Abstract
This retrospective study was launched to evaluate the efficacy of doxycycline and to find independent predictors of a clinical response in patients with ocular adnexal lymphoma of mucosa‐associated lymphoid tissue (OAML). Thirty‐eight patients with newly diagnosed, localized OAML received doxycycline for 3 weeks (12 patients) or 6 weeks (26 patients). Clinical factors including absolute lymphocyte count (ALC) and neutrophil count (ANC) were compared between responders and non‐responders. After a median follow‐up of 26.4 months, doxycycline resulted in an overall response rate of 47% and a 3‐year time‐to‐treatment failure (TTF) rate of 84%. Patients treated with doxycycline for 6 weeks versus 3 weeks tended to have a higher response rate (54%vs 33%). Absolute lymphocytosis (ALC > 3.01 × 109/L) and absolute neutrophilia (ANC > 1.92 × 109/L) were defined based on the median value of each count. Patients with (19 patients) versus without absolute lymphocytosis had significantly shorter 2‐year TTF (70%vs 100%, P = 0.021) and a lower response rate (32%vs 63%, P = 0.051). Absolute lymphocytosis (odds ratio [OR] = 4.7; 95% confidence interval [CI], 1.1–20.8; P = 0.043) and non‐conjunctival tumor (OR = 11.8; 95% CI, 1.1–122.5; P = 0.038) were negative predictors for response by multivariate analysis. Front‐line doxycycline is effective particularly in localized OAML patients without absolute lymphocytosis but with conjunctival involvement.
(Cancer Sci 2010; 101: 1199–1203)
The incidence of non‐Hodgkin’s lymphoma of the ocular adnexa has steadily increased, especially in Asia–Pacific Islanders, according to the Surveillance, Epidemiology, and End Results Program.( 1 ) Extranodal marginal zone lymphoma of mucosa‐associated lymphoid tissue (MALT lymphoma) constituted three‐fourths of lymphoproliferative diseases of the ocular adnexa in Korea,( 2 ) a higher proportion than that in Western countries. Although there is evidence that chronic antigenic stimulation by exogenous triggers or an autoimmune process leads to ocular adnexal MALT lymphoma (OAML),( 3 ) there is a variable association between Chlamydia psittaci (Cp) and this lymphoma in different geographical regions (Cp‐positivity, overall 25%; range, 0–88%).( 4 , 5 , 6 )
The first evidence for an association between Cp and OAML( 7 ) fostered a doxycycline trial demonstrating an overall response rate of 48% and a 2‐year failure‐free survival rate of 66% in localized OAML.( 8 , 9 ) An additional meta‐analysis, including 42 OAML patients, showed 20 (48%) experienced some improvement.( 6 ) Moreover, Cp‐eradicating antibiotic therapy is an alternative treatment in elderly patients with disseminated OAML, according to a small series.( 10 )
Despite a strong association between Cp and ocular adnexal lymphoma in Korea,( 11 ) no doxycycline study for OAML has been conducted. Poor prognostic factors have been suggested in a heterogeneous population with ocular adnexal lymphoma, and these factors include age, stage, histological grade, involved site, or tumor suppressor genes.( 12 ) However, clinical predictors of a doxycycline response have yet to be identified and would help in selecting the best candidates for this treatment. Therefore, we aimed to evaluate the efficacy of doxycycline in Korean patients with ocular adnexal lymphoma as well as to find independent factors that predict outcomes.
