Abstract
Hypoxia‐inducible factor‐1 (HIF‐1) is a transcription factor that plays an important role in tumor growth and metastasis by regulating energy metabolism and inducing angiogenesis. Elevated levels of HIF‐1α, a subunit of HIF‐1, are noted in various malignant tumors, but it is unclear whether this is so in esophageal carcinoma. The purpose of this study was to evaluate the implications of HIF‐1α expression in esophageal squamous cell carcinoma. In 215 patients with esophageal carcinoma, we examined immunoreactivity for HIF‐1α protein, vascular endothelial growth factor (VEGF) protein and p53 protein. In 38 patients, we examined the expression of HIF‐1α messenger ribonucleic acid (mRNA) (using the semiquantitative reverse transcriptase‐polymerase chain reaction [RT‐PCR]). A positive HIF‐1α protein expression was recognized in 95% of the patients, and was strongly apparent within both the nuclei and/or cytoplasm of tumor cells. The proportion of patients in the ‘high score’ group for HIF‐1α protein expression increased significantly with increasing VEGF protein expression. Immunoreactivity for HIF‐1α protein was found to have a significant effect on disease‐free survival rate in our univariate analysis, but no effect on overall survival rate. In RT‐PCR, HIF‐1α mRNA scores correlated significantly with scores for HIF‐1α protein expression, but not with any clinicopathologic factor or either of the survival rates. The detection of HIF‐1α protein and mRNA would appear to offer limited information as to progression and prognosis in esophageal carcinoma. (Cancer Sci 2005; 96: 176–182)
Tumor progression toward aggressive and metastatic potential is a fundamental process. Hypoxia is important in tumor progression, since it limits tumor growth, and tumors with poor vascularization fail to grow or form metastases. 1 , 2 Indeed, hypoxia has been negatively correlated with various indicators of tumor aggression, as well as metastasis and poor prognosis, in a number of tumors. 3 , 4 , 5 , 6 However, the mechanisms leading to the adaptation of tumor cells to these unfavorable environmental factors are still poorly understood.
Over the last decade or so, a number of angiogenic factors have been identified. Among these, one of the most important is vascular endothelial growth factor (VEGF). (7) Overexpression of VEGF is associated with angiogenesis and prognosis in several tumors, including esophageal carcinoma. 7 , 8 , 9 , 10 In addition, hypoxia‐inducible factor (HIF)‐1, one of the transcription factors, has been identified and found to play an essential role in oxygen homeostasis. 11 , 12 HIF‐1 is a heterodimer, composed of HIF‐1α (120 kDa) and HIF‐1β (91, 93, 94 kDa). HIF‐1β, also known as aryl hydrocarbon receptor nuclear translocator, is constitutively expressed, and usually does not change following hypoxic stimulation. However, under normoxic conditions, HIF‐1α is maintained at low levels due to continuous degradation via the ubiquitin‐dependent proteosome pathway, and this pathway is inhibited by hypoxia and by p53 or von Hippel‐Lindau tumor‐suppressor gene defects, leading to stabilization of the HIF‐1α protein. 13 , 14 , 15 , 16 Therefore, hypoxia can lead to a rapid increase in HIF‐1α protein levels. 11 , 12 , 13 , 16 Furthermore, HIF‐1α upregulates a number of factors important for tumor expansion, including VEGF. 17 , 18 In several cancers, overexpression of HIF‐1α protein has been found to be associated with tumor aggressiveness and with an unfavorable prognosis. 1 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 In esophageal carcinoma, however, few studies have yet dealt with the relationship between HIF‐1α protein expression and tumor progression or prognosis. The current study, using an immunohistochemical approach to examine formalin‐fixed, paraffin‐embedded, tumor‐tissue sections from 215 patients with esophageal squamous cell carcinoma, and using a semiquantitative reverse transcriptase‐polymerase chain reaction (RT‐PCR) approach to examine fresh frozen tissues (38 tumor tissues and 14 normal mucosal tissues from 38 patients), was aimed at ascertaining: (i) whether HIF‐1α protein is expressed in such sections; (ii) whether its expression shows any correlation with a variety of clinicopathologic findings, with the immunoreactivities for VEGF protein and p53 oncoprotein, and/or with clinical outcome in such patients; and (iii) whether expression of HIF‐1α messenger ribonucleic acid (mRNA) (as detected using semiquantitative RT‐PCR) is correlated with the expression of HIF‐1α protein (as detected using immunohistochemistry).
