Abstract
We have developed a novel enzyme‐linked immunosorbent assay (ELISA) system for the detection of N‐ERC/mesothelin in the serum of mesothelioma patients and have begun to examine its clinical usefulness. N‐ERC/mesothelin is a 31‐kDa protein that forms the N‐terminal fragment of the full‐length 71‐kDa ERC/mesothelin protein, and is physiologically secreted into the blood of mesothelioma patients where it can be detected using our sandwich ELISA containing two antibodies (rabbit polyclonal anti‐ERC/mesothelin antibody‐282 and mouse monoclonal antibody 7E7). Our ELISA system has thus far detected much higher serum levels of N‐ERC/mesothelin in mesothelioma patients than in healthy controls or patients with other lung or pleural diseases. In conclusion, N‐ERC/mesothelin is a promising candidate tumor marker for mesothelioma. (Cancer Sci 2006; 97: 928–932)
Mesothelioma is an aggressive tumor arising from serosal surfaces, such as the pleura and peritoneum. Its prognosis is dismal. Several recent studies have described safe utilization of aggressive multimodality therapy and have found evidence of the effectiveness of combination chemotherapy regimens using pemetrexed and cisplatin.( 1 , 2 , 3 ) However, the prognosis after this type of therapy remains poor.
A study by Sugarbaker et al. reporting a 5‐year survival of 40% for selected patients following trimodality therapy, which demonstrates that early diagnosis is vital for improving prognosis.( 1 ) However, in light of the difficulty of making an early diagnosis of mesothelioma using current diagnostic imaging techniques, the identification of tumor makers for mesothelioma is needed urgently. While no reliable serum marker for mesothelioma has yet been found, Robinson et al. have recently proposed soluble mesothelin‐related protein (SMRP) as a candidate.( 4 , 5 )
Previously, we discovered the renal carcinoma gene ERC, which we demonstrated was expressed highly in renal cancers in the Eker rat.( 6 ) Furthermore, we subsequently confirmed that ERC is a homolog of the human mesothelin gene, a gene expressed strongly in normal mesothelial cells, mesotheliomas, non‐mucinous ovarian carcinomas and pancreatic ductal adenocarcinomas.( 7 , 8 ) The human mesothelin gene codes for several proteins. Its primary product is a 71‐kDa precursor protein, which is cleaved physiologically by a furin‐like protease into a 40‐kDa C‐terminal fragment that remains membrane‐bound and a 31‐kDa N‐terminal fragment that is secreted into the blood.( 9 ) The resultant C‐terminal 40‐kDa fragment is classified as a mesothelin, and its presence in the serum has been reported as a useful tumor marker in mesothelioma patients.(10) In contrast, the N‐terminal 31‐kDa fragment, which is a secreted protein and has been cloned as a megakaryocyte‐potentiating factor, has not been reported as a useful tumor marker for mesothelioma or any other cancer. Consequently, we have focused on N‐terminal 31‐kDa fragment.
In the present study, we report on our development of an original sandwich enzyme‐linked immunosorbent assay (ELISA) system, utilizing specific antibodies that we prepared in our laboratory against the 31‐kDa N‐terminal fragment of ERC. We also present the results of our initial investigation into the usefulness of our assay. For the remainder of this paper, we will use the term ‘N‐ERC/mesothelin’ for the N‐terminal 31‐kDa fragment detected by our sandwich ELISA system, and the term ‘C‐ERC/mesothelin’ for the C‐terminal protein fragment detected using our sandwich ELISA system with specific antibodies against the C‐terminal fragment of the product of the ERC/mesothelin gene.