Materials and Methods
Patients. Thirty‐eight patients at Seoul National University Hospital with ocular adnexal lymphoma that was newly diagnosed between April 2005 and July 2008 met the following inclusion criteria: (1) typical histological features of MALT lymphoma according to the World Health Organization criteria,( 13 ) according to specialized hematopathologists (Y.K.J. and C.W.K.); (2) primary tumor confined to ocular adnexa; (3) doxycycline as a first‐line treatment; and (4) measurable disease by computed tomography (CT) or magnetic resonance imaging (MRI) of the orbit or by ophthalmic examination with a photograph taken at the time of doxycycline initiation. Cp DNA was detected in 25 paraffin‐embedded tumor tissues using touchdown enzyme time‐release polymerase chain reaction.( 14 ) Briefly, a touchdown method for thermal cycling combined with an enzyme time release protocol was used: cycling times at 95°C for 75 s; and 60 cycles of denaturation at 94°C for 45 s, annealing beginning at 62°C and ending at 52°C for 45 s, and extension at 72°C for 1 min. Cp DNA was kindly provided by Professor Seung‐Joon Lee (Kangwon National University). The primer sequences for Cp were 5′‐CCCAAGGTGAGGCTGA‐ TGAC‐3′ (forward) and 5′‐CAAACCGTCCTAAGACAGTTA‐3′ (reverse). Cp infection was considered positive for tumor tissues harboring at least one amplified Cp DNA in two amplifications.
A staging work‐up included complete blood count, blood chemistry, ophthalmic examination by an experienced ophthalmologist (S.I.K.), chest radiograph, CT/MRI of the orbit, CT of the chest and abdomen, and a bone marrow aspirate and biopsy. This retrospective study was approved by the Institutional Review Board at Seoul National University Hospital.
Doxycycline treatment and response evaluation. Doxycycline was given orally at a dose of 100 mg twice a day for 3 weeks (singe‐course therapy, n = 12) or at the same dose for 3 weeks, followed by 3 weeks off, and repeated for a second 3 weeks (double‐course therapy, n = 26). Double‐course therapy was assigned to patients with residual eye‐related symptoms who did not respond to singe‐course therapy. Response to doxycycline was evaluated 6 weeks after the first‐dose, then every 3 months for 2 years, and then every 6 months for 3 years. Ophthalmic examination and anterior segment photography were performed every 3 months. A CT of the orbit, and a complete blood count, chemistry, and lactate dehydrogenase (LDH) level were performed on every visit.
Response was assessed based on the CT or the ophthalmic examination with photography, using modified international workshop criteria.( 15 ) Complete remission (CR) is defined as complete disappearance of all detectable ophthalmic and radiographic evidence of disease and eye‐related symptoms if present before therapy. Partial remission (PR) denotes 50% or more decrease in the sum of the product of the greatest diameters. Progressive disease (PD) is defined by any new lesion or 50% or more increase from the smallest sum of the product of the greatest diameters. Stable disease is defined as an absence of CR, PR, and PD.
Statistical analysis. Absolute lymphocyte and neutrophil counts were determined within 1 week before doxycycline treatment at a time when the patient was free from active infection. Absolute lymphocytosis or neutrophilia was defined as an absolute lymphocyte or neutrophil count higher than the respective median value. The association between doxycycline outcome and clinical factors including absolute lymphocyte and neutrophil counts was evaluated by the Pearson’s chi‐square test or Fisher’s exact test, where appropriate. Overall survival was measured from the date of diagnosis to the date of death or the last follow‐up visit. Time‐to‐treatment failure (TTF) was measured from the date of initial treatment to the date of disease progression, the start date of second‐line treatment, or the last follow‐up visit. Time to response was calculated from the date of initial treatment to the date of documented CR or PR. The survival curve was derived by the Kaplan–Meier method.( 16 ) Comparisons between the groups were made using the log‐rank test. Factors independently associated with objective response were identified by binary logistic regression. Univariate and multivariate analyses of TTF were performed using the Cox proportional hazards regression model. Clinical variables by the binary logistic regression or Cox regression model were as follows: age, laterality, involved sites, hemoglobin level, platelet count, absolute lymphocytosis or neutrophilia, LDH level, performance status, and doxycycline duration. Two‐sided P‐values of <0.05 were considered significant. All statistical analyses were performed using SPSS version 13.0 (SPSS, Chicago, IL, USA).
