Abstract
Recent remarkable progress in hormonal therapy has provided great benefit to breast cancer patients, but it also evokes novel issues: how accurately can the efficacy of each hormonal therapy be predicted and how can hormonal therapy–resistant patients be treated? These clinically important issues must be closely related to the biological events in each cancer, such as the alteration of intracellular multiple estrogen signaling pathways and the estrogen‐related cancer microenvironment, which has recently revealed by molecular biological studies on estrogen and its receptors. However, the estrogen signaling status in individual breast cancers has not been clarified yet. Here we present the context of these issues and introduce our study of new tools which enable the visualization of estrogen signals in individual cancers. The assessment of estrogen receptor (ER)‐α activity in individual cancers or ER‐activating ability of the cancer microenvironment in each breast cancer patient revealed several new findings and interesting observations. We hope that these approaches provide new clues about the estrogen‐dependent mechanisms of breast cancer development, and will be useful to advance the diagnosis and treatment of breast cancer patients. (Cancer Sci 2009; 100: 1773–1778)
Estrogen receptor and hormonal therapy in breast cancer
Estrogen controls various physiological functions in many target tissues, and is also involved in diseases such as breast cancer, endometrial cancer, vascular disease, osteoporosis, and others. In particular, breast cancer is well recognized to be a crucial tumor in which estrogen and its receptor (ER) regulate development and progression in most cases, and are therefore the most important and effective targets for the therapy of breast cancer.( 1 ) In this article, ER refers to estrogen receptor α unless specified.
The efficacy of hormonal therapies targeting estrogen and ER has been established in numerous clinical trials. Current hormonal therapies are performed by two main strategies: inhibition of ER functions by anti‐estrogens and deprivation of estrogen production by luteinizing hormone‐releasing hormone agonist (pre‐menopause) or aromatase inhibitor (post‐menopause). Prediction of the efficiency of these therapies has involved using classical molecular biomarkers such as ERα and progesterone receptor.( 2 ) However, approximately one‐third of ER‐positive patients do not respond to endocrine therapy, while some ER‐negative patients are responsive.( 3 ) These discrepancies could result from different estrogen‐related intracellular signaling pathways in breast cancer cells.
One of the approaches to analyze the estrogen signaling status in breast cancer is expression profiling of estrogen‐regulated genes in cancer cells. Comprehensive gene expression analysis, such as DNA microarray, may be a useful tool to address this issue. So far, we have analyzed estrogen‐responsive gene expression profiles among ER‐positive cancer cells using comprehensive DNA microarray( 4 ) and, based on the obtained information, we have produced our own focused microarray using 200 estrogen‐regulated genes.( 5 ) Using this microarray, we have studied several basic issues regarding estrogen signaling, such as the effect of estrogen antagonists and endocrine disruptors on estrogen‐responsive gene expression profiles.( 6 ) Studies of the functional analysis of ERβ and identification of novel estrogen responsive genes were also carried out.( 7 , 8 ) These studies provided information on promising diagnostic or prognostic biomarkers( 9 ) for ER‐positive breast cancer, such as histone deacetylase 6,( 10 , 11 ) insulin‐like growth factor binding protein 4,( 12 ) and early growth responsive gene 3.( 13 ) As described above, the expression status of ER is not a perfect predictor for hormonal therapy. Since the cDNA microarray has several difficulties such as complicated method and cost performance for practical application, presently we are aiming to identify new biomarkers to predict the efficacy of hormonal therapy.
In these studies, we observed that most breast cancer specimens which showed a high expression of estrogen‐responsive genes were ER‐positive cases, but specimens which showed a low expression of estrogen‐responsive genes were not only ER‐negative. Namely, ER‐positive specimens did not always show a high expression of target genes, but some showed a low expression of target genes, and even the Allred score( 2 ) was high. There is some discrepancy between the expression levels of ER protein and the expression profile of target genes. This discrepancy might be the cause of the difference between the ER expression status and the efficacy of hormonal therapy. We next tried to analyze ER transcription activity in individual breast cancer patients using an adenovirus ERE–GFP reporter system.