Materials and Methods
Preparation of anti ERC/mesothelin antibodies
Mouse monoclonal anti‐ERC/mesothelin antibodies. N‐ERC/mesothelin, expressed in Escherichia coli as glutation S‐transferase‐tagged and histidine‐tagged fusion proteins, was purified by chromatography on Glutathione Sepharose 4B beads (GE‐Healthcare Bio‐Sciences, Piscataway, NJ, USA) and Ni‐NTA agarose (Qiagen GmbH, Hilden, Germany). The purified GST‐tagged and histidine‐tagged N‐ERC/mesothelins were injected into mice. Splenocytes from the immunized mice were fused with myeloma cell line X63‐Ag8.653. Using ELISA, the supernatants of the hybridoma cells were screened by their reactivity to the immunogen, and several positive clones were selected by the limiting dilution method. One clone, 7E7, was chosen for use in the present study.
Rabbit polyclonal anti‐ERC/mesothelin antibodies. Rabbits were immunized with the synthetic peptides RQPERTILRPRFRR and CPSGKKAREIDESLIFYKKWELEA, both of which were coupled with thyroglobulin. These peptide sequences corresponded to the regions R282 to R295 and C302 to A325 in the human ERC/mesothelin protein. The immunoglobulin (Ig) G fractions against these two peptide sequences were obtained from the sera of immunized rabbits using a column of antigen‐coupled Activated Thiol Sepharose 4B beads (Amersham Biosciences). The resulting purified IgG were designated PoAb‐282 and PoAb‐5‐1, respectively.
Cell culture, protein expression and western blot analysis
COS‐1, HeLa, MKN‐45 and NRC‐12 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Full‐length cDNA of the ERC/mesothelin coding region was inserted into the pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA, USA) to enable expression in COS‐1 cells. Transfection was carried out using FuGENE6 transfection reagent (Roche Diagnostics, Mannheim, Germany). After transient expression of ERC/mesothelin cDNA in the COS‐1 cells, culture supernatants and cells were harvested. COS‐1 cells containing the expressed ERC/mesothelin protein were lyzed in a solution containing 2% sodium dodecylsulfate, 10% glycerol, 50 mM Tris‐HCl (pH 6.8) and 100 mM dithiothreitol, and were then boiled. These crude lysates and culture supernatants were electrophoresed on 12.5% Laemmli gels and transferred to polyvinylidene fluoride (PVDF) membranes. Two identical membranes were blocked in 1% skim milk in phosphate‐buffered saline (PBS) with 0.1% Tween 20 (PBS‐T) for 1 h at room temperature. Next, one of the two membranes was incubated with 1 g/mL of 7E7 MoAb, and the other was incubated with PoAb‐282 and PoA‐5‐1 in 1% skim milk with PBS‐T for an additional hour at room temperature. Secondary antibodies in the form of goat antirabbit IgG or rabbit antimouse IgG conjugated to peroxidase (Nos 17508 and 17502, respectively; IBL, Gunma, Japan) were then added and allowed to react with the membranes at room temperature for 1 h longer. ERC/mesothelin on the membranes was visualized using the ECL detection system (Amersham Biosciences).
Human subjects
Our study for the tumor marker of mesothelioma was approved by the Institutional Review Board of Juntendo University School of Medicine, its hospital, Immuno‐Biological Laboratories, and the National Organization Tokyo Hospital. Patients and healthy volunteers (all Japanese) gave their signed informed consent.
Serum samples. Serum samples from seven patients with mesothelioma, four patients with carcinomatous pleuritis (lung cancer) and three patients with benign asbestos pleuritis were evaluated in the present study. Patients with mesothelioma were diagnosed immunohistochemically (all epithelial type) and there was no early International Mesothelioma Interest Group (IMIG) stage. Furthermore, the sera from 13 healthy volunteers were obtained. They consisted of seven men and six women with age range 28–50 years, four of whom were smokers and nine of whom were non‐smokers. Tissue sections were obtained from archival paraffin‐embedded tumor blocks from biopsies or surgical resection to evaluate mesothelin expression.