Results
Clinical features. The clinical characteristics of 38 patients are shown in Table 1. The median age was 47 years with a male to female ratio of 1:2.2. The median duration of symptoms was 4 months (range, 1–60 months). The most common presenting symptoms included mass or swelling (28 patients, 74%) and conjunctival redness or irritation (20, 53%), whereas proptosis (2, 5%), epiphora (2, 5%), and lid ptosis (1, 3%) were present in few patients. None of the patients had diplopia, pain, or decreased vision. Hepatitis C virus tests were all negative in 19 tested patients and no patients had autoimmune disorders. Measurable lesions at baseline were evaluated using CT/MRI of the orbit (13 patients, 34%) or ophthalmic examination with photography (25, 66%), when the lesion was unmeasurable by CT/MRI of the orbit.
Table 1.
>Characteristics of 38 patients with ocular adnexal mucosa‐associated lymphoid tissue lymphoma
| Characteristics | No. of patients | % |
|---|---|---|
| Age, n = 38 | ||
| 60 years or younger | 33 | 87 |
| Over 60 years | 5 | 13 |
| B symptoms, n = 38 | ||
| No | 38 | 100 |
| Yes | 0 | 0 |
| Ann Arbor stage, n = 38 | ||
| IE | 38 | 100 |
| IIE | 0 | 0 |
| Presentation of tumor, n = 38 | ||
| Orbit | 4 | 11 |
| Conjunctiva | 30 | 79 |
| Eyelid | 2 | 5 |
| Lacrimal gland | 2 | 5 |
| Performance status, n = 38 | ||
| 0 | 33 | 87 |
| 1 | 5 | 13 |
| Lactate dehydrogenase level, n = 26 | ||
| Normal | 23 | 88 |
| Elevated | 3 | 12 |
| Hemoglobin level, n = 38 | ||
| 12 g/dL or less | 3 | 8 |
| Over 12 g/dL | 35 | 92 |
| Platelet count, n = 38 | ||
| 150 000/μL or less | 2 | 5 |
| More than 150 000/μL | 36 | 95 |
| FLIPI, n = 35 | ||
| Low risk | 34 | 97 |
| Intermediate risk | 1 | 3 |
| IPI, n = 36 | ||
| Low risk | 35 | 97 |
| Low‐intermediate risk | 1 | 3 |
| Laterality, n = 38 | ||
| Unilateral | 26 | 68 |
| Bilateral | 12 | 32 |
| Absolute neutrophil count, n = 38 | ||
| Median | 3.01 × 109/L | |
| Range | 1.15–6.59 × 109/L | |
| Absolute lymphocyte count, n = 38 | ||
| Median | 1.92 × 109/L | |
| Range | 1.08–3.59 × 109/L | |
FLIPI, follicular lymphoma international prognostic index; IPI, international prognostic index.
Doxycycline outcomes. Collectively, of 38 patients initially treated with doxycycline, seven (18%) and 11 (29%) achieved CR and PR, respectively (overall response rate, 47%) (Fig. 1). After a median follow‐up of 26.4 months (range, 6.3–43.6 months), disease progression at the primary site was observed in four (20%) of 20 non‐responders (i.e. with stable disease) and in only one (6%) of 18 responders (CR + PR). Due to the persistent eye‐related symptoms, second‐line combination chemotherapy with cyclophosphamide, vincristine, and prednisolone (CVP)( 17 ) was given to 11 (55%) of 20 patients with stable disease, of whom six and one achieved CR and PR, respectively. One non‐responder received low‐dose electron beam radiotherapy and subsequently achieved CR. Among responders, one patient with a PR achieved a CR after CVP chemotherapy and another, who had PD after a PR following doxycycline therapy, attained a CR after radiotherapy. Treatment outcomes are summarized in Table 2, according to the doxycycline duration. Patients treated with a double course (vs a single course) of doxycycline showed a trend toward a higher response rate (54%vs 33%, respectively; P = 0.239) and a more rapid response (3.3 vs 10.7 months, respectively; P = 0.189). Doxycycline was interrupted after 1 week in only one patient due to grade 2 dizziness. Except for this, none of the patients had non‐hematologic or hematologic toxicities. Cp DNA was observed in 15 (60%) of 25 patients with available tumor tissues. However, Cp‐positivity had no association with doxycycline response (60%vs 60%, P = 1.000).