Imaging of transcription activity of nuclear receptor
Nuclear receptors such as glucocorticoid receptor (GR), mineralcorticoid receptor (MR), farnesol‐X receptor (FXR), vitamin D receptor (VDR), pregnane‐X receptor (PXR), androgen receptor (AR), and ER bind lipophilic ligands synthesized from cholesterol. ERα is a member of this nuclear receptor super‐family and regulates the transcription of various genes as a transcription factor,( 14 ) which binds to estrogen response elements (ERE) upstream of the target genes (Fig. 1). Usually, in vitro assay of transcriptional activity of ER is analyzed by the luciferase reporter plasmid connecting ERE in the enhancer region. However, this method has some disadvantages when analyzing samples prepared from tissue specimens, such as needle biopsy or surgical specimens. We therefore developed a new system to analyze ER‐transcription activity using green fluorescent protein (GFP) as a reporter gene.
Figure 1.

Biosynthesis and molecular action of estrogen and its receptor (ER). Steroid hormones are synthesized from cholesterol, and activate their specific nuclear receptors. Estrogen is synthesized from androgen by aromatase enzyme, and activates estrogen receptor as a ligand. The activated ER binds to the estrogen response element upstream of target genes, and enhances gene transcription. The induced gene products exhibit estrogenic physiological functions in cells.
We developed two kinds of GFP assay systems, as shown in Figure 2, one of which is an adenovirus ERE–GFP reporter system. This assay system enabled us to visualize and analyze the endogenous ER transcription activity of a small number of cancer cells in heterogeneous samples containing various stromal cells. The consensus ERE and TK‐promoter gene was inserted in front of GFP cDNA, constructed into an adenovirus vector (pAd/PL‐DEST; Invitrogen, Carlsbad, CA), and named pAd‐ERE‐tk‐GFP. The amplified adenovirus infected cells prepared from tissue specimens by collagenase treatment.( 15 ) The other assay system involves a reporter cell line which stably introduced the ERE–GFP reporter gene. We established a reporter cell line, named MCF‐7‐E10, to visualize ER activity stimulated by extracellular signals such as estrogen or other factors. To realize the quantitative analysis, specific GFP cDNA with a ubiqutination site was used so that the GFP protein had a very short half‐life. These GFP reporter cells were especially useful for analyzing the ER‐stimulating ability of stromal fibroblasts in the cancer microenvironment, as described later.
Figure 2.
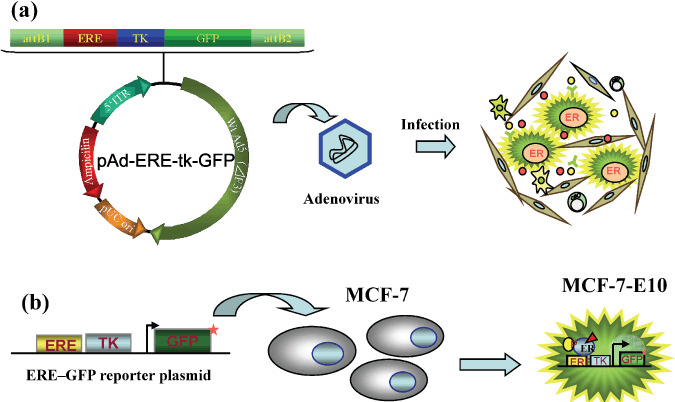
ERE–GFP assay systems for analyzing estrogen receptor (ER) activity in the samples from clinical specimens. (a) Adenovirus ERE–GFP reporter system which enables the assessment of endogenous ER activity in breast cancer cells obtained from clinical specimens. (b) Reporter cell line stably transfected ERE–GFP. ERE–GFP reporter cell line assay for the assessment of ER‐activating performance of stromal cells obtained from breast cancer tissues.
