Abstract
Degradation of extracellular matrix (ECM) is one of the first steps in tumor invasion and metastasis. Matrix metalloproteinases (MMP) have been strongly implicated in this step. Membrane‐type MMP‐1 (MT1‐MMP) was first identified as an activator of proMMP‐2 expressed on the surface of tumor cells and later, not only ECM macromolecules but also various biologically important molecules, were shown to serve as substrates for MT1‐MMP. Accumulated lines of evidence have demonstrated that MT1‐MMP expression level is closely associated with invasiveness and malignancy of tumors, suggesting that MT1‐MMP is one of the most critical factors for tumor invasion and metastasis. Despite enthusiasm for MMP inhibitors, phase III trials have not yet demonstrated significance in overall survival and side‐effects remain an issue. An understanding of the functions of MT1‐MMP could supply clues for developing novel therapeutic strategies targeting MT1‐MMP. (Cancer Sci 2005; 96: 212 –217)
Extracellular matrix (ECM) macromolecules are important for creating the cellular environments required during development and morphogenesis. Matrix metalloproteinases (MMP) play important roles in development and morphogenesis by participating in ECM re‐modeling. 1 , 2 , 3 Under normal physiological conditions, the activities of MMP are precisely regulated at the level of transcription, activation of the precursor zymogens, interaction with specific ECM components, and inhibition by endogenous inhibitors. (4) Cancer cells also make use of MMP for invasion and metastasis. The invading cells are forced to proliferate within an embedded dense three‐dimensional matrix composed largely of type I collagen or cross‐linked fibrin. Membrane type MMP‐1 (MT1‐MMP) has been thought to play a major role in this step. 5 , 6
Unique structure of MT1‐MMP. Currently 23 MMP have been identified in humans and they are divided into two groups; secretory and membrane‐type (Fig. 1). (7) While MT1‐MMP has a common MMP domain structure with pre‐, pro‐, catalytic and hemopexin‐like domains, it also has unique insertions. First there is an insertion of 11 amino‐acids between the pro‐peptide and the catalytic domains, which contains the recognition sequence for the Golgi‐associated processing enzyme furin. Another unique insertion at the C‐terminus contains a hydrophobic amino‐acid sequence that acts as a transmembrane domain. Thus, unlike secretory MMP, MT1‐MMP is expressed on the cell surface in an active form. (5)
Figure 1.
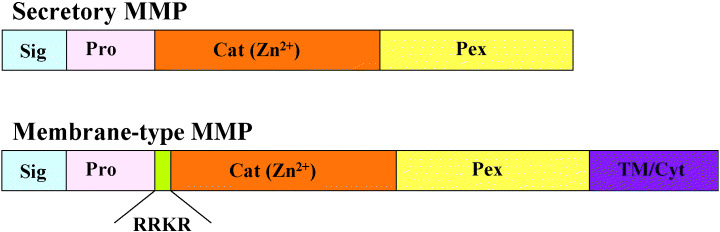
Domain structure of matrix metalloproteinases (MMP). The domain organization of MMP is as indicated: Sig, signal peptide; Pro, pro‐peptide; Cat(Zn2+), catalytic; Pex, hemopexin‐like; Tm/Cyt, transmembrane and cytoplasmic. RRKR is the recognition sequence for furin.
Implication of MT1‐MMP in tumor invasion and metastasis. MMP‐2 has been suspected to be associated with tumor invasion because it can degrade the type IV collagen, which is a major component of the basement membrane. An active form of MMP‐2 has frequently been detected in tumor tissues. 8 , 9 The expression of MT1‐MMP has been demonstrated in tumor tissues, in which MMP‐2 is always activated. 5 , 10 , 11 , 12 Furthermore, the MMP‐2 activation ratio correlates with the MT1‐MMP expression level, suggesting that MT1‐MMP is the MMP‐2 activator in tumor tissues. An analysis of MT1‐MMP‐deficient mice, which had severe defects in bone formation and died within a few weeks of birth, clearly showed that MT1‐MMP is the major activator of MMP‐2 during tissue morphogenesis. (13) Interestingly MMP‐2‐deficient mice do not show such severe defects, indicating that MT1‐MMP has critical functions in addition to MMP‐2 activation. ECM macromolecules including type I collagen and various biologically important molecules are now known to serve as substrates for MT1‐MMP, which will be discussed in the following sections (Table 1). 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
Table 1.