Figure 1.
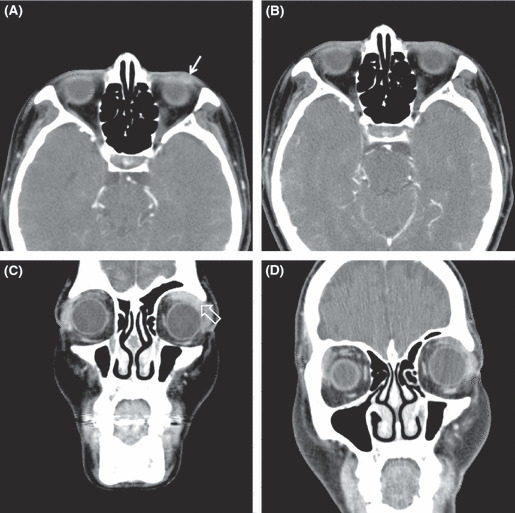
Contrast‐enhanced computed tomography (CT) scans of the orbit show (A) the neoplastic lesion of left lower eyelid (arrow) before treatment and (B) its complete remission at 4 months after 6‐week doxycycline. Contrasted CT scans of the orbit show (C) well‐enhancing soft tissue mass around left superior rectus muscle (open arrow) before treatment and (D) its partial regression at 6 months after 6‐week doxycycline.
Table 2.
Treatment outcomes according to the duration of doxycycline
| Treatment outcomes | No. of patients (%) | P‐values† | |
|---|---|---|---|
| 3‐week doxycycline (n = 12) | 6‐week doxycycline (n = 26) | ||
| Presentation of tumors | |||
| Conjunctival | 9 (75) | 21 (81) | 0.685 |
| Non‐conjunctival | 3 (25) | 5 (19) | |
| Best response | |||
| CR | 2 (17) | 5 (19) | 0.239 |
| PR | 2 (17) | 9 (35) | |
| SDz | 8 (66) | 12 (46) | |
| Time to response | |||
| Median, months | 10.7 | 3.3 | 0.189 |
| Range | 3.3–30.3 | 1.2–23.3 | |
| Progression | |||
| No | 11 (92) | 22 (85) | 0.550 |
| Yes | 1 (8) | 4 (15) | |
| 2‐year TTF | 89% | 82% | 0.882 |
†Pearson’s chi‐square or Fisher’s exact test. CR, complete remission; PR, partial remission; SDz, stable disease; TTF, time‐to‐treatment failure.
Absolute lymphocytosis or neutrophilia. Absolute neutrophil count (ANC) was higher in the responders than in non‐responders (mean ± SD, 3.58 ± 1.14 × 109/L vs 2.82 ± 1.19 × 109/L; P = 0.075). Absolute lymphocyte counts (ALCs) were 2.15 ± 0.65 × 109/L in the non‐responders and 1.85 ± 0.63 × 109/L in the responders (P = 0.090). Median value was used to define absolute lymphocytosis (ALC > 1.92 × 109/L) and neutrophilia (ANC > 3.01 × 109/L). Patients with rather than those without absolute lymphocytosis showed significantly worse outcomes (response rate, 32%vs 63%, respectively; P = 0.051; and disease progression, 26%vs 0%, respectively, P = 0.046) (Table 3). Contrarily, higher objective response was observed in patients with absolute neutrophilia (63%vs 32%, P = 0.051).
Table 3.