Individual transcription activity of ER analyzed by adenovirus ERE–GFP assay system
We have analyzed the individual transcription activity of ER in breast and endometrial cancer using the adenovirus ERE–GFP assay system. When the virus infected ERα‐positive breast cancer MCF‐7 cells, the GFP expression was dose‐dependently increased by the addition of estradiol, and this induced expression was strongly inhibited by the addition of a pure anti‐estrogen, fulvestrant (as shown in Fig. 3), indicating that this induction was mediated by ER and its quantitative evaluation was possible.
Figure 3.
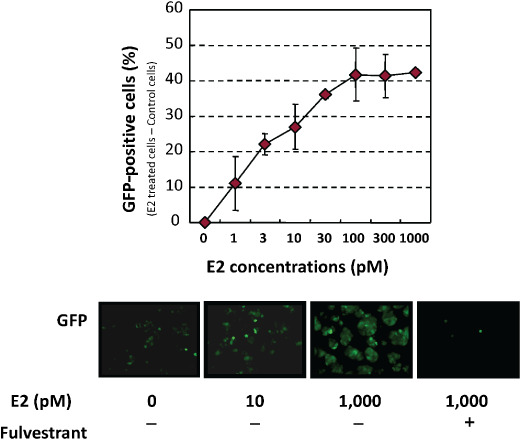
Adenovirus ERE–GFP expression in infected estrogen receptor (ER)‐positive breast cancer MCF‐7 cells. Virus‐infected cells expressed green fluorescent protein (GFP) in a dose‐dependent manner with estrogen, which was blocked by the addition of a pure anti‐estrogen, fulvestrant.
Our recent study of endometrial cancer using this system revealed that substantial ER activities were shown in tissue specimens of endometrial cancer, even compared with breast cancer.( 15 ) Furthermore, anti‐estrogen or aromatase inhibitors were effective for samples which showed high ER activities. Presently, hormonal therapy is not a standard option for endometrial cancer, because the used anti‐estrogen, tamoxifen, is known to have agonistic effects on endometrial cancer. However, a recently established option for breast cancer, such as third‐generation aromatase inhibitors or a pure anti‐estrogen, fulvestrant, might be useful for endometrial cancer patients with high estrogen signaling activity.
This adenovirus ERE–GFP assay was performed to analyze individual ER activity in breast cancers. So far, we have analyzed more than 60 primary breast cancer specimens, and they showed a vast variation of transcription activity (unpublished data). Interestingly, the profile of transcription activity was not identical to the ER‐protein expression analyzed immunohistochemically. As shown in Figure 4, ER protein, ER activity, and the expression of ER target genes are functionally and directly linked. Stomal fibroblasts activated ER in an estrogen‐mediated and phosphorylation‐mediated manner. Which one is more closely related to the response to hormonal therapy of breast cancers? Further analysis of the correlation with clinicopathological factors or the clinical outcome is anticipated for further personalization of ER‐positive primary breast cancers.
Figure 4.
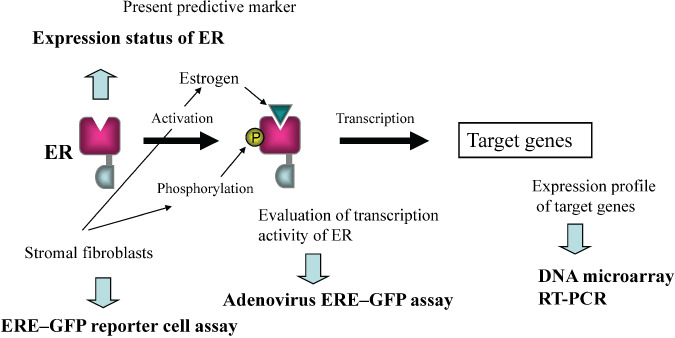
Direct connection among estrogen receptor (ER), its activity, and expression of the target genes. They are functionally linked, and the established predictive marker for breast cancer is the expression status of ER. Stromal fibroblasts in the tumor microenvironment activated ER via estrogen‐ and growth factor‐mediated signaling pathways.
