Abstract
The aim of the present study was to estimate optimum chemotherapeutic regimens for high‐grade mature B‐cell lymphoma cases with Burkitt‐like morphology (Burkitt's lymphoma [BL]/Burkitt‐like lymphoma [BLL]) patients. We analyzed 72 BL/BLL, including 36 with the c‐myc translocation (molecular BL [mBL]), 20 without it (mBL‐like), and 16 in whom we were uncertain regarding the existence of the c‐myc translocation, and compared them with 182 diffuse large B‐cell lymphoma (DLBCL) cases. On clinical and immunophenotypic analysis, the typical BL immunophenotype (CD10 positive, bcl‐2 negative, and Ki‐67 index ≥95%) was noted in 23 (66%) and 11 (55%) of the 35 mBL and 20 mBL‐like patients, respectively. The presence of the c‐myc translocation and typical immunophenotype in BL did not affect the overall survival of BL/BLL. There were no significant differences between the overall survival of DLBCL (45%) and BL/BLL (50%, P = 0.85). However, the overall survival of BL/BLL patients who received cyclophosphamide, doxorubicin, vincristine, and prednisolone‐related therapy (22%) was significantly lower than that of DLBCL patients (P = 0.01). In contrast, the overall survival of BL/BLL patients who received aggressive short‐term chemotherapy (75%) was better than that of the patients who received cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy (P < 0.01). The finding was confirmed by multivariate analysis (hazard ratio 4.4; confidence interval 2.0–9.7; P = 0.0003). We concluded that aggressive short‐term chemotherapy improves survival in BL/BLL, regardless of its genetic and immunophenotypic features. (Cancer Sci 2008; 99: 246–252)
Burkitt's lymphoma (BL) is a subtype of highly aggressive mature B‐cell lymphomas that have a rapid proliferative rate. Histopathologically, BL is characterized by a diffuse monotonous pattern of medium‐sized cells with round nuclei and clumped chromatin, a ‘starry sky’ appearance with numerous macrophages, and a cohesive ‘jigsaw puzzle‐like’ pattern.
For many years it has been difficult to categorize some cases of high‐grade mature B‐cell lymphomas that exhibit Burkitt‐like morphology and intermediate clinical and histopathological characteristics between those of BL and diffuse large B‐cell lymphoma (DLBCL). In the Revised European–American Lymphoma classification published in 1994,( 1 ) Burkitt‐like lymphoma (BLL) was proposed to be an independent subtype of highly aggressive B‐cell lymphomas showing intermediate clinical and immunophenotypic features between BL and DLBCL. Other terms used for BLL include ‘undifferentiated non‐Burkitt's lymphoma’ in the Rappaport classification and ‘small non‐cleaved non‐Burkitt's lymphoma (SNC‐NB)’ in the Working Formulation.( 2 )
Because the histopathological classification of high‐grade mature B‐cell lymphomas with Burkitt‐like morphology has low reproducibility and concordance rate between diagnosticians, BLL is defined as a variant of BL, and the c‐myc translocation is necessary for the diagnosis of BLL in the new World Health Organization (WHO) classification.( 3 ) However, several groups have indicated the existence of BL (or BLL) cases without the c‐myc translocation, and the percentage of BLL cases with the c‐myc translocation varies from 41 to 80%.( 4 , 5 , 6 ) Yano et al. analyzed 11 cases of SNC‐NB and reported that none of them had the c‐myc translocation.( 7 ) Furthermore, the c‐myc translocation has occasionally been detected in other types of B‐cell lymphomas,( 6 , 8 ) indicating that it is not specific to BL. In addition, Spina et al. demonstrated that cell proliferation in BL is not controlled solely by c‐myc; it depends on other genes and alterations as well.( 9 ) Other investigators argued that additional genomic changes could be involved in the biology of BL.( 9 , 10 )
The typical BL immunophenotype exhibits expression of the B‐cell‐associated antigen CD10, a proliferation (Ki‐67) index of nearly 100%, and lack of expression of bcl‐2 and TdT. CD10 expression indicates that the lymphoma cells in BL originate from the follicular center. The lack of expression of bcl‐2 reflects enhanced apoptosis, whereas the overexpression of MIB‐1 (Ki‐67) signifies rapid cell proliferation and increased mitosis in BL. Thus, the high rates of apoptosis and mitosis are morphologically compatible with the typical ‘starry sky’ appearance in BL. However, in BLL, the expression of MIB‐1, bcl‐2, and CD10 varies among reports.( 4 , 5 )
Recently, the survival rates of patients with BL or BLL, particularly children and adolescents, have improved following the use of aggressive short‐term chemotherapy.( 11 , 12 ) However, optimum chemotherapeutic regimens for high‐grade mature B‐cell lymphomas with Burkitt‐like morphology lacking the c‐myc translocation have not yet been well established because only a few studies have investigated the relationship between the clinical features and the c‐myc translocation in high‐grade mature B‐cell lymphomas with Burkitt‐like morphology.