Materials and Methods
The material used comprised: (i) 215 surgically resected tumor specimens for immunohistochemistry, and (ii) fresh materials (38 tumor tissues and 14 corresponding esophageal normal mucosal tissues) for semiquantitative RT‐PCR. Materials (i) and (ii) were obtained from patients with esophageal squamous cell carcinoma between 1980 and 2002, and between 1996 and 2002, respectively, at the Department of Surgery II, National Defense Medical College Hospital, Tokorozawa, Japan. No patients had received preoperative treatment. Their clinicopathologic findings were determined according to the criteria proposed by the International Union Against Cancer (UICC). (28)
For immunohistochemistry, each specimen was obtained from a block, including the tumor center and the invasive front. Immunohistochemical staining for three antibodies (HIF‐1α, VEGF, p53 oncoprotein) was performed on specimens obtained from the same block. For immunohistochemistry of HIF‐1α protein, we used the catalyzed signal‐amplification system method (DAKO) on deparaffinized sections, employing mouse monoclonal antibodies against HIF‐1α protein (NB100‐105; Nobus Biological Inc.) diluted 1:500. Autoclave pretreatment in 0.1 M citrate buffer, pH 6.0, was performed for 10 min at 105°C. For the negative control, the incubation step with the primary antibody was omitted. Since HIF‐1α protein was expressed in either the nuclei or cytoplasm (or both) tumor cells, we evaluated its expression by a method modified as previously described. 23 , 29 The intensity of cytoplasmic staining was scored as absent, weak, moderate or strong. The extent of cytoplasmic staining was expressed as the percentage of positive cancer cells, from 0 to 100%. Tumors were scored using a four‐scale system according to the intensity and extent of staining. Score 1: tumors with absent or weak cytoplasmic reactivity and no nuclear staining; score 2: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells lower than the mean value, and with no nuclear staining; score 3: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells higher than the mean value; and score 4: tumors with a clear nuclear reactivity (with or without cytoplasmic reactivity and regardless of the intensity). The evaluation was performed twice by two investigators (T. M., K. N.) who were blind to both tumor stage and grade.
For immunohistochemistry of VEGF protein and p53 oncoprotein, we used the polymer‐peroxidase method (EnVision+/HRP; DakoCytomation) on deparaffinized sections, employing a previously described rabbit polyclonal antibody against VEGF protein (8) diluted 1:250, and a diluted mouse monoclonal antibody against p53 oncoprotein (DO‐7; DAKO). Autoclave pretreatment for p53 oncoprotein in 0.1 M citrate buffer, pH 6.0, was performed for 10 min at 105°C. For the assessment of VEGF protein, the extent of staining was scored as negative expression (indicating <30% of tumor area stained) or positive expression (>30% stained), as previously described. (8) For the assessment of p53 oncoprotein, the extent of staining was scored as negative expression (<10% of tumor area stained) or positive expression (>10% stained).
For RT‐PCR, the total RNA in tumor tissues and the corresponding normal mucosal tissues was isolated using Trizol (Invitrogen). RT‐PCR was performed using an amplification reagent kit (TaqMan EZRT‐PCR kit; Applied Biosystems) with several primers. The sense, antisense and TaqMan probe sequences for the HIF‐1α primers were: 5′‐TTACCATGCCCCAGATTCAG‐3′, 5′‐CTTGCGGAACTGCTTTCTAATG‐3′, and 5′‐AGACACCTAGTCCTTCCGATGGAAGCACT‐3′, respectively, and those for glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) were: 5′‐GAAGGTGAAGGTCGGAGTC‐3′, 5′‐GAAGATGGTGATGGGATTTC‐3′, and 5′‐CAAGCTTCCCGTTCTCAGCC‐3′, respectively. The four primers were synthesized using an automated DNA synthesizer. TaqMan probes were labeled at the 5′ end with the reporter dye molecule, 6‐carboxyfluorescein (FAM), and at the 3′ end with the quencher dye, (6‐carboxytetramethylrodamine) (TAMRA). The reaction master mix was prepared according to the manufacturer's protocol to give final concentrations of 1× reaction buffer, 300 µM deoxyadenosine triphosphate, 300 µM deoxycytidine triphosphate, 300 µM deoxyguanosine triphosphate, 600 µM deoxyuridine triphosphate (dUTP), 3 mM manganese (II) acetate, 0.1 U/µl rTth DNA polymerase, 0.01 U/µl AmpErase UNG, 900 nM primers, and 200 nM TaqMan probe. PCR was performed using 40 amplification cycles for HIF‐1α and 40 amplification for GAPDH mRNA (at 95°C for 20 s and at 62°C or 60°C for 1 min) using an ABI PRISM 7700 Sequence detector (Applied Biosystems). During each cycle of the PCR, the 5′→3′ exonuclease activity of rTth DNA polymerase cleaves the TaqMan probe, thereby increasing the fluorescence of the reporter dye at the appropriate wavelength. The increase in fluorescence was proportional to the concentration of template in the PCR. The threshold cycle was calculated by determining the point at which the fluorescence exceeded a threshold limit (10 times the standard deviation of the baseline). Therefore, signals were regarded as positive if the fluorescence intensity exceeded 10 times the standard deviation of the baseline fluorescence. The standard curve was obtained using the threshold cycle established separately for four wells for each applied RNA amount. PCR products were separated by electrophoresis in a 3% agarose gel and stained with ethidium bromide.
In the statistical analysis, disease‐free and overall survival rates were the two main dependent variables tested in this study. ‘Disease‐free survival’ was defined as the period between the initial radical operation and the subsequent appearance of recurrence or metastasis. The end‐point was either recurrence/metastasis of the tumor or the closing date of the study, whichever came first. ‘Overall survival’ was defined as the interval between surgery and death, the end‐point for this variable being either death or the closing date of the study.
Disease‐free and overall survival curves for the univariate analyses were assessed using the Kaplan–Meier method. Comparisons between survival curves were assessed using the log‐rank test. Multivariate analysis of the clinicopathologic parameters was performed using the Cox stepwise‐regression model. The above analyses were performed using the StatView 5.0 software package (SAS Institute Inc.).
Comparisons between HIF‐1α protein immunoreactivity and other tumor characteristics (see Table 1) were performed using the Chi‐squared analysis or Mann–Whitney U‐test. Regression analysis of the final score for HIF‐1α protein immunoreactivity and the ratio of HIF‐1α mRNA over GAPDH mRNA was performed using Spearman's correlation coefficient by rank. Differences were considered significant at P < 0.05.