Substrates for MT1‐MMP and cleavage sequences
| Protein | Cleavage sequences* | Reference |
|---|---|---|
| ECM Components | ||
| Type I Collagen α1(I) Chain | 771PGPQG↓IAGQR | 14 |
| Type I Collagen α2(I) Chain | 771PGPQG↓LLGAPG↓ILGLP | 14 |
| Laminin 5 γ2 chain | 409DCYSG↓DENPD | 15 |
| Lumican | 66PGIKY↓LYLRN | 16 |
| 80HIDEK↓AFENV | ||
| 271TVNEN↓LENYY | ||
| 281LEVNQ↓LEKFD | ||
| Cell‐Surface Receptors | ||
| Integrin aV | 487ITKRD↓LALSE | 17 |
| Transglutaminase | 371CGPVP↓VRAKI | 17 |
| 454EAFTR↓AN↓LNKLA | ||
| CD44H | 158KGEYR↓TNPED | 19 |
| 182SSSER↓SSTRG | ||
| 188STSGG↓YIFYT | ||
| Syndecan 1 | 78PEPTG↓LEATA | 20 |
| 241GASQG↓LLDRK | ||
| Ligands and Others | ||
| KiSS 1 | 114WNSFG↓LRFGK | 21 |
| Interleukin 8 | 23AVLPR↓SAKEL | 22 |
| Secretory Leukocyte Protease Inhibitor | 20WAVEG↓SGKSF | 22 |
| Pro Tumor Necrosis Factor α | 65RDLSL↓ISP↓LA↓QA↓VRSSS | 22 |
| Connective Tissue Growth Factor | 177PALAA↓YRLEDTF | 22 |
| Amyloid Precursor Protein | 575DVLAN↓MISEP | 23 |
MT1‐MMP, membrane type matrix metalloproteinases‐1; ECM, extracellular matrix. *MT1‐MMP cleavage sites are indicated by the arrow.
Induction of MT1‐MMP expression by transformation of epithelial cells. MT1‐MMP is associated with not only tumor invasion and metastasis but also tissue morphogenesis, which is clearly demonstrated in vitro by the Madin‐Darby canine kidney (MDCK) epithelial cell system (Fig. 2). MDCK cells are kidney‐derived normal epithelial cells that show typical cobblestone morphology when cultured in a plastic dish. MDCK cells form cysts when cultured in three‐dimensional type I collagen gel, and grow to form branching tubules in the presence of hepatocyte growth factor. (24) MT1‐MMP expression is induced at a low level in tube‐forming MDCK cells. Collagenolytic activity of MT1‐MMP plays an essential role in tube formation in three‐dimensional collagen gel. (25) Oncogenic transformation of MDCK cells by v‐src or erbB2 induces loss of cell‐to‐cell adhesion and a high‐level expression of MT1‐MMP. (26) Transformed cells acquire the capacity to invade collagen gels, tumorigenicity and metastatic ability in nude mice. Inhibition of invasive growth of transformed cells in collagen gel by MMP inhibitors indicates that MT1‐MMP plays a major role in their invasive growth.
Figure 2.