To estimate optimum chemotherapeutic regimens for high‐grade mature B‐cell lymphomas with Burkitt‐like morphology patients, we retrospectively analyzed the clinical, genetic, and immunophenotypic features and the overall survival of 72 cases with high‐grade mature B‐cell lymphoma with Burkitt‐like morphology and compared them with those of 182 cases of DLBCL in the present study.
Patients and Methods
Patient selection and material. We selected 72 BL/BLL patients and 182 DLBCL patients diagnosed between 1980 and 2005 from the list of lymphoma files in the Departments of Pathology of Fukuoka University and Kurume University. We had previously reported some patients.( 13 , 14 , 15 ) In the current study, DLBCL patients were reviewed by four experienced hematopathologists (T. Y., K. O., N. S., and M. K.) according to the criteria of the new WHO classification. Biopsy was carried out after obtaining informed consent from all patients. None of the cases included in the present study had acquired immunodeficiency syndrome. All clinical and laboratory data of the patients along with the follow‐up data were obtained from the medical records at each institution.
Morphological analysis. The histopathological diagnoses of all cases were obtained solely on the basis of the examination of hematoxylin–eosin‐stained sections with no other additional data; these were reviewed by the abovementioned four experienced hematopathologists according to the morphological criteria of the new WHO classification, namely, a diffuse monotonous growth pattern composed of medium‐sized cells with round nuclei, clumped chromatin, multiple medium‐sized nucleoli, and basophilic cytoplasm. A ‘starry sky’ pattern is generally present, owing to the presence of numerous benign macrophages and is associated with an extremely high rate of both cell proliferation and apoptosis (Fig. 1a,b). Atypical Burkitt's/Burkitt‐like lymphoma (BLL) is defined as a variant of BL. In contrast to classical BL, the morphology of this variant includes greater pleomorphism in nuclear size and shape with fewer and more prominent nucleoli. In the present study, we regarded both BL and BLL as high‐grade mature B‐cell lymphoma with Burkitt‐like morphology (BL/BLL), which had a ‘starry sky’ pattern and many mitotic figures.
Figure 1.
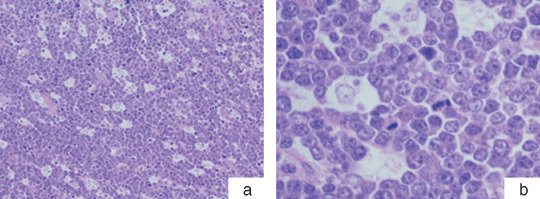
Classical Burkitt's lymphoma. (a) Hematoxylin–eosin‐stained paraffin sections of the lymphoma show a ‘starry sky’ pattern on examination under low power (100× magnification). (b) The tumor is composed of medium‐sized cells with monomorphous round nuclei, clumped chromatin, and indistinct nucleoli when viewed under high power (400× magnification).