Table 1.
Relationships between the expressions of hypoxia‐inducible factor‐1α messenger ribonucleic acid and protein and other tumor characteristics (pathologic findings, expression of vascular endothelial growth factor protein, and p53 oncoprotein) in esophageal carcinoma
| HIF protein expression (n = 215) | HIF mRNA expression (n = 38) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. cases | High‐score † | Low‐score | P‐value* | No. cases | High‐score ‡ | Low‐score | P‐value | |
| Depth of invasion | ||||||||
| T1 | 28 | 16 | 12 | 4 | 1 | 3 | ||
| T2 | 23 | 13 | 10 | 5 | 3 | 2 | ||
| T3 | 119 | 66 | 53 | 24 | 13 | 11 | ||
| T4 | 45 | 33 | 12 | 0.210 | 5 | 3 | 2 | 0.667 |
| Lymph node involvement | ||||||||
| Present | 155 | 92 | 63 | 27 | 14 | 13 | ||
| Absent | 60 | 36 | 24 | 0.931 | 11 | 5 | 6 | 0.721 |
| Blood vessel invasion | ||||||||
| v (+) | 165 | 100 | 65 | 32 | 17 | 15 | ||
| v (–) | 50 | 28 | 22 | 0.561 | 6 | 2 | 4 | 0.302 |
| Lymphatic vessel invasion | ||||||||
| Ly (+) | 195 | 118 | 77 | 35 | 19 | 16 | ||
| Ly (–) | 20 | 10 | 10 | 0.362 | 3 | 0 | 3 | 0.071 |
| Differentiation | ||||||||
| Well differentiated | 72 | 49 | 23 | 13 | 7 | 6 | ||
| Moderately differentiated | 116 | 64 | 52 | 22 | 10 | 12 | ||
| Poorly differentiated | 27 | 15 | 12 | 0.196 | 3 | 2 | 1 | 0.744 |
| VEGF protein expression | ||||||||
| VEGF(+) | 115 | 76 | 39 | 27 | 15 | 12 | ||
| VEGF(–) | 100 | 52 | 48 | 0.036 | 11 | 4 | 7 | 0.283 |
| p53 oncoprotein expression | ||||||||
| p53 (+) | 122 | 79 | 43 | 23 | 9 | 14 | ||
| p53 (–) | 93 | 49 | 44 | 0.074 | 15 | 10 | 5 | 0.097 |
| Recurrence type | ||||||||
| Distant metastasis (+) | 46 | 29 | 17 | 9 | 3 | 6 | ||
| Distant metastasis (–) | 47 | 33 | 14 | 0.463 | 7 | 4 | 3 | 0.341 |
† Hypoxia‐inducible factor (HIF)‐1α protein scores were classified as follows: The intensity of cytoplasmic staining was scored as absent, weak, moderate, and strong. The extent of cytoplasmic staining was expressed as the percentage of positive cancer cells, from 0 to 100%. Tumors were scored using a four‐scale system according to the intensity and extent of staining. Score 1: tumors with absent or weak cytoplasmic reactivity and no nuclear staining; score 2: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells lower than the mean value, and with no nuclear staining; score 3: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells higher than the mean value; score 4: tumors with a clear nuclear reactivity (with or without cytoplasmic reactivity and regardless of the intensity). Tumors with scores 1 and 2 for HIF‐1α immunostaining were classified as the low‐score group, while tumors wwith scores 3 and 4 were classified as the high‐score group. ‡ The ratio of HIF‐1α messenger ribonucleic acid (mRNA) over glyceraldehyde‐3‐phosphate dehydrogenase mRNA was calculated for each tumor sample. The samples of normal mucosa from the esophagus were classified as ‘high score’ if the score was ≥0.99, since 0.99 was the median value for the carcinomas studied. *Comparisons with respect to lymph node involvement, blood vessel invasion, lymphatic vessel invasion, vascular endothelial growth factor (VEGF) protein expression, p53 oncoprotein expression, and recurrence type were performed using the Chi‐squared analysis. Comparisons with respect to depth of invasion and differentiation were performed using the Mann–Whitney U‐test.
Results
The patients’ age at diagnosis (215 cases) was within the range 35–88 years, with a median age of 64 years. Ninety of the 215 patients died as a result of their tumors 1–83 months after surgery (mean, 17 months; median, 12 months). The remainder had survived 1–118 months after surgery at the time of the study (mean, 30 months; median, 21 months). Among the 215 cases, seven tumors (3%) were in the cervical esophagus, 23 (11%) were in the upper one‐third of the esophagus, 109 (51%) were in its middle one‐third, 66 (31%) were in its lower one‐third, and 10 (4%) were in the abdominal esophagus. The initial management of the 215 patients consisted of resection of the esophagus with lymph node dissection, but without preoperative supplemental therapy.
The tumors were divided into four stages according to the criteria proposed by UICC. There were 28 cases (13%) of T1 (tumors invading the lamina propria or submucosa), 23 cases (11%) of T2 (tumors invading the muscularis propria), 119 cases (55%) of T3 (tumors invading the adventitia), and 45 cases (21%) of T4 (tumors invading adjacent structures). In the 93 patients with recurrence and/or metastasis, the mean follow up was 17 months (median, 12 months; range, 1–96 months), whereas the mean follow up for the 122 patients without recurrence or metastasis was 30 months (median, 18 months; range, 1–118 months).