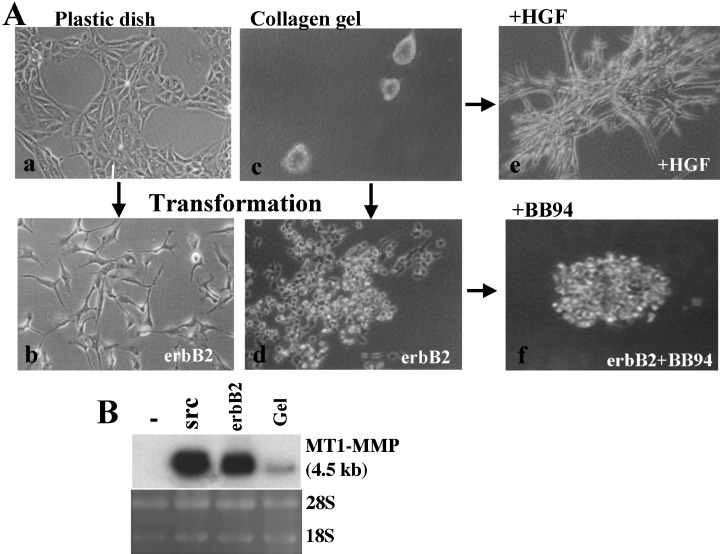
Induction of membrane type matrix metalloproteinases‐1 (MT1‐MMP). A: Madin‐Darby canine kidney (MDCK) cells cultured on plastic dish show a normal epithelial morphology (a), and loose cell‐cell adhesion by transformation with erbB2 gene (b). MDCK cells form cysts in type I collagen gel (c), and erbB2‐transformed cells show invasive growth in it (d). Addition of hepatocyte growth factor to the collagen‐gel culture of MDCK cells induces formation of branching tubules (e). Addition of MMP‐specific inhibitor BB94 to the collagen culture of erbB2‐transformed cells suppressed spreading of cells into collagen (f). B: MT1‐MMP mRNA expression is compared among control MDCK cells (–), v‐src‐transformed cells (src), erbB2‐transformed cells (erbB2) and MDCK cells cultured in type I collagen gel (Gel).
MT1‐MMP and extra‐cellular signal‐regulated kinase activation. Collagen matrix acts as physical barrier for tumor‐cell invasion, but at the same time it provides cells with various signals for growth, survival, differentiation, migration and so on. Extra‐cellular signal‐regulated kinase (ERK) is activated in cells cultured in type I collagen matrix or on collagen sheet through interaction with integrins. ERK activated by collagen culture of cells in turn stimulates MT1‐MMP expression (Fig. 3a). (27) MT1‐MMP thus induced by ERK plays an essential role in sustained activation of ERK. Activation of ERK is not maintained in the presence of MT1‐MMP inhibitor. Thus, MT1‐MMP functions in a positive feedback loop to induce sustained ERK activation and subsequent MT1‐MMP accumulation, which collectively promotes cell migration on collagen (Fig. 3b).
Figure 3.
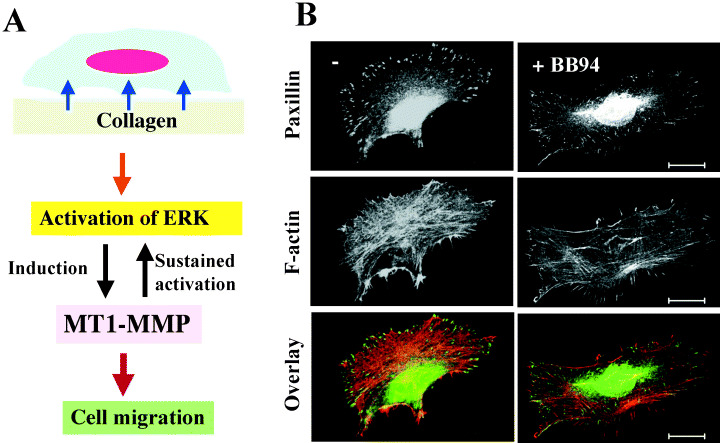
Role of membrane type matrix metalloproteinases‐1 (MT1‐MMP) in cell migration. a, Schematic representation of extra‐cellular signal‐regulated kinase (ERK) and MT1‐MMP induction on type I collagen. Note that MT1‐MMP functions in a positive feedback loop to induce sustained ERK activation and subsequent MT1‐MMP accumulation, which collectively promotes cell migration on collagen b, MMP inhibitor BB94 suppresses formation of focal adhesion and actin stress fiber in HT1080 cells cultured on collagen, which results in inhibition of cell migration.
Decorin and lumican as substrates for MT1‐MMP. ECM regulates cell growth/survival and differentiation, which vary between tissues. Some ECM components such as decorin and lumican, both of which belong to a small lecine‐rich proteoglycan family, negatively regulate cell growth and are classified as tumor suppressors. 28 , 29 Both docorin and lumican have been shown to induce p21/Waf‐1, an inhibitor of cyclin‐dependent kinases in tumor cells that down‐regulates cell growth and tumorigenicity (Fig. 4). (16) MT1‐MMP expressed on the surface of tumor cells degrades decorin and lumican, which restores tumorigenicity. (16) Lumican is the most abundant proteoglycan among small lecine‐rich proteoglycan family members in breast cancer. (30) Although lumican mRNA expression is high within the tumor and tumor margin, lumican protein is not immunolocalized within the tumor but within the collagenous stroma. As MT1‐MMP is predominantly found within breast cancer tumors, 5 , 12 lumican protein expressed by tumor cells would be degraded by MT1‐MMP. This may account for the observation that differences between lumican levels in normal and malignant breast tissues observed by both immunohistochemistry and Western blot are not as marked as those seen at the level of mRNA expression. (30)
Figure 4.