Immunohistochemical staining. Paraffin sections from each specimen were immunostained using monoclonal antibodies against CD10 (Novocastra Laboratories, Newcastle upon Tyne, UK), bcl‐2 (DakoCytomation, Glostrup, Denmark), Ki‐67 (MIB‐1; Immunotech, Hialeah, FL, USA), and TdT (Supertec, Bethesda, MD, USA) (Fig. 2a–c). All immunohistochemical reactions and analyses were carried out in a single laboratory, as described previously.( 14 )
Figure 2.
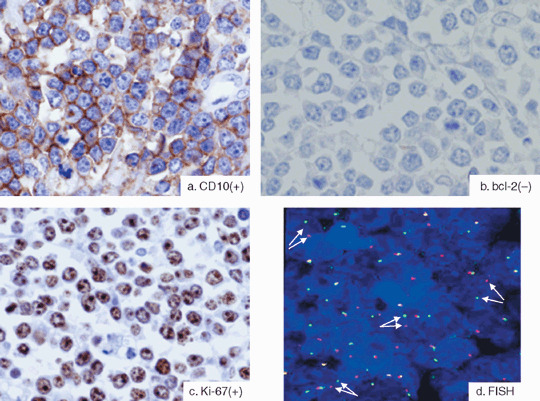
(a–c) Typical immunophenotype of Burkitt's lymphoma. Burkitt's lymphomas typically exhibit the following immunophenotype: (a) CD10 positive, (b) bcl‐2 negative, and (c) Ki‐67 positive in almost 100% cases (Ki‐67 index ≥95%). (d) Burkitt's lymphoma showing the c‐myc translocation by fluorescence in situ hybridization (FISH). To detect the c‐myc translocation by FISH analysis, we used the LSI MYC (8q24) Dual Color Break Apart Rearrangement probe. In the nuclei of normal cells, the probe generally appears as two distinct orange and green fusion signals. If the nuclei have the c‐myc translocation, the signals appear to have split (arrows).
Immunohistological scoring. The percentages of cells that stained positive for Ki‐67 were averaged to yield an immunohistological score of 0–100% (MIB‐1 index). With regard to CD10 expression, the following two categories were defined: negative (positively stained tumor cells <20%) and positive (positively stained tumor cells ≥20%). With regard to bcl‐2 expression, the categorization was carried out as follows: negative (positively stained tumor cells <40%) and positive (positively stained tumor cells ≥40%). With regard to TdT expression, we defined two categories as follows: negative (positively stained tumor cells <10%) and positive (positively stained tumor cells ≥10%). The criteria were defined according to individual antibody types due to the differences in staining with these antibodies.
Cytogenetic and molecular genetic analysis. We used the LSI MYC Dual Color Break Apart Rearrangement probe (Vysis, Downers Grove, IL, USA) to detect the c‐myc translocation (Fig. 2d) by carrying out routine fluorescence in situ hybridization (FISH) analysis on frozen and paraffin sections, as described previously.( 16 , 17 ) We divided the patients with high‐grade mature B‐cell lymphomas with Burkitt‐like morphology into molecular BL (mBL) or mBL‐like groups according to the presence of the c‐myc translocation: the former included patients in whom the c‐myc translocation was detected, and the latter included patients in whom the c‐myc translocation was not detected.
Statistical analysis. Survival curves were constructed using the Kaplan–Meier method (SAS system; SAS Institute, Cary, NC, USA), and the level of significance of the differences in the survival rates were tested using the generalized log‐rank test. Correlations between the two groups were examined using the χ2‐test and Student's t‐test. Differences were considered significant if the P‐value was < 0.05. Univariate and multivariate analyses were carried out using the Cox proportional hazard regression model, and variables were selected by the stepwise method. Data were analyzed using STATA statistical software version 9.1 (StataCorp, College Station, TX, USA).