Treatment outcomes according to absolute lymphocytosis or neutrophilia
| Treatment outcomes | Absolute lymphocytosis, no. of patients (%) | P‐values† | Absolute neutrophilia, no. of patients (%) | P‐values† | ||
|---|---|---|---|---|---|---|
| No (n = 19) | Yes (n = 19) | No (n = 19) | Yes (n = 19) | |||
| Best response | ||||||
| CR | 5 (26) | 2 (11) | 0.051 | 3 (16) | 4 (21) | 0.051 |
| PR | 7 (37) | 4 (21) | 3 (16) | 8 (42) | ||
| SDz | 7 (37) | 13 (68) | 13 (68) | 7 (37) | ||
| Progression | ||||||
| No | 19 (100) | 14 (74) | 0.046 | 15 (79) | 18 (95) | 0.340 |
| Yes | 0 (0) | 5 (26) | 4 (21) | 1 (5) | ||
| 2‐year TTF | 100% | 70% | 0.021 | 73% | 94% | 0.077 |
†Pearson’s chi‐square or Fisher’s exact test. CR, complete remission; PR, partial remission; SDz, stable disease; TTF, time‐to‐treatment failure.
Survival analysis and clinical predictors. All patients were alive at the time of analysis, and the 3‐year TTF rate was 84% (Fig. 2). Non‐conjunctival OAML, when compared with conjunctival lymphoma, had a proclivity towards reduced TTF (2‐year TTF 64%vs 88%, respectively; P = 0.077). There was no significant difference in TTF between bilateral and unilateral diseases (2‐year TTF 79%vs 86%, respectively; P = 0.590). However, absolute lymphocytosis significantly reduced TTF (2‐year TTF 70%vs 100%, P = 0.021 [Fig. 3A]), whereas absolute neutrophilia inclined toward improved TTF (2‐year TTF 94%vs 73%, P = 0.077 [Fig. 3B]). The clinical factors associated with objective response in univariate analysis were conjunctival tumor (odds ratio [OR] = 9.2, 95% confidence interval [CI], 1.0–84.0; P = 0.050), presence of absolute neutrophilia (OR = 3.7, 95% CI, 1.0–14.2; P = 0.056), and absence of absolute lymphocytosis (OR = 3.7, 95% CI, 1.0–14.2; P = 0.056). Absence of absolute lymphocytosis (OR = 4.7, 95% CI, 1.1–20.8; P = 0.043) and conjunctival tumor (OR = 11.8, 95% CI, 1.1–122.5; P = 0.038) were identified as independent predictors for doxycycline response by multivariate analysis. No clinical factors independently affected TTF by the Cox proportional hazards regression model (Ps > 0.050).
Figure 2.
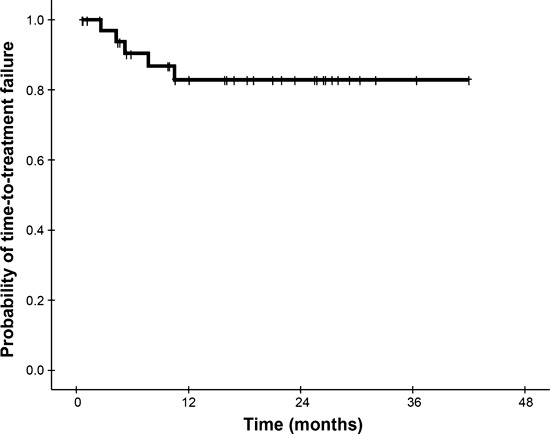
Kaplan–Meier plot of time‐to‐treatment failure in all patients.
Figure 3.
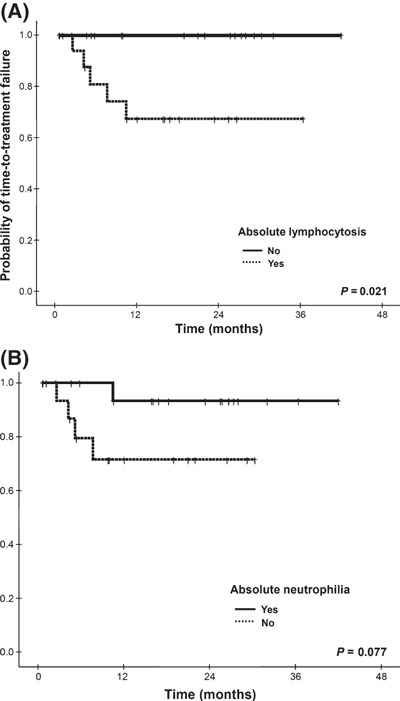
Kaplan‐Meier plots of time‐to‐treatment failure according to (A) the presence of absolute lymphocytosis and (B) the presence of absolute neutrophilia.