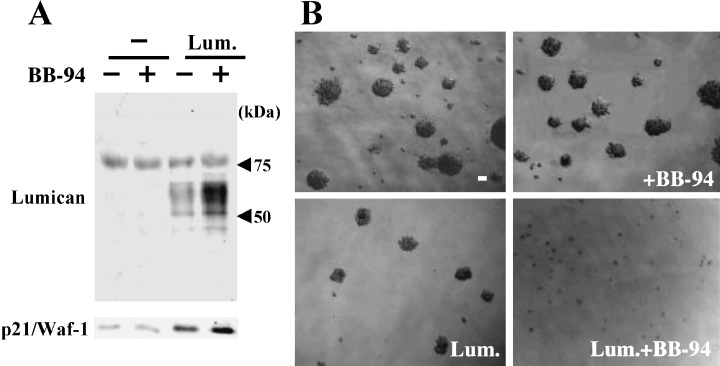
Degradation of lumican by membrane type matrix metalloproteinases‐1 (MT1‐MMP) abrogates suppression of tumorigenicity. (a) Control HT1080 (–) or cells stably transfected with lumican gene (Lum) were cultured with or without BB94, and the supernatants and cell lysates were examined for the expression of lumican and p21/Waf‐1, respectively. b, control HT1080 (–) or HT1080/Lum cells were plated into 0.3% agarose with or without BB94, and colonies were observed under microscopy 2‐weeks after incubation. Note that the accumulation level of lumican protein correlates with p21/Waf‐1 expression level and reversely with colony forming ability in agarose.
Cd44. CD44 is a major receptor for hyaluronan and its association with tumor malignancy has been investigated for a long time. Furthermore, recent evidence indicates the importance of CD44 cleavage in tumor invasion and metastasis. Cleavage of CD44 by MT1‐MMP has been demonstrated with in vitro experiments, (19) and the analysis of cleaved fragments of CD44 in human tumor tissues indicated the involvement of MT1‐MMP. (31) CD44 is also cleaved by structurally related ADAM metalloproteinases (ADAM: a disintegrin and metalloproteinase). The mechanism and significance of CD44 cleavage were reviewed by Nagano and Saya. (32)
Syndecan‐1. Another interesting cell‐surface receptor cleaved by MT1‐MMP is syndecan‐1. Syndecans are transmembrane heparan sulfate proteoglycans expressed on all adherent cells, and have been proposed to play important roles in tissue morphogenesis by virtue of their ability to bind, through their covalently attached glycosaminoglycan chains, to a variety of ECM molecules including fibronectin, thrombospondin, various collagens, and heparin‐binding growth‐associated molecules and growth factors, such as basic fibroblast growth factor. As the expression of syndecans appears to be controlled during both development and the progression of tumor cells to the metastatic phenotype, it has been proposed that syndecans are important regulators of the migratory and invasive behaviors of both normal and transformed cells. 33 , 34 Syndecan‐1 is abundant in normal epithelial cells and tissues, localizing to both basal and suprabasal cell layers. Disruption of syndecan‐1 expression in cultured cells leads to an epithelial mesenchymal transformation, with associated changes in cell polarity, cell‐cell adhesion and altered epithelial‐specific gene expression. 