Clinical analysis. All relevant data were available, including the individual risk factors that constitute the International Prognostic Index (IPI) (age, clinical stage, performance status, lactate dehydrogenase level, and the number of extranodal disease sites). The IPI score ranged from 0 to 5, and based on this, the cases were classified into low‐risk (IPI score 0–2) and high‐risk (IPI score 3–5) categories. Clinical staging was based on the Ann Arbor classification. With regard to treatment, we divided the patients with high‐grade mature B‐cell lymphomas with Burkitt‐like morphology into two groups, namely, A and B. Group A included patients treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), which is the standard chemotherapeutic regimen for DLBCL, or with the modified CHOP regimen. Group B included patients treated with intensive short‐term chemotherapeutic regimens used in children with BL, for instance, cyclophosphamide, vincristine, doxorubicin, and high‐dose methotrexate (MTX), and ifosfamide, etoposide, and high‐dose cytarabine. The characteristics of the patients in groups A and B are shown in Table 1. The median age of the group B patients was lower than that of the group A patients and, compared to group A, the number of patients in group B under 16 years of age was significantly greater. Central nervous system (CNS) or bone marrow involvement was significantly more frequent in the patients of group A than in those of group B. There were no significant differences between group A and B patients with regard to other clinical characteristics. Immunohistochemical analysis revealed that compared to mBL‐like patients, a significantly greater number of mBL patients were CD10 positive
Table 1.
| Characteristic | Group A (n = 30) | Group B (n = 39) | P‐value |
|---|---|---|---|
| Median age (years) | 69 | 10 | |
| Median age range (years) | 14–88 | 3–60 | |
| Child ≤15 years | 2 (6.7%) | 27 (69.2%) | <0.001 |
| Sex | |||
| Male | 16 (53%) | 23 (59%) | |
| Female | 14 (47%) | 16 (41%) | 0.639 |
| Stage | |||
| I/II | 12 (40%) | 16 (41%) | |
| III/VI | 18 (60%) | 23 (59%) | 0.931 |
| IPI § score | |||
| High risk | 20 (67%) | 21 (54%) | |
| Low risk | 10 (33%) | 18 (46%) | 0.282 |
| Site | |||
| Nodal | 14 (47%) | 13 (33%) | |
| Extranodal | 16 (53%) | 26 (67%) | 0.26 |
| Central nervous system or bone marrow involvement | 4 (13%) | 18 (46%) | 0.004 |
| c‐Myc translocation ¶ | 14 (54%) | 22 (76%) | 0.086 |
| Typical BL immunophenotype †† | 16 (57%) | 28 (74%) | 0.159 |
Patients with high‐grade B cell lymphoma with Burkitt‐like morphology (BL/BLL) treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP), or the modified CHOP regimen.
Patients with BL/BLL treated with intensive short‐term chemotherapeutic regimen.
The International Prognostic Index (IPI) (age, clinical stage, performance status, lactate dehydrogenase level, and the number of extranodal disease sites): low risk (score 0–2) and high risk (score 3–5).
We were able to analyze the presence of the c‐myc translocation in 26 of 30 group A patients and 29 of 39 group B patients.
CD10 positive + bcl‐2 negative + MIB‐1 index ≥ 95%. We were able to analyze theimmunohistochemical findings of 28 group A patients and 38 group B patients for typical BL immunophenotype.
Results
Comparison between BL/BLL and DLBCL.
Patient characteristics The characteristics of patients with DLBCL and BL/BLL are shown in Table 2. The patients with BL/BLL were younger than those with DLBCL, and clinical stage was significantly more progressive in the former than in the later. High‐risk IPI score, more than one site of extranodal disease, and the c‐myc translocation were significantly more frequent in patients with BL/BLL than in those with DLBCL. Immunohistochemical analysis showed that compared to DLBCL patients, a significantly higher percentage of BL/BLL patients were positive for CD10. Conversely, a significantly lower percentage of BL/BLL patients were positive for bcl‐2 compared with the DLBCL patients.
Table 2.