A positive expression of HIF‐1α protein was recognized in 95% of the patients studied (11 cases showed no staining by immunohistochemistry). HIF‐1α protein was expressed in the nuclei and/or the cytoplasm of tumor cells (Fig. 1a). When the tumors with scores 1 and 2 for HIF‐1α immunostaining were classified as the low‐score group, and tumors with scores 3 and 4 were classified as the high‐score group, the high‐score HIF‐1α group was contained 60% of the patients studied. A positive expression of VEGF protein was recognized in 53% of the patients; the immunoreactivity for this protein being confined to the cytoplasm of tumor cells (Fig. 1b). A positive expression of p53 oncoprotein was recognized in 57% of the patients. For this protein, the immunoreactivity was confined to the nuclei of tumor cells, no cytoplasmic staining being observed at all (Fig. 1c).
Figure 1.
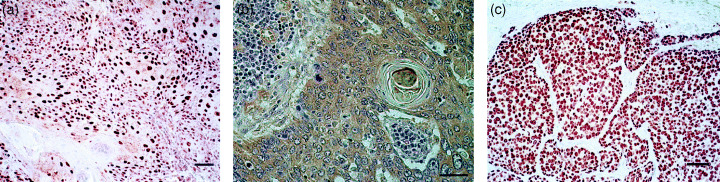
(a) Expression of hypoxia‐inducible factor‐1α (HIF‐1α) protein, (b) vascular endothelial growth factor (VEGF) protein, and (c) p53 oncoprotein in esophageal squamous cell carcinoma. Scale bar: 100 µm.
In the assessment of the relationship between HIF‐1α protein expression and clinicopathologic findings, the proportion of patients in the high‐score HIF‐1α group increased significantly with increasing VEGF protein expression (Table 1).
In the assessment of disease‐free and overall survivals, 45 cases with non‐curative resection were excluded because of the difference in their clinical course. Disease‐free and overall survival curves are shown in Figure 2. The survival rates for the high‐ and low‐score HIF‐1α protein‐immunoreactivity groups were as follows: for a 5‐year disease‐free survival, 47% and 55%, respectively, and for a 5‐year overall survival, 43% and 52%, respectively. In our univariate analysis of overall survival rates, depth of invasion, lymph node involvement, blood vessel invasion factor and lymphatic vessel invasion factor, all had a significant effect (Table 2). In our univariate analysis of disease‐free rates, the expression of HIF‐1α protein, depth of invasion, lymph node involvement, blood vessel invasion factor and lymphatic vessel invasion factor, all had a significant effect (Table 2).
Figure 2.
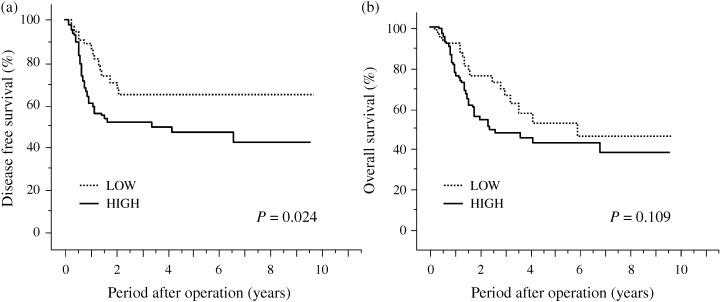
Disease‐free and overall survival curves for patients with esophageal squamous cell carcinoma (subdivided according to hypoxia‐inducible factor‐1α protein status [HIF‐1α]). (a) Disease‐free survival curve: patients with low (n = 75) or high (n = 95) expression of HIF‐1α protein, and (b) overall survival curve: patients with low (n = 75) or high (n = 95) expression of HIF‐1α protein.
Table 2.
Univariate analysis of overall and disease‐free survival rates
| Prognostic indicator | Overall survival (n = 170) | Disease‐free survival (n = 170) | ||||
|---|---|---|---|---|---|---|
| Risk ratio | 95% Confidence interval | P‐value | Risk ratio | 95% Confidence interval | P‐value | |
| Depth of invasion | ||||||
| T1–2/3–4 | 2.475 | 1.255–4.878 | 0.009 | 2.294 | 1.179–4.464 | 0.015 |
| Lymph node involvement | ||||||
| Present/absent | 3.293 | 1.621–6.691 | 0.001 | 4.003 | 1.804–8.884 | 0.001 |
| Blood vessel invasion | ||||||
| v +/– | 2.031 | 1.071–3.849 | 0.030 | 2.570 | 1.205–5.479 | 0.015 |
| Lymphatic vessel invasion | ||||||
| Ly +/– | 2.679 | 4.966–7.432 | 0.058 | 4.200 | 1.019–17.323 | 0.047 |
| HIF‐1α expression | ||||||
| High score/low score † | 1.554 | 0.903–2.624 | 0.112 | 1.963 | 1.078–3.573 | 0.027 |
Hypoxia‐inducible factor (HIF)‐1α protein scores were classified as follows: The intensity of cytoplasmic staining was scored as absent, weak, moderate, and strong. The extent of cytoplasmic staining was expressed as the percentage of positive cancer cells, from 0 to 100%. Tumors were scored using a four‐scale system according to the intensity and extent of staining. Score 1: tumors with absent or weak cytoplasmic reactivity and no nuclear staining; score 2: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells lower than the mean value, and with no nuclear staining; score 3: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells higher than the mean value; score 4: tumors with a clear nuclear reactivity (with or without cytoplasmic reactivity and regardless of the intensity). Tumors with scores 1 and 2 for HIF‐1α immunostaining were classified as the low‐score group, while tumors with scores 3 and 4 were classified as the high‐score group.