Immunohistochemistry
Tissue sections 4 m thick were prepared from formalin‐fixed, paraffin‐embedded specimens. After deparaffinization, the tissue sections were heated in 10 mM citrate buffer (pH 6.0) for antigen retrieval and then treated with 3% hydrogen peroxide. Next, the sections were incubated with primary antibody solution (1 g/mL PoAb‐5‐1) in PBS‐T overnight at room temperature. Goat antirabbit Ig conjugated to peroxidase was applied to the tissue sections as the secondary antibody. Diaminobenzidine was used as the substrate for peroxidase.
Sandwich ELISA
Microtiter plates (96 wells) were coated with 100 L/well of 100 mM carbonate buffer (pH 9.5) containing purified 7E7 MoAb and allowed to adhere overnight at 4°C. The plates were washed with PBS‐T and blocked with 200 L/well of 1% (w/v) bovine serum albumin (BSA) in PBS containing 0.05% NaN3 for 1 h at room temperature. Following three washes with PBS‐T, 100‐L aliquots of test samples or recombinant N‐ERC/mesothelin as a standard, serially diluted in 1% BSA in PBS‐T, were added in duplicate to the wells and incubated at 37°C for 1 h. After seven washes with PBS‐T, 100 L of horse radish peroxidase (HRP)‐conjugated PoAb‐282 rabbit IgG was added to each well and incubated for 30 min at 4°C. The wells were washed nine times with PBS‐T, and then 100 L of freshly prepared tetramethyl benzidine solution was added to each well as a substrate and incubated in the dark for 30 min at room temperature. The reaction was terminated by the addition of 100 L of 1 M H2SO4. Absorbance of the solution at 450 nm was measured in an ELISA reader (E‐Max; Molecular Devices, Sunnyvale, CA, USA). Recombinant N‐ERC/mesothelin used as a standard protein in the ELISA system was purified from culture supernatants of CHO‐K1 cells transfected with ERC/mesothelin cDNA, using a formyl‐cellulofine affinity column (Seikagaku, Tokyo, Japan) coupled with anti‐ERC/mesothelin PoAb‐282. The concentration of affinity‐purified N‐ERC/mesothelin was determined using the Bradford assay (Bio‐Rad Laboratories, Hercules, CA, USA, Code. 500–0006).
Results
Characterization of the anti‐ERC/mesothelin antibodies
7E7 MoAb and PoAb‐282 both detected N‐ERC/mesothelin in the culture supernatants of COS‐1 cells transfected with full‐length cDNA from ERC/mesothelin, HeLa and MKN‐45 cells (Fig. 1A). PoAb‐5‐1 also detected C‐ERC/mesothelin in the lysates of these cells (data not shown).
Figure 1.
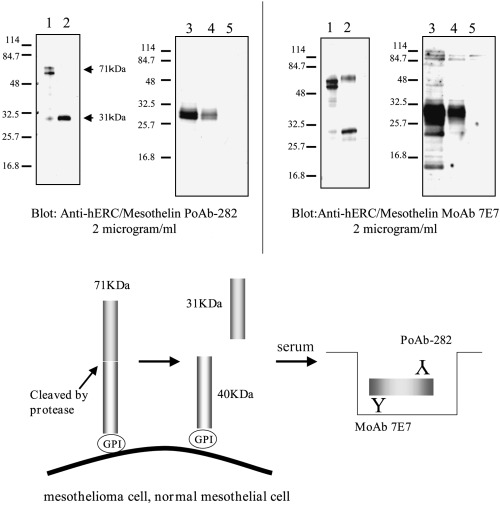
(A) Characterization of anti‐ERC/mesothelin antibodies by western blot analysis. Lanes 1 and 2, lysate and culture supernatant, respectively, of COS‐1 cells transfected with ERC/mesothelin cDNA; lanes 3, 4 and 5, culture supernatants of HeLa, MKN‐28 and NRC‐12 cells, respectively. (B) Products of the ERC/mesothelin gene and enzyme‐linked immunosorbent assay (ELISA) system. The primary product of the ERC/mesothelin gene is a 71‐kDa precursor protein. This protein is cleaved physiologically, releasing its 31‐kDa N‐terminal fragment into the blood. Our system detects the 31‐kDa N‐terminal fragment using a sandwich ELISA containing two antibodies against the N‐terminal fragment.