Alternative estrogen signaling and aromatase inhibitor‐resistant cancer
So far, multiple signaling pathways for ER activation are known (Fig. 5). The main signaling pathway is the binding of estrogen to nuclear ER. Recently, the presence of estrogen receptor in the cell membrane has been suggested.( 16 ) However, it is still unclear whether this membrane‐associated binding site consists of the same protein molecule as nuclear ERα/β and how this pathway works, because a reliable method has not yet been established to distinguish these signals from localized receptors.
Figure 5.

Multiple estrogen signaling pathways. Estrogen‐dependent and ‐independent activation of estrogen receptor (ER).
Alternative signaling pathways independent of estrogen are also proposed. The growth factor receptor pathway followed by the phosphorylation cascade has been reported to activate ER.( 17 , 18 , 19 ) The signal is mediated by various growth factors, such as epidermal growth factor (EGF), insulin‐like growth factor (IGF), and others, eventually resulting in the phosphorylation of ER with or without binding estrogen. Elevation of ERK1/ERK2 activities has also been observed.( 20 ) Another pathway provoked by androgen metabolites has been considered.( 21 ) In postmenopausal women, estrogens are mainly supplied by aromatase from androgens (testosterone and androstenedione) biosynthesized in adrenal glands. Aromatase inhibitor (AI) therapy prevents this estrogen supply route, but does not alter circulating androgen levels. It is well known that the main metabolizing reaction of androgen is reduction of the Δ4‐3‐keto system on Ring A, converting testosterone to dihydrotestosterone (DHT; active androgen). DHT is further metabolized to 3β,17‐dihydroxy‐5α‐androstanediol and its 3α‐isomer.( 22 ) These metabolites have rather weak affinity to ERα and ‐β,( 23 ) but can directly act as ligands to cause cell proliferation in culture in a dose‐dependent manner.( 24 ) In AI‐resistant cancer, these alternative pathways may play an important role in cell growth signaling. Signaling diversity has been observed and we have listed the most possible hypotheses for the mechanism of AI‐resistant cancer in Table 1.
Table 1.
Possible hypotheses for mechanism of aromatase inhibitor‐resistant breast cancer
| Hypothesis | Possible therapy |
|---|---|
| 1. Promoted metabolism or excretion of the employed AI | Switching to another AI or SERM therapy |
| 2. Structural or qualitative change of aromatase | |
| 3. Ligand dependency on other than estrogen • Androgen metabolites act as a ligand | SERM therapy |
| 4. ER activation via phosphorylation cascade independent of ligands • Activated growth factor signaling pathways | SERM therapy or combination of SERM and kinase inhibitor |
| 5. Acquired growth ability independent of ER • ER positive but sensitivity completely lost • ER negative | Chemotherapy or other molecular targeting therapy |
AI, aromatase inhibitor; ER, estrogen receptor; SERM, selective estrogen receptor modulator.
We think that the main signaling pathway shifts to the alternative (estrogen‐independent) pathways mentioned above in many cases of cancer recurrence in hormonal therapy. Thus, we tried to assess the ER activity of AI‐refractory patients using the adenovirus ERE–GFP assay system described in the previous section, and found that ER activity was maintained in most cases. Furthermore, the addition of anti‐estrogens (tamoxifen, tormifen, or fulvestrant) suppressed ER activity in some cases (unpublished data); therefore, ER must be activated by alternative pathways. These results suggest that anti‐estrogen treatment can be effective even if cells have lost estrogen dependency and the signal is mediated by androgen metabolites. In cases unresponsive to anti‐estrogens, ER may be activated by a phosphorylation cascade. In such cases, protein kinase inhibitors and/or growth factor receptor inhibitors would be effective. Thus, we expect that this GFP assay system will be a useful tool to clarify the mechanisms of acquiring AI resistance in hormonal therapy.