Only those variables that appeared significant in the univariate analyses of disease‐free survival (170 cases) and overall survival (170 cases) were entered into the final models of the multivariate analysis. Only lymph node involvement was found to be a prognostic factor for either overall or disease‐free survival (Table 3).
Table 3.
Multivariate analysis of overall and disease‐free survival rates
| Prognostic indicator | Overall survival (n = 170) | Disease‐free survival (n = 170) | ||||
|---|---|---|---|---|---|---|
| Risk ratio | 95% Confidence interval | P‐value | Risk ratio | 95% Confidence interval | P‐value | |
| Depth of invasion | ||||||
| T1–2/3–4 | 1.541 | 0.734–3.236 | 0.253 | 0.845 | 0.406–1.756 | 0.651 |
| Lymph node involvement | ||||||
| Present/absent | 2.838 | 1.361–5.916 | 0.005 | 3.424 | 1.480–7.922 | 0.004 |
| Blood vessel invasion | ||||||
| v +/– | 1.686 | 0.856–3.319 | 0.253 | 1.904 | 0.836–4.338 | 0.125 |
| Lymphatic vessel invasion | ||||||
| Ly +/– | 2.225 | 0.516–9.881 | 0.280 | |||
| HIF‐1α expression | ||||||
| High group/low group † | 1.574 | 0.859–2.884 | 0.142 | |||
Hypoxia‐inducible factor (HIF)‐1α protein scores were classified as follows: The intensity of cytoplasmic staining was scored as absent, weak, moderate, and strong. The extent of cytoplasmic staining was expressed as the percentage of positive cancer cells, from 0 to 100%. Tumors were scored using a four‐scale system according to the intensity and extent of staining. Score 1: tumors with absent or weak cytoplasmic reactivity and no nuclear staining; score 2: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells lower than the mean value, and with no nuclear staining; score 3: tumors with moderate or strong cytoplasmic reactivity in a percentage of positive cancer cells higher than the mean value; and score 4: tumors with a clear nuclear reactivity (with or without cytoplasmic reactivity and regardless of the intensity). Tumors with scores 1 and 2 for HIF‐1α immunostaining were classified as the low‐score group, while tumors with scores 3 and 4 were classified as the high‐score group.
In the 38 cases in which we used semiquantitative RT‐PCR, the patients’ age at diagnosis was within the range 48–84 years, with a median age of 65 years. Eighteen of the 38 patients died as a result of their tumors 1–30 months after surgery (mean, 13 months; median, 11 months). The remainder had survived 1–51 months after surgery at the time of the study (mean, 20 months; median, 14 months). Among the 38 cases, one tumor (3%) was in the cervical esophagus, four (11%) were in the upper one‐third of the esophagus, 16 (42%) were in its middle one‐third, 14 (37%) were in its lower one‐third, and three (8%) were in the abdominal esophagus. HIF‐1α mRNA was recognized in all 38 tumors and in all 14 of the corresponding normal mucosal tissues (Fig. 3). The ratio of HIF‐1α mRNA over GAPDH mRNA was found to be statistically higher in the tumors (range, 0.30–6.80; mean, 1.30; median, 0.99) than in the corresponding normal mucosal tissues (range, 0.183–0.654; mean, 0.339; median, 0.314) when the Mann–Whitney U‐test was employed (Fig. 4). Furthermore, the ratio of HIF‐1α mRNA over GAPDH mRNA correlated significantly with HIF‐1α protein scores (by immunohistochemistry) when Spearman's correlation coefficient by rank was employed (r 2 = 0.087, P = 0.0054). Moreover, for HIF‐1α, the ratio of tumor mRNA over normal mRNA ranged from 0.46 to 21.1 (mean, 3.9; median, 3.1), with a ratio over 1.0 being recognized in 78.6% of the 14 patients studied. In our assessment of the relationship between the ratio of HIF‐1α mRNA over GAPDH mRNA (HIF‐1α mRNA score) and clinicopathologic findings, the HIF‐1α mRNA score was classified as ‘high’ if it was >0.99, since 0.99 was the median value for the carcinomas studied. No relationship between HIF‐1α mRNA scores and clinicopathologic findings, VEGF protein expression or p53 oncoprotein expression was seen (Table 1). In our assessment of disease‐free and overall survivals, 38 cases who had no metastasis at surgery and in whom the malignant tumor was excised totally by surgery were included in the analysis. The rates for a 3‐year disease‐free survival and a 3‐year overall survival were 50% and 42%, respectively. In a univariate analysis of disease‐free and overall survival rates, no correlations were found between HIF‐1α mRNA score and prognosis (data not shown).
Figure 3.
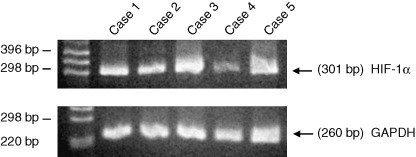
Reverse transcription‐polymerase chain reaction (RT‐PCR) results for hypoxia‐inducible factor‐1α and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) messenger ribonucleic acid in tumors on five patients with esophageal squamous cell carcinoma.