Characteristics of patients with diffuse large B cell lymphoma (DLBCL) and high‐grade B cell lymphoma with Burkitt‐like morphology (BL/BLL)
| Characteristic | DLBCL | BL/BLL | P‐value |
|---|---|---|---|
| Age | n = 156‡ | n = 72 | |
| Median age (years) | 63 | 46 | |
| Median age range (years) | 0–87 | 3–88 | |
| Child ≤15 years | 5 (3.2%) | 29 (40%) | <0.001 |
| Senior ≥60 years | 93 (60%) | 26 (36%) | <0.001 |
| Sex | n = 156 ‡ | n = 72 | 0.61 |
| Male | 81 (52%) | 40 (56%) | |
| Female | 75 (48%) | 32 (44%) | |
| Stage | n = 161 ‡ | n = 71 ‡ | 0.09 |
| I/II | 85 (53%) | 29 (41%) | |
| III/VI | 76 (47%) | 42 (59%) | |
| IPI † score | n = 161 ‡ | n = 70 ‡ | 0.006 |
| High risk | 65 (40%) | 42 (60%) | |
| Low risk | 96 (60%) | 28 (40%) | |
| Extranodal disease | n = 161 ‡ | n = 72 | 0.009 |
| ≥2 sites | 64 (40%) | 42 (58%) | |
| c‐Myc translocation | n = 137 § | n = 56§ | <0.001 |
| Positive | 14 (10%) | 36 (64%) | |
| Negative | 123 (90%) | 20 (36%) | |
| CD10 | n = 138 ¶ | n = 67 ¶ | <0.001 |
| Positive | 39 (28%) | 57 (85%) | |
| Negative | 99 (72%) | 10 (15%) | |
| bcl‐2 | n = 158 ¶ | n = 70 ¶ | <0.001 |
| Positive | 90 (57%) | 15 (21%) | |
| Negative | 68 (43%) | 55 (79%) |
The International Prognostic Index (IPI) (age, clinical stage, performance status, lactate dehydrogenase level, and the number of extranodal disease sites): low risk (score 0–2) and high risk (score 3–5).
We were able to obtain clinical information of 156 (age and sex) and 161 (stage, IPI score, and extranodal disease) of 182 DLBCL, and 71 (stage) and 70 (IPI score) of BL/BLL.
We were able to analyze the presence of the c‐myc translocation in 137 DLBCL and 56 of 72 BL/BLL.
We were able to analyze the immunohistochemical findings for 138 DLBCL and 67 BL/BLL for CD10, and for 158 DLBCL and 70 BL/BLL for bcl‐2.
Overall survival and response to chemotherapy. Clinical follow‐up data were obtained from 182 patients with DLBCL and from 72 with BL/BLL. In the living patients, the median follow‐up periods were 14.2 and 16.5 months for DLBCL and BL/BLL, respectively. Univariate analysis showed no significant difference between the overall survival in DLBCL (45%) and that in BL/BLL (50%) (P = 0.85; Fig. 3a). Furthermore, we found that 30 (43%) of the 69 patients with BL/BLL received CHOP or CHOP‐like chemotherapy (group A). We compared these patients (i.e. group A) (Fig. 3b) with the 182 DLBCL patients (Fig. 3a) in order to eliminate the disparity with regard to the intensity of treatment. Patients belonging to group A showed a significantly poorer prognosis (overall survival, 22%) than the DLBCL patients (P = 0.01).
Figure 3.
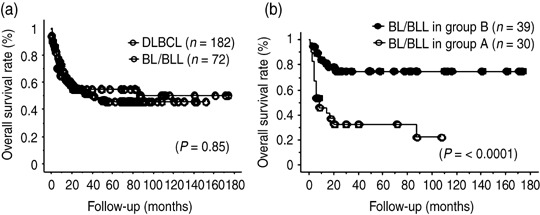
Comparison between high‐grade mature B‐cell lymphomas with Burkitt‐like morphology (BL/BLL) and diffuse large B‐cell lymphoma (DLBCL) with respect to overall survival rate and response to chemotherapy. (a) Overall survival rate of DLBCL and BL/BLL. (b) Overall survival rate of BL/BLL patients in group A, who were treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or modified CHOP, and group B, who were treated with highly intensive short‐term chemotherapeutic regimens.