Discussion
Our study demonstrated that front‐line doxycycline is well‐tolerated and effective against ocular adnexal lymphoma, especially in patients without absolute lymphocytosis or in conjunctival lymphoma patients. In addition, chemotherapy or radiotherapy can be reserved for relapsed or progressive cases. Moreover, patients who received double‐course therapy showed a trend towards an earlier response and higher response rate than did those who received single‐course therapy. Absolute lymphocytosis significantly reduces TTF as well as doxycycline response in localized ocular adnexal lymphoma. However, Cp‐positivity did not affect doxycycline outcome.
Cumulative evidence has indicated that a pre‐treatment, high neutrophil‐to‐lymphocyte ratio or elevated neutrophil count, suggestive of systemic inflammatory response, is significantly associated with survival in non‐small‐cell lung cancer,( 18 ) renal cell carcinoma,( 19 ) melanoma,( 20 ) and colorectal cancer.( 21 ) However, the role of the neutrophil count in predicting survival has not been well described in B‐cell lymphomas. A pretreatment high lymphocyte count, implying host immunocompetence, was an independent prognostic factor for survival in follicular lymphoma( 22 ) as well as in diffuse large cell lymphoma.( 23 ) These findings are inconsistent with ours in which poor outcomes were observed in patients with relatively low neutrophil or high lymphocyte counts. Considering that the growth of gastric MALT lymphoma is stimulated by Helicobacter pylori‐specific intratumoral T cells,( 24 ) a high blood lymphocyte count may contribute to the antigen‐specific T‐cell population in the microenvironment of ocular adnexal MALT lymphoma. With regard to the role of neutrophil, viable Cp is detected in CD68‐positive monocytes/macrophages in OAML.( 25 ) Additionally, it was suggested that Chlamydia pneumoniae multiplies in short‐lived neutrophils, which can extend their lifespan.( 26 ) Taken together, the elevation of circulating neutrophils by Cp infection may indirectly predict a good response to doxycycline because a trend toward a higher response rate was observed in Cp‐positive OAML patients.( 9 ) However, Cp infection in our study had no association with doxycycline response, suggesting a questionable role of Cp in OAML unlike Helicobacter pylori in gastric MALT lymphoma. We have, nevertheless, identified independent outcome predictors for OAML patients treated with front‐line doxycycline. Absence of absolute lymphocytosis and conjunctival lymphoma were positive predictors of doxycycline outcome. Furthermore, patients with OAML with absolute lymphocytosis had relatively brief TTF. Accordingly, pre‐treatment absolute lymphocytosis can be a predictive biomarker for the outcome of doxycycline therapy in ocular adnexal lymphoma. Regarding conjunctival MALT lymphoma, more favorable outcome may be attributable to a small tumor burden in our study, inconsistent with previous study that demonstrated comparable outcome between conjunctival and nonconjunctival lymphomas.( 9 )
In an Italian study, Cp‐eradicating doxycycline for 3 weeks was associated with a gradual response, with a median time to best response of 6 months and an overall response rate of 48%.( 9 ) However, another trial found that blind antibiotic therapy was not effective in an Austrian population with OAML, suggesting a potential geographic difference in Cp‐positive rates.( 27 ) In contrast to previous studies with short follow‐up periods and a small number of newly diagnosed cases,( 9 , 27 ) our study followed patients over a somewhat longer period and had an adequate number of cases to identify a predictive factor. Moreover, patients treated with 6 weeks of doxycycline had a tendency toward a higher response rate and more rapid response, compared with those who received 3 weeks of therapy. Considering the high proportion of stable disease, 6 weeks of doxycycline, despite having a similar TTF rate, is optional for patients who cannot tolerate persistent eye‐related symptoms. In addition, because few patients with absolute lymphocytosis responded to doxycycline, radiotherapy or chemotherapy should be considered in these patients. Inversely, OAML patients without absolute lymphocytosis but with conjunctival involvement are good candidates for front‐line doxycycline. However, doxycycline efficacy and comparison of treatment schedules might be biased due to the absence of a control arm and non‐randomization of treatment arm, respectively.