Estrogen signaling in the cancer microenvironment
The tumor microenvironment, composed of a variety of stromal cells such as inflammatory cells, fibroblasts, and endothelial cells, is widely recognized to have a critical role in the initiation and progression of tumors, and their cellar interactions provide growth factors, cytokines, and chemokines in the microenvironment.( 25 , 26 , 27 , 28 ) In addition to these factors, stromal fibroblasts in postmenopausal breast cancers express aromatase, a key enzyme in estrogen biosynthesis, resulting in intratumoral estrogen production (Fig. 6).( 29 , 30 ) Since growth factors such as EGF and IGF‐1 also activate ER via phosphorylation, the stromal fibroblasts in breast cancer cells regulate ER activity via both estrogen‐dependent and ‐independent pathways.( 31 , 32 , 33 , 34 )
Figure 6.
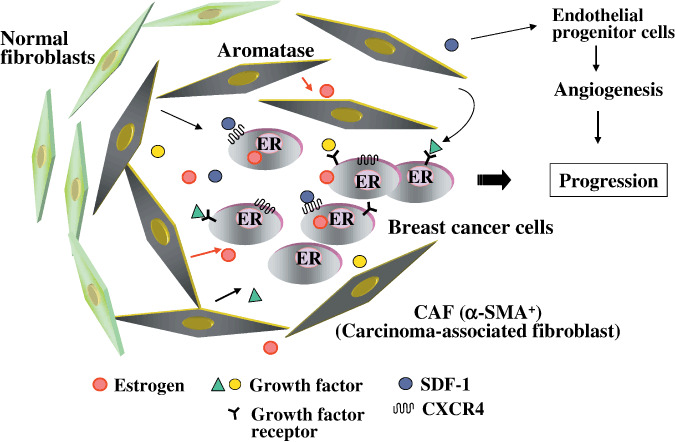
In the microenvironment of breast carcinoma, fibroblasts adjacent to tumor cells, named carcinoma‐associated fibroblasts (CAFs), produce various growth factors, and estrogen via aromatase expression, to activate estrogen receptor (ER). They also secrete stronal‐derived factor (SDF)‐1 to directly and indirectly promote the growth of breast cancer cells. CXCR4, CXC chemokine receptor 4.
To visualize and quantify the ER‐activating ability of stromal fibroblasts for individual breast cancers, we established an MCF‐7 clone reporting ER activity via the expression of GFP, as previously mentioned.( 35 ) MCF‐7 cells are transfected with the ERE–GFP reporter plasmid which has an ubiquitination site to make a half‐life of approximately 2 h, and we isolated several clones that showed specific expression of GFP in the presence of estrogen. We used a clone named MCF‐7‐E10 to analyze the ER‐activating ability of stromal fibroblasts because the background level of GFP detected in the absence of estrogen is lower than others.
Estrogen receptor (ER)‐activating ability of stromal fibroblasts for individual breast cancers was analyzed under co‐culture with MCF‐7‐E10 cells in the presence of testosterone, a substrate for aromatase using insert wells. Although the co‐culture system required more incubation time to detect GFP expression in MCF‐7‐E10 cells than estrogen treatment, a comparable maximal level of GFP was observed after 4 days of culture. GFP could not be observed in the co‐culture without testosterone. To more easily detect GFP expression, we developed an automated image analysis system of GFP expression in collaboration with Olympus Life Science Company (Tokyo, Japan).( 36 )
Using this system, we characterized the stromal fibroblasts of individual human breast cancers, and found that the ability of stromal fibroblasts to activate ER varied among cases, and that ER‐activating ability was higher in postmenopausal cases than in premenopausal cases, and was lower in Grade 3 than in Grade 1.( 35 ) Aromatase inhibitors dose‐dependently inhibited GFP expression in the co‐culture system.