Overall survival of high‐grade BL/BLL.
Clinical characteristics We analyzed the overall survival based on various clinical parameters, such as age, sex, CNS or bone marrow involvement, IPI, and treatment modality. There were significant differences in overall survival with respect to age (≤15 years, 75%; >15 years, 27%; P = 0.002), IPI score (low, 83%; high, 39%; P = 0.002), and treatment modality (group A, 22%; group B, 75%; P < 0.0001) (Fig. 3b). However, sex and CNS or bone marrow involvement did not influence overall survival.
To define the role of the treatment regimen based on the parameters that showed a significant influence on overall survival, we compared the overall survival of BL/BLL patients aged >15 years in group A with those in group B; we also compared the overall survival of the patients with low IPI scores and those with high IPI scores (Fig. 4a,b). The overall survival of patients aged >15 years in group B (72%) was significantly better than that in group A (20%, P = 0.03). Because most patients aged 15 years belonged to group B (group A, two patients; group B, 27 patients), we could not analyze their overall survival. With regard to IPI, the overall survival was significantly better in the high‐risk patients in group B (65%) than in the high‐risk patients in group A (12%, P = 0.0004). The overall survival tended to be better in the low‐risk patients in group B (88%) than in the low‐risk patients in group A (70%); however, the difference was not significant (P = 0.172).
Figure 4.
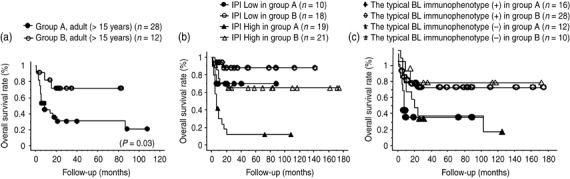
Overall survival rate of patients with high‐grade mature B‐cell lymphomas with Burkitt‐like morphology (BL/BLL) based on clinical parameters, immunohistochemical findings, and intensity of treatment. (a) Overall survival rate of adult BL/BLL patients (aged >15 years) treated with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or modified CHOP (group A) and treated with highly intensive short‐term chemotherapeutic regimens (group B). (b) Overall survival rate of BL/BLL patients with low and high International Prognostic Index (IPI) scores in treatment groups A and B. (c) Overall survival rate of patients with BL/BLL with regard to results of immunohistochemical examination for the occurrence of the typical BL immunophenotype (CD10 positive, bcl‐2 negative, and Ki‐67 index = 95%) in treatment with CHOP or modified CHOP (group A) and with highly intensive short‐term chemotherapeutic regimens (group B).
Immunohistochemical findings.
Based on the occurrence of the typical BL immunophenotype, we analyzed the overall survival of 68 BL/BLL patients whose clinical follow‐up data could be obtained. There was no significant difference in overall survival on immunohistochemical examination (typical BL immunophenotype positive, 58%; typical BL immunophenotype negative, 40%; P = 0.70).
To eliminate the disparity caused by the use of different treatment modalities, we compared the overall survival in BL/BLL treatment groups A and B on the basis of the result of immunohistochemical examination (Fig. 4c) and found that there was no significant difference in overall survival between the two groups (group A typical BL immunophenotype positive, 35%; group A typical BL immunophenotype negative, 18%; P = 0.58; group B typical BL immunophenotype positive, 72%; group B typical BL immunophenotype negative, 78%; P = 0.65). Although there was no significant difference, overall survival tended to be better for group B patients compared with group A patients.
