1998; 19: 365–8. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi M, Nishikawa A, Furukawa F, Enami T, Hasegawa T, Hayashi Y. Dose‐dependent promoting effects of sodium chloride (NaCl) on rat glandular stomach carcinogenesis initiated with N‐methyl‐N′‐nitro‐N‐nitrosoguanidine. Carcinogenesis 1994; 15: 1429–32. [DOI] [PubMed] [Google Scholar]
- 24. Nakae D, Mizumoto Y, Kobayashi E, Noguchi O, Konishi Y. Improved genomic/nuclear DNA extraction for 8‐hydroxydeoxyguanosine analysis of small amounts of rat liver tissue. Cancer Lett 1995; 97: 233–9. [DOI] [PubMed] [Google Scholar]
- 25. Pou S, Tsai P, Porasuphatana S et al. Spin trapping of nitric oxide by ferro‐chelates: kinetic and in vivo pharmacokinetic studies. Biochim Biophys Acta 1999; 1427: 216–26. [DOI] [PubMed] [Google Scholar]
- 26. Porasupharatana S, Weaver J, Budzichowski TA, Tsai P, Rosen GM. Differential effects of buffer on the spin trapping of nitric oxide by iron chelates. Anal Biochem 2001; 298: 50–6. [DOI] [PubMed] [Google Scholar]
- 27. Hirose M, Kawabe M, Shibata M, Takahashi S, Okazaki S, Ito N. Influence of caffeic acid and other O‐dihydroxybenzene derivatives on N‐methyl‐N′‐nitro‐N‐nitrosoguanidine‐initiated rat forestomach carcinogenesis. Carcinogenesis 1992; 13: 1825–8. [DOI] [PubMed] [Google Scholar]
- 28. Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H. Formation of 8‐nitroguanine by the reaction of guanine with peroxynitrite in vitro . Carcinogenesis 1995; 16: 2045–50. [DOI] [PubMed] [Google Scholar]
- 29. Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett 1995; 371: 86–8. [DOI] [PubMed] [Google Scholar]
- 30. Radi R, Peluffo G, Alvarez MN, Naviliat M, Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic Biol Med 2001; 30: 463–88. [DOI] [PubMed] [Google Scholar]
- 31. Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls: The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 1991; 266: 4244–50. [PubMed] [Google Scholar]
- 32. Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation‐induced carcinogenesis. Arch Biochem Biophys 2003; 417: 3–11. [DOI] [PubMed] [Google Scholar]
- 33. Cerutti PA. The role of active oxygen in tumor promotion. In: Curtis C, Harris C, eds. Biochemical and Molecular Epidemiology of Cancer. New York: Alan R Liss, 1986; 167–76. [Google Scholar]
- 34. Kensler TW, Egner PA, Taffe BG, Trush MA. Role of free radicals in tumor promotion and progression. In: Slaga TJ, Klein‐Szanto AJP, Boutwell RK, Stevenson DE, Spitzer HL, D'Motto B, eds. Skin Carcinogenesis: Mechanisms and Human Relevance. New York: Alan R Liss, 1989; 233–48. [PubMed] [Google Scholar]
- 35. Potter DW, Tran T‐B. Apparent rates of glutathione turnover in rat tissues. Toxicol Appl Pharmacol 1993; 120: 186–92. [DOI] [PubMed] [Google Scholar]
- 36. Munday R, Munday CM. Induction of phase II enzymes by 3H‐1,2‐dithiole‐3‐thione: dose–response study in rats. Carcinogenesis 2004; 25: 1721–5. [DOI] [PubMed] [Google Scholar]
- 37. Zhang S, Hunter DJ, Forman MR et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst 1999; 91: 547–56. [DOI] [PubMed] [Google Scholar]
- 38. Michels KB, Holmberg L, Bergkvist L, Ljung H, Bruce A, Wolk A. Dietary antioxidant vitamins, retinol, and breast cancer incidence in a cohort of Swedish women. Int J Cancer 2001; 91: 563–7. [DOI] [PubMed] [Google Scholar]
- 39. Knekt P, Ritz J, Pereira MA et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr 2004; 80: 1508–20. [DOI] [PubMed] [Google Scholar]
- 40. Norman HA, Butrum RR, Feldman E et al. The role of dietary supplements during cancer therapy. J Nutr 2003; 133: 3794–9. [DOI] [PubMed] [Google Scholar]
- 41. White E, Patterson RE, Kristal AR et al. Vitamins and lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol 2004; 159: 83–93. [DOI] [PubMed] [Google Scholar]