We evaluated the response based on modified international workshop criteria( 15 ) using imaging studies as well as an ophthalmic examination with photography. Because a change in clearly measurable disease by a CT scan constituted nearly one‐third of cases using this criteria, the true response rate may have been underestimated. Moreover, in a case of conjunctival lymphoma, although only the thickness or short diameter of the tumor decreased with ameliorated symptoms, this patient’s disease was considered stable. Therefore, it is crucial to establish the response criteria of OAML.( 6 )
In conclusion, our study demonstrated that absolute lymphocytosis and nonconjunctival tumor in ocular adnexal MALT lymphoma are independent predictors for poor response after first‐line doxycycline. Furthermore, patients with absolute lymphocytosis showed an early progression. Particularly when confined to cases without absolute lymphocytosis or with conjunctival lymphoma, doxycycline may be the best choice of treatment for localized ocular adnexal lymphoma. In view of the association between chronic inflammation and this lymphoma, future efforts should be directed toward finding the roles of neutrophil and lymphocyte subsets either in peripheral blood or in the tumor microenvironment.
Acknowledgments
This study was supported by a grant from the Innovative Research Institute for Cell Therapy, Republic of Korea (no. A0622660). We acknowledge the assistance of the Seoul National University Hospital Medical Research Collaborating Center for statistical consultation.
References
- 1. Moslehi R, Devesa SS, Schairer C, Fraumeni JF Jr. Rapidly increasing incidence of ocular non‐hodgkin lymphoma. J Natl Cancer Inst 2006; 98: 936–9. [DOI] [PubMed] [Google Scholar]
- 2. Oh DE, Kim YD. Lymphoproliferative diseases of the ocular adnexa in Korea. Arch Ophthalmol 2007; 125: 1668–73. [DOI] [PubMed] [Google Scholar]
- 3. Ferreri AJ, Dolcetti R, Du MQ et al. Ocular adnexal MALT lymphoma: an intriguing model for antigen‐driven lymphomagenesis and microbial‐targeted therapy. Ann Oncol 2008; 19: 835–46. [DOI] [PubMed] [Google Scholar]
- 4. Rosado MF, Byrne GE Jr, Ding F et al. Ocular adnexal lymphoma: a clinicopathologic study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood 2006; 107: 467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chanudet E, Zhou Y, Bacon CM et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J Pathol 2006; 209: 344–51. [DOI] [PubMed] [Google Scholar]
- 6. Husain A, Roberts D, Pro B, McLaughlin P, Esmaeli B. Meta‐analyses of the association between Chlamydia psittaci and ocular adnexal lymphoma and the response of ocular adnexal lymphoma to antibiotics. Cancer 2007; 110: 809–15. [DOI] [PubMed] [Google Scholar]
- 7. Ferreri AJ, Guidoboni M, Ponzoni M et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004; 96: 586–94. [DOI] [PubMed] [Google Scholar]
- 8. Ferreri AJ, Ponzoni M, Guidoboni M et al. Regression of ocular adnexal lymphoma after Chlamydia psittaci‐eradicating antibiotic therapy. J Clin Oncol 2005; 23: 5067–73. [DOI] [PubMed] [Google Scholar]
- 9. Ferreri AJ, Ponzoni M, Guidoboni M et al. Bacteria‐eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: a multicenter prospective trial. J Natl Cancer Inst 2006; 98: 1375–82. [DOI] [PubMed] [Google Scholar]