Figure 4.
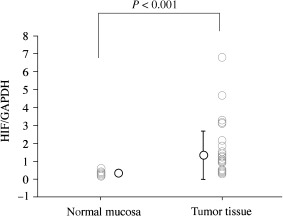
Hypoxia‐inducible factor‐1α/glyceraldehyde‐3‐phosphate dehydrogenase messenger ribonucleic acid expression in tumors (n = 38) and in the corresponding normal mucosal tissues (n = 14).
Discussion
The purpose of our investigation was to look for a possible relation between the expression of HIF‐1α mRNA or protein, and clinicopathologic findings or clinical outcome in esophageal squamous cell carcinoma. Our analysis of clinicopathologic findings revealed positive relationships between the expression of HIF‐1α protein and VEGF protein expression. Furthermore, a significant correlation was found between HIF‐1α protein expression and disease‐free survival rate in the univariate analysis, but not in the multivariate analysis. In the RT‐PCR study, the ratio of HIF‐1α mRNA over GAPDH mRNA was statistically higher in tumors than in the corresponding normal mucosal tissues. However, this ratio showed no relation to clinicopathological factors, or to either disease‐free or overall survival. Therefore, the detection of HIF‐1α protein and mRNA would appear to be of limited value in informing the prognosis in esophageal squamous cell carcinoma.
A high expression of HIF‐1α protein has been found to be associated with both tumor aggressiveness and an unfavorable prognosis in a variety of tumors, including carcinomas of the uterine cervix, colon, ovary, lung, oropharyngus and breast. 1 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 With regard to the expression of HIF‐1α protein in esophageal carcinoma, only three reports have been published to our knowledge, 29 , 30 , 31 and the results are controversial. Koukourakis et al. examined the expression of HIF‐1α and HIF‐2α proteins using immunohistochemistry in 37 early stage esophageal cancers treated with photodynamic therapy, and reported that expressions of HIF‐1α and HIF‐2α proteins could be recognized in 51% and 14%, respectively, of the patients studied. However, they did not examine the relation between the expression of HIF‐1α protein and clinicopathologic findings or survivals. (29) Kurokawa et al. examined the expression of HIF‐1α protein using immunohistochemistry in 130 resected specimens of esophageal squamous cell carcinoma, and demonstrated that a high expression of HIF‐1α protein correlated with pTNM stage, depth of invasion, lymph node metastasis, distant metastasis, lymphatic invasion factor and positive surgical margin. Furthermore, they also showed that the overall survival was worse in the high‐expression group than in low‐expression group, but the expression of HIF‐1α protein was not found to be an independent prognostic factor in their multivariate analysis. (30) Kimura et al. examined the expression of HIF‐1α protein using immunohistochemistry in 82 resected specimens of esophageal squamous cell carcinoma, and reported a significant relationship between the expression of HIF‐1α protein and blood vessel invasion factor. They alsoshowed that overexpression of HIF‐1α was associated with a high VEGF expression and with angiogenesis. Moreover, they mentioned that the outcome was significantly poorer in those with high HIF‐1α‐expressing tumors than those with low HIF‐1α‐expressing tumors. They therefore concluded that HIF‐1α might be a potential target as an angiogenic factor for the treatment of esophageal squamous cell carcinoma. (31) In the present study, the expression of HIF‐1α protein was found to be associated only with VEGF protein expression, not with depth of tumor invasion, lymph node involvement or blood vessel invasion factor. Furthermore, although a significant correlation was found between HIF‐1α protein expression and clinical outcome in our univariate analysis of disease‐free survival rate, no such correlation was found in the multivariate analysis. On this basis, the detection of HIF‐1α protein would appear to be of limited value in predicting tumor progression in esophageal squamous cell carcinoma. At present, the reason for the discrepancies among the above four studies (see ref. 29, 30, 31 and the present study) is not clear. However, one possible factor is that the different investigations employed a variety of examination methods, and indeed the positive rate of HIF‐1α protein expression was quite variable among the investigations. Since it can be assumed that nuclear HIF‐1α protein is the active form, our analysis focused on the nuclear expression, as in some previous reports. 23 , 29
It is well known that HIF‐1α is an important mediator of the response to hypoxia shown by tumor cells, and that it up‐regulates VEGF expression. 1 , 2 , 14 , 17 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 29 The VEGF gene contains a number of HIF‐1‐binding sites in its regulatory region, and HIF‐1α has been shown to activate the VEGF promoter in vitro. 32 , 33 The expression of HIF‐1α correlates directly with both VEGF expression and tumor vascularity in a variety of tumors. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 29 Furthermore, HIF‐1α activation may lead to a stimulation of tumor growth and to metastasis. In the present study, a significant correlation was found between the expressions of HIF‐1α protein and VEGF protein. However, we could not find evidence of a relationship between the expression of HIF‐1α protein and the aggressiveness of the tumor (such as depth of invasion, lymph node metastasis, and distant metastasis). Therefore, our results are consistent with the earlier findings.