35 , 36 Syndecan‐1 is cleaved by MT1‐MMP at a juxtamembrane site, which results in shedding of its ectodomain and stimulates cell migration. (20) Syndecan‐1 shedding is also mediated by MMP‐7/matrilysin. (37) High cancer cell syndecan‐1 expression in tissue biopsies has been found to be associated with a favorable outcome. Low syndecan‐1 expression in immunostaining of cancer tissues is associated with a poor histological grade of differentiation in various cancer tissues. High serum syndecan‐1 levels at diagnosis were reported to be associated with poor outcome in lung cancer. (38) It should be hypothesized that syndecan‐1 ectodomain might be shed by MT1‐MMP and/or MMP‐7 expressed in tumor cells, because expression levels of MT1‐MMP and MMP‐7 are also associated with the malignancy of tumors including lung cancer. (12)
KiSS‐1/Metastin. The KiSS‐1 gene was identified originally as being differentially up‐regulated in C8161 melanoma cells that have lost the potential to metastasize after microcell‐mediated transfer of human chromosome 6. 39 , 40 A C‐terminally amidated peptide with 54 amino acid residues corresponding to residues 68–121 of the full‐length KiSS‐1 protein was isolated from human placenta as the endogenous ligand of an orphan G‐protein‐coupled receptor (hOT7T175/AXOR12/human GPR54), and was named metastin, because it suppressed metastasis of B16‐BL6 melanoma cells expressing hOT7T175. (41) Metastin peptide is cleaved by various MMP including MT1‐MMP. (21) Metastin down‐regulates migration of receptor‐expressing tumor cells and cleavage of metastin by MT1‐MMP inactivates its ligand activity. Thus, cleavage of metastin by MT1‐MMP abrogates metastin‐mediated inhibition of tumor metastasis.
Conclusion
A recent new idea indicates the importance of the physical three‐dimensionality of the matrix in regulating normal cell behavior, including cell proliferation and Hotary et al. emphasized the contribution of MT1‐MMP in tumor cell growth in a three‐dimensional matrix composed largely of type I collagen or crosslinked fibrin. (6) Various substrates for MT1‐MMP other than ECM components have been discovered, such as adherence molecules, cytokines/growth factors and cell surface receptors (Fig. 5). While the physiological significance of these activities of MT1‐MMP still remain to be explored, MT1‐MMP appears to be involved in regulation of various events taking place at the cell–ECM interface. These multifunctions of MT1‐MMP may play important roles in not only the initial step of tumor invasion but also cell growth in distant organs where ECM environments are different from that of the original tissue. Although MMP has been expected to be a molecular target for preventing tumor metastasis, initial clinical tests of broad‐spectrum MMP inhibitors have been disappointing. The development of novel therapeutic strategies targeting MMP is expected.
Figure 5.
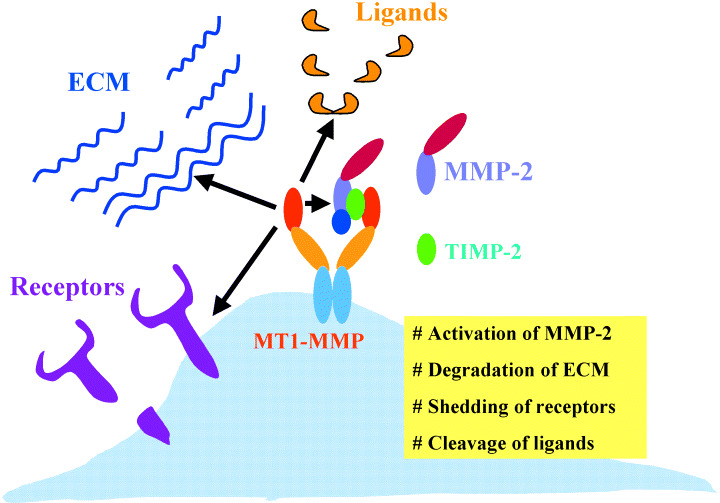
Schematic representation of membrane type matrix metalloproteinases‐1 (MT1‐MMP) functions on invasive tumor cells. MT1‐MMP contributes to invasion of tumor cells by activating MMP‐2, degrading various extracellular matrix components, shedding cell‐surface receptors and cleaving ligand molecules.