c‐Myc translocation (review and overall survival of mBL and mBL‐like patients). Table 3 shows the clinical characteristics of mBL and mBL‐like patients: age, sex, site, CNS or bone marrow involvement, IPI score, treatment modality, and the result of immunohistochemical staining. The median age of the mBL patients was lower than that of the mBL‐like patients and, compared to mBL‐like patients, the number of patients with mBL under 16 years of age was significantly greater. There were no significant differences between mBL and mBL‐like patients with regard to other clinical characteristics. Immunohistochemical analysis revealed that compared to mBL‐like patients, a significantly greater number of mBL patients were CD10 positive. We could not detect significant differences between mBL and mBL‐like patients with respect to the occurrence of the typical BL immunophenotype (CD10 positive, bcl‐2 negative, and MIB‐1 index ≥95%).
Table 3.
Characteristics of patients with classified high‐grade B cell lymphoma with Burkitt‐like morphology according to the presence of c‐myc translocation
| Characteristic | c‐Myc+ (mBL) | c‐Myc− (mBL‐like) | P‐value |
|---|---|---|---|
| Age | n = 36 | n = 20 | |
| Median (range) | 22 years (3–81) | 58 years (5–88) | |
| Child ≤15 years | 17 (47.2%) | 4 (20%) | 0.043 |
| Sex | n = 36 | n = 20 | 1 |
| Male | 18 (50%) | 10 (50%) | |
| Female | 18 (50%) | 10 (50%) | |
| Site | n = 36 | n = 20 | 0.311 |
| Nodal | 13 (36%) | 10 (50%) | |
| Extranodal | 23 (64%) | 10 (50%) | |
| CNS or bone marrow involvement | n = 36 | n = 19 ¶ | 0.451 |
| Positive | 11 (31%) | 4 (21%) | |
| Negative | 25 (69%) | 15 (79%) | |
| IPI † score | n = 36 | n = 19 ¶ | 0.685 |
| High risk | 21 (58%) | 10 (53%) | |
| Low risk | 15 (42%) | 9 (47%) | |
| Treatment ‡ | n = 36 | n = 19 ¶ | 0.086 |
| Group A | 14 (39%) | 12 (63%) | |
| Group B | 22 (61%) | 7 (37%) | |
| CD10 | n = 34 †† | n = 20 | 0.016 |
| Positive | 31 (91%) | 13 (65%) | |
| bcl‐2 | n = 36 | n = 20 | 0.197 |
| Negative | 29 (81%) | 13 (65%) | |
| MIB‐1 index | n = 36 | n = 20 | 0.87 |
| ≥95% | 30 (83%) | 17 (85%) | |
| Typical BL immunophenotype § | n = 35 †† | n = 20 | 0.431 |
| Positive | 23 (66%) | 11 (55%) |
The International Prognostic Index (IPI) (age, clinical stage, performance status, lactate dehydrogenase level, and the number of extranodal disease sites): low‐risk (score 0–2) and high‐risk (score 3–5).
Treatment: Group A included patients treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), or the modified CHOP regimen. Group B included patients treated with intensive short‐term chemotherapeutic regimen.
CD10 positive + bcl‐2 negative + MIB‐1 index ≥95%.
We were able to obtain clinical information of CNS or bone marrow involvement, IPI, and treatment modality from 19 of 20 mBL‐like.
We were able to analyze the immunohistochemical findings of 34 of 36 mBL for CD10, and 35 mBL for typical BL immunophenotype. CNS, central nervous system.
To estimate the effect of c‐myc translocation on BL/BLL, we compared the overall survival of mBL and mBL‐like patients (Fig. 5a); however, no significant difference was observed between the overall survival of the two groups with respect to the c‐myc translocation (mBL, 52%; mBL‐like, 62%; P = 0.70).
Figure 5.