It is well known that wild‐type p53 induces apoptosis. (34) Furthermore, hypoxia may induce p53‐dependent apoptosis and inhibit tumor growth. 4 , 35 In 1998, Kenekal et al. reported that HIF‐1α increases the stability of p53 protein (18) and a loss of wild‐type p53 is associated with a marked reduction in hypoxia‐mediated apoptosis. Both HIF‐1α overexpression and p53 protein dysfunction seem to be necessary for HIF‐1α sufficiently to stimulate tumor progression in early cancerogenesis (through angiogenesis and the induction of adaptive intracellular responses to hypoxia without a supporting proapoptotic mechanism). Zhong et al. (27) demonstrated that HIF‐1α overexpression is associated with aberrant p53 accumulation in 75 human cancers, including the majority of colon and breast cancers. They therefore suggested that in addition to hypoxia, an inactivation of tumor suppressor genes is associated with increased HIF‐1α expression. However, we failed to demonstrate a correlation between the expression of HIF‐1α protein and that of p53 oncoprotein in esophageal carcinoma.
Recently, HIF‐1α mRNA and protein levels were found to be upregulated in primary tumors (as compared with normal specimens) in urinary bladder and kidney. 36 , 37 Furthermore, Zhong et al. (38) demonstrated that HIF‐1α mRNA was overexpressed in six rat prostate cancer cell lines (as compared with the normal prostate), and that metastatic potential correlated with HIF‐1α mRNA levels in those cell lines. In the present study, the expression score for HIF‐1α protein was observed to be significantly related to the ratio of HIF‐1α mRNA over GAPDH mRNA when Spearman's correlation coefficient by rank was employed. Furthermore, the ratio of HIF‐1α mRNA over GAPDH mRNA was significantly higher in tumors than in the corresponding normal mucosal tissues. However, no relationships were found between HIF‐1α mRNA score and blood vessel invasion factor in the tumor, the depth of tumor invasion, the expressions of VEGF protein and p53 oncoprotein or prognosis, while the expression of HIF‐1α protein was associated with VEGF protein expression and disease‐free survival rate in the univariate analysis. We cannot be sure whether this accounts for the discrepancy between the apparent implications of our HIF‐1α protein data and our HIF‐1α mRNA data, but we should point out that the materials examined for the expression of HIF‐1α mRNA were limited to only 38 cases (against 215 cases for HIF‐1α protein expression).
In conclusion, the finding of a high expression of HIF‐1α protein and mRNA in resected specimens may provide limited information as to progression and prognosis in esophageal carcinoma. In the last few years, several investigators have demonstrated HIF‐2α expression in tumor‐associated macrophages and noted that the critical determinant of HIF activity is the level of HIF‐1α and HIF‐2α proteins. 13 , 39 , 40 Furthermore, the expression of HIF‐2α protein is associated with a poor prognosis in non‐small cell lung carcinoma and breast cancer. 23 , 41 Therefore, future studies should examine HIF‐2α expression and seek to elucidate the relationship between its expression and clinicopathologic findings or clinical outcome in esophageal carcinoma.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Cancer Research 14–20 from the Ministry of Health, Labor, and Welfare, Japan, and by a Grant‐in‐Aid from the 14th Research Foundation of the Surgical Association of Saitama Prefecture.
References
- 1. Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia‐inducible factor‐1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci USA 1997; 94: 8104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryan HE, Lo J, Johnson RS. HIF‐1α is required for solid tumor formation and embryonic vascularization. EMBO J 1998; 17: 3005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56: 4509–15. [PubMed] [Google Scholar]
- 4. Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia‐mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996; 379: 88–91. [DOI] [PubMed] [Google Scholar]
- 5. Brize DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res 1996; 56: 941–3. [PubMed] [Google Scholar]
- 6. Stadler P, Becker A, Feldmann HJ, Hansgen G, Dunst J, Wurschmidt F, Molls M. Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiat Oncol Biol Phys 1999; 44: 749–54. [DOI] [PubMed] [Google Scholar]
- 7. Ferrara N, Davis‐Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997; 18: 4–25. [DOI] [PubMed] [Google Scholar]
- 8. Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer 1997; 79: 206–13. [DOI] [PubMed] [Google Scholar]
- 9. Shimada H, Takeda A, Nabeya Y, Okazumi SI, Matsubara H, Funami Y, Hayashi H, Gunji Y, Kobayashi S, Suzuki T, Ochiai T. Clinical significance of serum vascular endothelial growth factor in esophageal squamous cell carcinoma. Cancer 2001; 92: 663–9. [DOI] [PubMed] [Google Scholar]
- 10. Uchida S, Shimada Y, Watanabe G, Tanaka H, Shibagaki I, Miyahara T, Ishigami S, Imamura M. In oesophageal squamous cell carcinoma, vascular endothelial growth factor is associated with p53 mutation, advanced stage and poor prognosis. Br J Cancer 1998; 77: 1704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang GL, Semenza GL. General involvement of hypoxia‐inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 1993; 90: 4304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang GL, Semenza GL. Purification and characterization of hypoxia‐inducible factor 1. J Biol Chem 1995; 270: 1230–7. [DOI] [PubMed] [Google Scholar]
- 13. Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia‐inducible transcription factor depends primarily upon redox‐sensitive stabilization of its alpha subunit. J Biol Chem 1996; 271: 32253–9. [DOI] [PubMed] [Google Scholar]
- 14. Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53‐induced degradation of hypoxia‐inducible factor 1 alpha. Genes Dev 2000; 14: 34–44. [PMC free article] [PubMed] [Google Scholar]