Using our co‐culture system, we analyzed stromal fibroblasts obtained from endometrial cancer, another tumor for which estrogen is considered a risk factor.( 15 ) We found the similar result that while GFP levels varied among cases, stromal fibroblasts could activate ER in the presence of testosterone, which was inhibited by aromatase inhibitors. These results suggest that aromatase inhibitor might be useful for hormonal therapy in endometrial cancers.
In recent studies, fibroblasts isolated from carcinoma, named carcinoma‐associated fibroblasts (CAF), have been seen to exhibit biological characteristics distinct from normal mammary fibroblasts, which are myofibroblasts expressing α‐smooth‐muscle actin.( 37 ) CAFs but not normal fibroblasts promoted tumor growth in a xenograft model of breast cancer cells, mediated in part through the secretion of chemokine stromal‐derived factor (SDF‐1) (Fig. 6).( 37 ) SDF‐1 directly stimulates the growth of breast cancer cells highly expressing CXC chemokine receptor 4, a G‐protein‐coupled receptor for SDF‐1,( 27 , 28 , 38 ) and indirectly stimulates the growth of breast cancer through angiogenesis by recruiting endothelial progenitor cells to breast cancer (Fig. 6).( 37 ) Other studies have also reported that CAFs play an important role in the onset and progression of various types of tumor.( 25 , 28 , 39 ) Although the origin of CAFs has not been clarified, at least some have been suggested to be bone‐marrow derived.( 40 , 41 ) Further studies will reveal the relationship between aromatase‐expressing fibroblasts and CAFs, and the mechanism by which normal fibroblasts convert to CAFs.
Perspectives
The recently established classification ‘intrinsic subtypes’, identified by comprehensive DNA microarray analysis of breast cancer tissues, has greatly contributed to the individualization of breast cancers.( 42 ) Furthermore, new molecular diagnostic tools, such as ‘Oncotype DX’ (Genomic Health) based on RT‐PCR technology, and ‘MammaPrint’ (Agendia), based on microarray technology, will advance future personalized medicine for breast cancer. New molecular technologies often open up a new vista of basic, translational, and clinical research in medicine. In the field of breast cancer, a more accurate predictive method or novel predictive biomarker from among ER and PgR is desired to predict hormonal therapy. Comprehensive analytical techniques, such as microarray analysis, may be useful to search for such new biomarkers. However, these expression analyses are not able to reveal the specific pathway of estrogen signals working in cells. Another novel method, such as phosphorylation‐specific proteomics or the in vitro bioassay system described here will be needed to analyze the signaling pathway at work in individual cancer cells. The adenovirus ERE–GFP assay system and MCF‐7‐E10 reporter cells may be applicable to assess individual ER activity and the estrogen‐related microenvironment in individual breast cancers as diagnostic tools. These methods may also be useful to determine secondary therapy for patients refractory to hormonal therapy. However, further strict studies using a large number of cases will be needed before clinical application.
Acknowledgments
This study was supported in part by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan; a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labor, and Welfare, Japan; and a grant from the Smoking Research Foundation.