Acknowledgment
This work was supported by a grant for cancer research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Woessner JFJ. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 1991; 5: 2145–54. [PubMed] [Google Scholar]
- 2. Stetler‐Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 1993; 9: 541–73. [DOI] [PubMed] [Google Scholar]
- 3. Seiki M, Yana I. Roles of pericellular proteolysis by membrane type‐1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci 2003; 94: 569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990; 6: 121–5. [DOI] [PubMed] [Google Scholar]
- 5. Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994; 370: 61–5. [DOI] [PubMed] [Google Scholar]
- 6. Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three‐dimensional extracellular matrix. Cell 2003; 114: 33–45. [DOI] [PubMed] [Google Scholar]
- 7. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res 2003; 92: 827–39. [DOI] [PubMed] [Google Scholar]
- 8. Brown PD, Bloxidge RE, Stuart NSA, Gatter KC, Carmichael J. Association between expression of activated 72‐Kilodaloton gelatinase and tumor spread in non‐small‐cell lung carcinoma. J Natl Cancer Inst 1993; 85: 574–8. [DOI] [PubMed] [Google Scholar]
- 9. Azzam HS, Arand G, Lippman ME, Thompson EW. Association of MMP‐2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP‐2 activation. J Natl Cancer Inst 1993; 85: 1758–64. [DOI] [PubMed] [Google Scholar]
- 10. Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane‐type matrix metalloproteinase in human gastric carcinomas. Cancer Res 1995; 55: 3263–6. [PubMed] [Google Scholar]
- 11. Ueno H, Nakamura H, Inoue M, Imai K, Noguchi M, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane‐type 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinoma. Cancer Res 1997; 57: 2055–60. [PubMed] [Google Scholar]
- 12. Tokuraku M, Sato H, Murakami S, Okada Y, Watanabe Y, Seiki M. Activation of the precursor of gelatinase A/72 kDa type IV collagenase/MMP‐2 in lung carcinomas correlates with the expression of membrane‐type matrix metalloproteinase (MT‐MMP) and with lymph node metastasis. Int J Cancer 1995; 64: 355–9. [DOI] [PubMed] [Google Scholar]
- 13. Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal‐Hansen H. MT1‐MMP‐deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999; 99: 81–92. [DOI] [PubMed] [Google Scholar]
- 14. Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane‐type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem 1997; 272: 2446–51. [DOI] [PubMed] [Google Scholar]
- 15. Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Vito Quaranta V. Role of cell surface metalloprotease MT1‐MMP in epithelial cell migration over laminin‐5. J Cell Biol 2000; 148: 615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Aoki T, Mori Y, Ahmad M, Miyamori H, Takino T, Sato H. Cleavage of lumican by membrane‐type matrix metalloproteinase‐1 abrogates this proteoglycan‐mediated suppression of tumor cell colony formation in soft agar. Cancer Res 2004; 64: 7058–64. [DOI] [PubMed] [Google Scholar]
- 17. Ratnikov BI, Rozanov DV, Postnova TI, Baciu PG, Zhang H, DiScipio RG, Chestukhina GG, Smith JW, Deryugina EI, Strongin AY. An alternative processing of integrin αv subunit in tumor cells by membrane type‐1 matrix metalloproteinase. J Biol Chem 2002; 277: 7377–85. [DOI] [PubMed] [Google Scholar]
- 18. Belkin AM, Akimov SS, Zaritskayai LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrix‐dependent proteolysis of surface transglutaminase by membrane‐type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem 2001; 276: 18415–22. [DOI] [PubMed] [Google Scholar]
- 19. Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane‐type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol 2001; 153: 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan‐1 by membrane‐type matrix metalloproteinase‐1 stimulates cell migration. J Biol Chem 2003; 278: 40764–70. [DOI] [PubMed] [Google Scholar]
- 21. Takino T, Koshikawa N, Miyamori H, Tanaka M, Sasaki T, Okada Y, Seiki M, Sato H. Cleavage of metastasis suppressor gene product KiSS‐1 protein/metastin by matrix metalloproteinases. Oncogene 2003; 22: 4617–26. [DOI] [PubMed] [Google Scholar]