- 10. Ferreri AJ, Dognini GP, Ponzoni M et al. Chlamydia‐psittaci‐eradicating antibiotic therapy in patients with advanced‐stage ocular adnexal MALT lymphoma. Ann Oncol 2008; 19: 194–5. [DOI] [PubMed] [Google Scholar]
- 11. Yoo C, Ryu MH, Huh J et al. Chlamydia psittaci infection and clinicopathologic analysis of ocular adnexal lymphomas in Korea. Am J Hematol 2007; 82: 821–3. [DOI] [PubMed] [Google Scholar]
- 12. Decaudin D, De Cremoux P, Vincent‐Salomon A, Dendale R, Rouic LL. Ocular adnexal lymphoma: a review of clinicopathologic features and treatment options. Blood 2006; 108: 1451–60. [DOI] [PubMed] [Google Scholar]
- 13. Harris NL, Jaffe ES, Diebold J et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting‐Airlie House, Virginia, November 1997. J Clin Oncol 1999; 17: 3835–49. [DOI] [PubMed] [Google Scholar]
- 14. Madico G, Quinn TC, Boman J, Gaydos CA. Touchdown enzyme time release‐PCR for detection and identification of Chlamydia trachomatis, C. pneumoniae, and C. psittaci using the 16S and 16S‐23S spacer rRNA genes. J Clin Microbiol 2000; 38: 1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheson BD, Horning SJ, Coiffier B et al. Report of an international workshop to standardize response criteria for non‐Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244. [DOI] [PubMed] [Google Scholar]
- 16. Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 17. Song EK, Kim SY, Kim TM et al. Efficacy of chemotherapy as a first‐line treatment in ocular adnexal extranodal marginal zone B‐cell lymphoma. Ann Oncol 2008; 19: 242–6. [DOI] [PubMed] [Google Scholar]
- 18. Paesmans M, Sculier JP, Libert P et al. Prognostic factors for survival in advanced non‐small‐cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol 1995; 13: 1221–30. [DOI] [PubMed] [Google Scholar]
- 19. Negrier S, Escudier B, Gomez F et al. Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d’Immunotherapie. Ann Oncol 2002; 13: 1460–8. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt H, Suciu S, Punt CJ et al. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol 2007; 25: 1562–9. [DOI] [PubMed] [Google Scholar]
- 21. Neal CP, Mann CD, Sutton CD et al. Evaluation of the prognostic value of systemic inflammation and socioeconomic deprivation in patients with resectable colorectal liver metastases. Eur J Cancer 2009; 45: 56–64. [DOI] [PubMed] [Google Scholar]
- 22. Siddiqui M, Ristow K, Markovic SN et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol 2006; 134: 596–601. [DOI] [PubMed] [Google Scholar]
- 23. Kim DH, Baek JH, Chae YS et al. Absolute lymphocyte counts predicts response to chemotherapy and survival in diffuse large B‐cell lymphoma. Leukemia 2007; 21: 2227–30. [DOI] [PubMed] [Google Scholar]
- 24. Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev 2004; 4: 644–53. [DOI] [PubMed] [Google Scholar]
- 25. Ponzoni M, Ferreri AJ, Guidoboni M et al. Chlamydia infection and lymphomas: association beyond ocular adnexal lymphomas highlighted by multiple detection methods. Clin Cancer Res 2008; 14: 5794–800. [DOI] [PubMed] [Google Scholar]
- 26. Van Zandbergen G, Gieffers J, Kothe H et al. Chlamydia pneumoniae multiply in neutrophil granulocytes and delay their spontaneous apoptosis. J Immunol 2004; 172: 1768–76. [DOI] [PubMed] [Google Scholar]
- 27. Grunberger B, Hauff W, Lukas J et al. ‘Blind’ antibiotic treatment targeting Chlamydia is not effective in patients with MALT lymphoma of the ocular adnexa. Ann Oncol 2006; 17: 484–7. [DOI] [PubMed] [Google Scholar]