References
- 1. Kitajima I et al . Current topics in endocrine therapy for breast cancer. Breast Cancer 2008; 15: 253–90. [DOI] [PubMed] [Google Scholar]
- 2. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 3. Veronesi U, Cascinelli N, Greco M et al . A reappraisal of oophorectomy in carcinoma of the breast. Ann Surg 1987; 205: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayashi S. Prediction of hormone sensitivity by DNA microarray. Biomed Pharmacotherapy 2004; 58: 1–9. [DOI] [PubMed] [Google Scholar]
- 5. Inoue A, Yoshida N, Omoto Y et al . Development of cDNA microarray for expression profiling of estrogen‐responsive genes. J Mol Endocrinol 2002; 9: 175–92. [DOI] [PubMed] [Google Scholar]
- 6. Terasaka S, Aita Y, Inoue A et al . Expression profiling of the estrogen responsive genes for evaluation of estrogen activity among natural estrogens and industrial chemicals. Environ Health Persp Toxicogenomics 2004; 112: 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Omoto Y, Eguchi H, Yamaguchi Y, Hayashi S. Estrogen receptor (ER) β1 and ERβcx/β2 inhibit ERα function differently in breast cancer cell line MCF7. Oncogene 2003; 22: 5011–20. [DOI] [PubMed] [Google Scholar]
- 8. Inoue A, Omoto Y, Yamaguchi Y, Kiyama R, Hayashi S. Transcription factor EGR3 is involved in the estrogen‐signaling pathway in breast cancer cell. J Mol Endocrinol 2004; 32: 649–61. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida N, Omoto Y, Inoue A et al . Prediction of prognosis of estrogen receptor‐positive breast cancer with combination of selected estrogen‐regulated genes. Cancer Sci 2004; 95: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saji S, Hayashi S, Yoshida N et al . Therapeutic utility of histone deacetylase 6 regulation via estrogen signaling in human breast cancer MCF‐7. Oncogene 2005; 24: 4531–9. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Z, Yamashita H, Toyama T et al . HDAC6 expression is correlated with better prognosis in breast cancer. Clin Cancer Res 2004; 10: 6962–8. [DOI] [PubMed] [Google Scholar]
- 12. Mita K, Zhang Z, Ando Y et al . Prognostic significance of insulin‐like growth factor binding protein (IGFBP) ‐4 and IGFBP‐5 expression in breast cancer. Jpn J Clin Oncol 2007; 37: 575–82. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki T, Inoue A, Miki Y et al . Early growth responsive gene 3 (EGR3) in human breast carcinoma: a regulator of estrogen‐mediated invasion and a potent prognostic factor. Endocrine-Related Cancer 2007; 14: 279–92. [DOI] [PubMed] [Google Scholar]
- 14. Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 1994; 63: 451–86. [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto M, Yamaguchi Y, Seino Y et al . Estrogen signaling ability in human endometrial cancer through the cancer‐stromal interaction. Endocrine-Related Cancer 2008; 15: 451–63. [DOI] [PubMed] [Google Scholar]
- 16. Márquez DC, Pietras RJ. Membrane‐associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene 2001; 20: 5420–30. [DOI] [PubMed] [Google Scholar]
- 17. Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human cancer cells adapted to long‐term estrogen deprivation. Cancer Res 2005; 65: 3903–10. [DOI] [PubMed] [Google Scholar]
- 18. Santen RJ, Song RX, Masamura S et al . Adaptation to estradiol deprivation causes up‐regulaiton of growth factor pathways and hypersensitivity to estradiol in breast cancer cells. Adv Exp Med Biol 2008; 630: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yue W, Fan P, Wang J et al . Mechanisms of acquired resistance to endocrine therapy in hormone‐dependent breast cancer cells. J Steroid Biochem Mol Biol 2007; 106: 102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin L‐A, Farmer I, Johnston SRD, Ali S, Dowsett M. Elevated ERK1/ERK2/estrogen receptor cross‐talk enhances estrogen‐mediated signaling during long term estrogen deprivation. Endocrine-Related Cancer 2005; 12: S75–84. [DOI] [PubMed] [Google Scholar]