- 15. Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia‐inducible factors for oxygen‐dependent proteolysis. Nature 1999; 399: 271–5. [DOI] [PubMed] [Google Scholar]
- 16. Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. HIF‐1 is expressed in normoxic tissue and displays an organ‐specific regulation under systemic hypoxia. FASEB J 2001; 15: 2445–53. [DOI] [PubMed] [Google Scholar]
- 17. Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF‐1α in hypoxia‐mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394: 485–90. [DOI] [PubMed] [Google Scholar]
- 18. An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild‐type p53 by hypoxia‐inducible factor 1α. Nature 1998; 392: 405–8. [DOI] [PubMed] [Google Scholar]
- 19. Birner P, Schindl M, Obermai A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia‐inducible factor 1α is a marker for an unfavorable prognosis in early‐stage invasive cervical cancer. Cancer Res 2000; 60: 4693–6. [PubMed] [Google Scholar]
- 20. Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, Ito M, Chayama K. Expression of hypoxia‐inducible factor‐1α is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer 2003; 105: 176–81. [DOI] [PubMed] [Google Scholar]
- 21. Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G. Expression of hypoxia‐inducible factor 1α in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res 2001; 7: 1661–8. [PubMed] [Google Scholar]
- 22. Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL. Expression of hypoxia‐inducible factor‐1α: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Clin Cancer Res 2001; 61: 2911–6. [PubMed] [Google Scholar]
- 23. Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non‐small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001; 85: 881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krishnamachary B, Berg‐Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia‐inducible factor 1. Cancer Res 2003; 63: 1138–43. [PubMed] [Google Scholar]
- 25. Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, Gatter KC, Johnson PJ, Harris AL. Coexpression of hypoxia‐inducible factors 1α and 2α, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res 2002; 8: 2595–604. [PubMed] [Google Scholar]
- 26. Bos R, Van Der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, Van Diest PJ, Van Der Wall E. Levels of hypoxia‐inducible factor‐1α independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 2003; 97: 1573–81. [DOI] [PubMed] [Google Scholar]
- 27. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia‐inducible factor 1α in common human cancers and their metastases. Cancer Res 1999; 59: 5830–5. [PubMed] [Google Scholar]
- 28. Sobin LH, Wittekind C, eds. UICC TNM Classification of Malignant Tumours, 5th edn. New York: Wiley‐Liss Inc., 1997;. 54–8. [Google Scholar]
- 29. Koukourakis MI, Giatromanolaki A, Skarlatos J, Corti L, Blandamura S, Piazza M, Gatter KC, Harris AL. Hypoxia inducible factor (HIF‐1α and HIF‐2α) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res 2001; 61: 1830–2. [PubMed] [Google Scholar]
- 30. Kurokawa T, Miyamoto M, Kato K, Cho Y, Kawarada Y, Hida Y, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of hypoxia‐inducible‐factor 1alpha (HIF‐1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer 2003; 89: 1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kimura S, Kitadai Y, Tanaka S, Kuwai T, Hihara J, Yoshida K, Toge T, Chayama K. Expression of hypoxia‐inducible factor (HIF)‐1 alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer 2004; 40: 1904–12. [DOI] [PubMed] [Google Scholar]
- 32. Shima DT, Kuroki M, Deutsch U, Ng YS, Adamis AP, D’Amore PA. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post‐transcriptional regulatory sequences. J Biol Chem 1996; 271: 3877–83. [DOI] [PubMed] [Google Scholar]
- 33. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia‐inducible factor 1. Mol Cell Biol 1996; 16: 4604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ginsberg D, Mechta F, Yaniv M, Oren M. Wild‐type p53 can down‐modulate the activity of various promoters. Proc Natl Acad Sci USA 1991; 88: 9979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ Jr, Giaccia AJ. Hypoxia induces accumulation of p53 protein, but activation of a G1‐phase checkpoint by low‐oxygen conditions is independent of p53 status. Mol Cell Biol 1994; 14: 6264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones A, Fujiyama C, Blanche C, Moore JW, Fuggle S, Cranston D, Bicknell R, Harris AL. Relation of vascular endothelial growth factor production to expression and regulation of hypoxia‐inducible factor‐1 alpha and hypoxia‐inducible factor‐2 alpha in human bladder tumors and cell lines. Clin Cancer Res 2001; 7: 1263–72. [PubMed] [Google Scholar]
- 37. Turner KJ, Moore JW, Jones A, Taylor CF, Cuthbert‐Heavens D, Han C, Leek RD, Gatter KC, Maxwell PH, Ratcliffe PJ, Cranston D, Harris AL. Expression of hypoxia‐inducible factors in human renal cancer. relationship to angiogenesis and to the von Hippel‐Lindau gene mutation. Cancer Res 2002; 62: 2957–61. [PubMed] [Google Scholar]
- 38. Zhong H, Agani F, Baccala AA, Laughner E, Rioseco‐Camacho N, Isaacs WB, Simons JW, Semenza GL. Increased expression of hypoxia inducible factor‐1alpha in rat and human prostate cancer. Cancer Res 1998; 58: 5280–4. [PubMed] [Google Scholar]
- 39. Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia‐inducible factors HIF‐1alpha and HIF‐2alpha in normal human tissues, cancers, and tumor‐associated macrophages. Am J Pathol 2000; 157: 411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia‐inducible factor 1alpha is mediated by an O2‐dependent degradation domain via the ubiquitin‐proteasome pathway. Proc Natl Acad Sci USA 1998; 95: 7987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL. Relation of hypoxia‐inducible factor‐2α (HIF‐2α) expression in tumor‐infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Res 2002; 62: 1326–9. [PubMed] [Google Scholar]