- 22. Tam EM, Morrison CJ, Wu YI, Stack MS, Overall CM. Membrane protease proteomics: Isotope‐coded affinity tag MS identification of undescribed MT1–matrix metalloproteinase substrates. Proc Natl Acad Sci USA 2004; 101: 6917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higashi S, Miyazaki M. Identification of a region of β‐amyloid precursor protein essential for its gelatinase a inhibitory activity. J Biol Chem 2003; 278: 14020–8. [DOI] [PubMed] [Google Scholar]
- 24. Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast‐derived epithelial morphogen as hepatocyte growth factor. Cell 1991; 67: 901–8. [DOI] [PubMed] [Google Scholar]
- 25. Kadono Y, Shibahara K, Namiki M, Watanabe Y, Seiki M, Sato H. Membrane type 1‐matrix metalloproteinase (MT1‐MMP) is involved in the formation of hepatocyte growth factor/scatter factor‐induced branching tubules in Madin‐Darby Canine Kidney (MDCK) epithelial cells. Biochem Biophys Res Commun 1998; 251: 681–7. [DOI] [PubMed] [Google Scholar]
- 26. Kadono K, Okada Y, Namiki M, Seiki M, Sato H. Transformation of epithelial MDCK cells with pp60v‐src induces expression of membrane‐type 1‐matrix metalloproteinase and the invasiveness. Cancer Res 1998; 58: 2240–4. [PubMed] [Google Scholar]
- 27. Takino T, Miyamori H, Watanabe Y, Yoshioka K, Sato H. Membrane‐Type 1 Matrix Metalloproteinase Regulates Collagen‐dependent Mitogen‐Activated Protein/Extracellular Signal‐Related Kinase Activation and Cell Migration. Cancer Res 2004; 64: 1044–9. [DOI] [PubMed] [Google Scholar]
- 28. Kolettas E, Rosenberger RF. Suppression of decorin expression and partial induction of anchorage‐independent growth by the v‐src oncogene in human fibroblasts. Eur J Biochem 1998; 254: 266–74. [DOI] [PubMed] [Google Scholar]
- 29. Yoshioka N, Inoue H, Nakanishi K, Oka K, Yutsudo M, Yamashita A, Hakura A, Nojima H. Isolation of transformation suppressor genes by cDNA subtraction: lumican suppresses transformation induced by v‐src and v‐K‐ras. J Virol 2000; 74: 1008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leygue E, Snell L, Dotzlaw H, Troup S, Hiller‐Hitchcock T, Murphy LC, Roughley PJ, Watson PH. Lumican and decorin are differentially expressed in human breast carcinoma. J Pathol 2000; 192: 313–20. [DOI] [PubMed] [Google Scholar]
- 31. Nakamura H, Suenaga N, Taniwaki K, Matsuki H, Yonezawa K, Fujii M, Okada Y, Seiki M. Constitutive and induced CD44 shedding by ADAM‐like proteases and membrane‐type 1 matrix metalloproteinase. Cancer Res 2004; 64: 876–82. [DOI] [PubMed] [Google Scholar]
- 32. Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci 2004; 95: 930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol 1992; 8: 365–93. [DOI] [PubMed] [Google Scholar]
- 34. Inki P, Jalkanen M. The role of syndecan‐1 in malignancies. Ann Med 1996; 28: 63–7. [DOI] [PubMed] [Google Scholar]
- 35. Rapraeger AC. Molecular interactions of syndecans during development. Semin Cell Dev Biol 2001; 12: 107–16. [DOI] [PubMed] [Google Scholar]
- 36. Dobra K, Andang M, Syrokou A, Karamanos NK, Hjerpe A. Differentiation of mesothelioma cells is influenced by the expression of proteoglycans. Exp Cell Res 2000; 258: 12–22. [DOI] [PubMed] [Google Scholar]
- 37. Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan‐1 shedding of syndecan‐1 regulates chemokine mobiblization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002; 111: 635–46. [DOI] [PubMed] [Google Scholar]
- 38. Joensuu H, Anttonen A, Eriksson M, Makitaro R, Alfthan H, Kinnula V, Leppa S. Cancer Res 2002; 62: 5210–7. [PubMed] [Google Scholar]
- 39. Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS‐1, a novel human malignant melanoma metastasis‐suppressor gene. J Natl Cancer Institute 1996; 88: 1731–1737. Erratum in J Natl Cancer Institute 1997; 89: 1549. [DOI] [PubMed] [Google Scholar]
- 40. Lee JH, Welch DR. Identification of highly expressed genes in metastasis‐suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. Int J Cancer 1997; 71: 1035–44. [DOI] [PubMed] [Google Scholar]
- 41. Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS‐1 encodes peptide ligand of a G‐protein‐coupled receptor. Nature 2001; 411: 613–7. [DOI] [PubMed] [Google Scholar]