- 21. Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5α‐androstane‐3β,17β‐diol (3βAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat 2009; 115: 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pirog EC, Collins DC. Metablism of dihydrotestosterone in human liver: importance of 3α‐ and 3β‐hydroxysteroid dehydrogenase. Endocrinol Metab 1999; 84: 3217–21. [DOI] [PubMed] [Google Scholar]
- 23. Kuiper GGJM, Carlsson B, Grandien KC et al . Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 1997; 138: 863–70. [DOI] [PubMed] [Google Scholar]
- 24. Maggiolini M, Donzé O, Jeannin E, Andò S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor α. Cancer Res 1999; 59: 4864–9. [PubMed] [Google Scholar]
- 25. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature Rev Cancer 2006; 6: 392–401. [DOI] [PubMed] [Google Scholar]
- 26. Allinen M, Beroukhim R, Cai L et al . Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004; 6: 17–32. [DOI] [PubMed] [Google Scholar]
- 27. Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor‐promoting cell type. Cell Cycle 2006; 5: 1597–601. [DOI] [PubMed] [Google Scholar]
- 28. Yamaguchi Y. Microenvironmental regulation of estrogen signals in breast cancer. Breast Cancer 2007; 14: 175–81. [DOI] [PubMed] [Google Scholar]
- 29. O’Neill JS, Miller WR. Aromatase activity in breast adipose tissue from women with benign and malignant breast disease. Br J Cancer 1987; 56: 601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santen RJ, Santner SJ, Pauley RJ et al . Estrogen production via the aromatase enzyme in breast carcinoma: which cell type is responsible? J Steroid Biochem Mol Biol 1997; 61: 267–71. [PubMed] [Google Scholar]
- 31. Osborne CK, Shou J, Massarweh S et al . Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistancein breast cancer. Clin Cancer Res 2005; 11: 865s–870s. [PubMed] [Google Scholar]
- 32. Le Goff P, Montano MM, Schodin DJ et al . Phosphorylation of the human estrogen receptor. Identification of hormone‐regulated sites and examination of their influence on transcriptional activity. J Biol Chem 1994; 269: 4458–66. [PubMed] [Google Scholar]
- 33. Kato S, Endoh H, Masuhiro Y et al . Activation of the estrogen receptor through phosphorylation by mitogen‐activated protein kinase. Science 1995; 270: 1491–4. [DOI] [PubMed] [Google Scholar]
- 34. Hayashi S, Yamaguchi Y. Estrogen signaling and prediction of endocrine therapy. Cancer Chemother Pharmacol 2005; 56: 27–31. [DOI] [PubMed] [Google Scholar]
- 35. Yamaguchi Y, Takei H, Suemasu K et al . Tumor‐stromal interaction through the estrogen‐signaling pathway in human breast cancer. Cancer Res 2005; 65: 4653–62. [DOI] [PubMed] [Google Scholar]
- 36. Yamaguchi Y, Hayashi S. Estrogen‐related cancer microenvironment of breast cancer. Endocrine J 2009; 56: 1–7. [DOI] [PubMed] [Google Scholar]
- 37. Orimo A, Gupta PB, Sgroi DC et al . Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF‐1/CXCL12 secretion. Cell 2005; 121: 335–48. [DOI] [PubMed] [Google Scholar]
- 38. Hall JM, Korach KS. Stromal cell‐derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol 2003; 17: 792–803. [DOI] [PubMed] [Google Scholar]
- 39. Gaggioli C, Hooper S, Hidalgo‐Carcedo C et al . Fibroblast‐led collective invasion of carcinoma cells with differing roles for RhoGTPase in leading and following cells. Nature Cell Biol 2007; 9: 1392–400. [DOI] [PubMed] [Google Scholar]
- 40. Ishii G, Sangai T, Oda T et al . Bone‐marrow‐derived myofibroblasts contribute to the cancer‐induced stromal reaction. Biochem Biophys Res Commun 2003; 309: 232–40. [DOI] [PubMed] [Google Scholar]
- 41. Direkze NC, Hodivala‐Dilke K, Jeffery R et al . Bone marrow contribution to tumor‐associated myofibroblasts and fibroblasts. Cancer Res 2004; 64: 8492–5. [DOI] [PubMed] [Google Scholar]
- 42. Sorlie T, Perou CM, Tibshirani R et al . Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci 2001; 98: 10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