Establishment of ELISA for the 31‐kDa fragment of ERC/mesothelin
For detection of the 31‐kDa fragment of ERC/mesothelin, we developed ELISA combinations using 7E7 MoAb and PoAb‐282 (Fig. 1B). This ELISA combination was evaluated by measuring the expression of the 31‐kDa fragment of ERC/mesothelin in the culture supernatant of COS‐1 cells transfected with ERC/mesothelin cDNA. The standard dose–response curve of the ELISA system exhibited a linear shape when plotted on a log/log scale over a range from 0.081 to 5.2 ng/mL or 2.62–168 pM calculated with a 31‐kDa molecular weight (data not shown). Repeated freezing and thawing of samples did not affect the results (data not shown). The intra‐assay and interassay variations were in the acceptable range (data not shown).
ERC/mesothelin in blood samples and tissues
The median serum level of 13 healthy controls, four carcinomatous pleuritis (lung cancer) patients and three benign asbestos pleuritis paitents was 1.4 ng/mL (range 0.67 ± 4.14 ng/mL), 1.31 ng/mL (range 0.68 ~ 4.33 ng/mL) and 1.10 ng/mL (range 0.30 ~ 1.19 ng/mL), respectively. Substantially higher concentrations of serum N‐ERC/mesothelin were found in the seven patients with mesothelioma (median 25.12 ng/mL; range 10.32 ± 53.13 ng/mL; Fig. 2A). Furthermore, supporting the results of ELISA, immunohistochemical studies using PoAb‐5‐1 showed strong mesothelin positivity in these patients (Fig. 2B).
Figure 2.
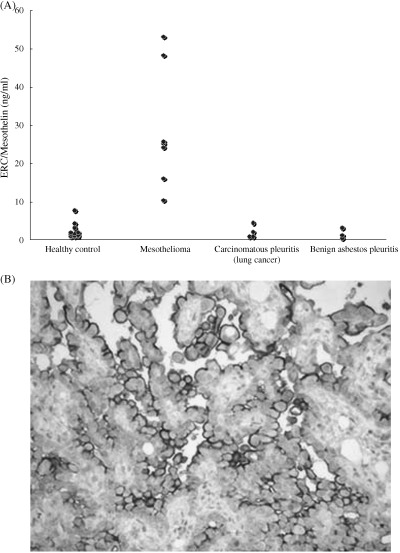
(A) Scatter plot of serum N‐ERC/mesothelin. The serum concentrations of N‐ERC/mesothelin were much higher in mesothelioma patients than in the healthy controls, carcinomatous pleuritis (lung cancer) patients and benign asbestos pleuritis patients. (B) Immunohistochemical staining for mesothelin using rabbit polyclonal antibody (PoAb)‐5‐1 in one sample of pleural mesotheliomas. Mesothelin was expressed highly in tumor cells and membranous reactivity was evident.
Discussion
Several potential tumor markers for mesothelioma have previously been described, including CA125, CA15‐3 and hyaluronic acid. Recently, osteopontin and SMRP have also be reported to have potential utility for early diagnosis and monitoring of mesothelioma.( 4 , 11 ) Of them, SMRP is perhaps the most promising.
Published studies indicate that the human mesothelin gene produces several proteins. The primary product of the human mesothelin gene is a 71‐kDa precursor protein, which is cleaved physiologically by proteases to yield a 31‐kDa N‐terminal fragment that is secreted into the blood, and a 40‐kDa C‐terminal fragment (mesothelin) that is a cell‐surface glycoprotein. SMRP is another protein product of the human mesothelin gene. SMRP can be detected by sandwich ELISA containing two MoAbs (OV569 and 4H3), which also bind to different epitopes of the membrane‐bound portion of mesothelin (C‐terminal fragment). In short, SMRP is a soluble form of mesothelin and is probably one of its splicing variants. However, we still have inadequate knowledge about the behavior of SMRP. Furthermore, very recently, another ELISA system for ‘serum mesothelin’ has been reported.( 11 )
In contrast, N‐ERC/mesothelin protein is detectable by a sandwich ELISA containing two antibodies (PoAb‐282 and MoAb 7E7), which bind to different epitopes of the 31‐kDa N‐terminal fragment that is released physiologically into the blood from mesothelioma cells. Consequently, we can detect our target proteins with high reliability and identify the inflection point more easily.
In the present study, we found substantial differences in the values of serum N‐ERC/mesothelin between patients with mesothelioma and healthy controls. Moreover, we confirmed similar results in a comparison of mesotheliomas with other lung and pleural diseases. Currently, we are engaged in a prospective study to prove the clinical utility of our sandwich ELISA system. We are seeking to determine the relationship between serum N‐ERC/mesothelin values and clinicopathological features, cut‐offs, sensitivity and specificity, and the possibility of early diagnosis.
Conclusion
We have developed a novel measurement system (sandwich ELISA) for serum N‐ERC/mesothelin. Using this system, we found that mesothelioma patients exhibited higher serum ERC/mesothelin concentrations than healthy controls. Currently, we are examining the utility of this ELISA system on a large scale and in several clinical settings.
Acknowledgments
We thank Masaaki Abe, Naoko Aoki, Mizuka Segawa and Toshiyuki Kobayashi for helping in the management of this study and critical reading of this manuscript. This work is supported by a Grant‐in‐Aid for Cancer Research and Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports and Science and Technology of Japan and the Ministry of Health, Labor and Welfare of Japan.
References
- 1. Sugarbaker DJ, Flores RM, Jaklitsch MT et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long‐term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999; 117: 54–63. [DOI] [PubMed] [Google Scholar]
- 2. Sugarbaker DJ, Jaklitsch MT, Bueno R et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004; 128: 138–46. [DOI] [PubMed] [Google Scholar]
- 3. Vogelzang NJ, Rusthoven JJ, Symanowski J et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–44. [DOI] [PubMed] [Google Scholar]
- 4. Robinson BW, Creaney J, Lake R et al. Soluble mesothelin‐related protein − a blood test for mesothelioma. Lung Cancer 2005; 49: 109–11. [DOI] [PubMed] [Google Scholar]
- 5. Robinson BW, Creaney J, Lake R et al. Mesothelin‐family proteins and diagnosis of mesothelioma. Lancet 2003; 362: 1612–16. [DOI] [PubMed] [Google Scholar]
- 6. Hino O, Kobayashi E, Nishizawa M et al. Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol 1995; 121: 602–5. [DOI] [PubMed] [Google Scholar]
- 7. Yamashita Y, Yokoyama M, Kobayashi E, Takai S, Hino O. Mapping and determination of the cDNA sequence of the Erc gene preferentially expressed in renal cell carcinoma in the Tsc2 gene mutant (Eker) rat model. Biochem Biophys Res Commun 2000; 275: 134–40. [DOI] [PubMed] [Google Scholar]
- 8. Hino O. Multistep renal carcinogenesis in the Eker (Tsc 2 gene mutant) rat model. Curr Mol Med 2004; 4: 807–11. [DOI] [PubMed] [Google Scholar]
- 9. Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004; 10: 3937–42. [DOI] [PubMed] [Google Scholar]
- 10. Hassan R, Remaley AT, Sampson ML et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006; 12: 447–53. [DOI] [PubMed] [Google Scholar]
- 11. Pass HI, Lott D, Lonardo F et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med 2005; 353: 1564–73. [DOI] [PubMed] [Google Scholar]