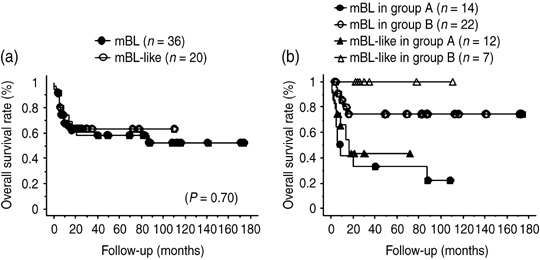
Overall survival rate and response to chemotherapy in patients of molecular Burkitt's lymphoma (mBL; high‐grade mature B‐cell lymphomas with Burkitt‐like morphology [BL/BLL] in which c‐myc translocation was present) and mBL‐like (c‐myc translocation was absent). (a) Overall survival rate of BL/BLL with regard to the presence of the c‐myc translocation. (b) Overall survival rate of BL/BLL with regard to the presence of the c‐myc translocation in treatment with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or modified CHOP (group A) and with highly intensive short‐term chemotherapeutic regimens (group B).
To eliminate the disparity caused by the use of different treatment modalities, we compared the overall survival of mBL cases with that of mBL‐like cases in the treatment groups A and B (Fig. 5b). However, in group A, the overall survival of mBL patients (22%) did not differ significantly from that of the mBL‐like patients (44%, P = 0.49). Because all of the group B patients who died had the c‐myc translocation, we could not make comparisons in this group (overall survival of mBL patients, 74%). Although there was no significant difference in overall survival based on the presence of the c‐myc translocation, the overall survival was better for group B patients compared with group A patients, similar to the trend observed by immunohistochemical examination for the occurrence of the typical BL phenotype.
Multivariate analysis Univariate Cox analysis identified the following prognostic factors affecting overall survival: age, performance status (PS), type of chemotherapy, and IPI (Table 4). Multivariate analysis excluding the IPI categories showed that the chemotherapeutic regimen followed (CHOP or modified CHOP) and PS >1 were significant and independent prognostic factors (Table 4).
Table 4.
Prognostic factors affecting overall survival of high‐grade B‐cell lymphoma with Burkitt‐like morphology
| Variable | Unfavorable factors | Univariate | Multivariate † | ||
|---|---|---|---|---|---|
| Hazard ratio (CI) | P | Hazard ratio (CI) | P | ||
| Comparison with risk factors | |||||
| Chemotherapy | CHOP therapy | 4.4 (2.0–9.6) | 0.0003 | 4.4 (2.0–9.7) | 0.0003 |
| PS | 2–4 | 2.5 (1.2–5.1) | 0.01 | 2.3 (1.1–5.0) | 0.03 |
| Age | >60 years | 2.7 (1.4–5.5) | 0.004 | – | |
| LDH | >Normal | 4.2 (1.00–17.5) | 0.05 | – | |
| Stage | III/IV | 1.6 (0.75–3.3) | 0.23 | – | |
| Extranodal disease | ≥2 sites | 1.9 (1.1–3.5) | 0.35 | – | |
| CNS/BM involvement | Positive | 1.3 (0.63–2.6) | 0.48 | – | |
| Sex | Male | 1.2 (0.60–2.4) | 0.6 | – | |
| c‐Myc | Rearranged | 1.2 (0.48–2.9) | 0.71 | – | |
| Immunophenotype | Atypical | 1.1 (0.55–2.4) | 0.71 | – | |
| Comparison with IPI category | |||||
| Chemotherapy | Conventional | 4.4 (2.0–9.6) | 0.0003 | 3.7 (1.6–8.2) | 0.002 |
| IPI category | H‐I/H | 3.1 (1.5–6.6) | 0.01 | 2.3 (1.03–4.9) | 0.04 |
Final model. BM, bone marrow; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; CI, confidence interval; CNS, central nervous system; H, high; H‐I, high–intermediate; IPI, International Prognostic Index; LDH, lactate dehydrogenase; PS, performance status.
When the IPI score was considered instead of its constitutive factors, the chemotherapeutic regimen followed (relative risk [RR] = 3.7; confidence interval [CI], 1.6–8.2; P = 0.002) and IPI score (RR = 2.3; CI, 1.03–4.9; P = 0.04) were identified as independent and significant prognostic factors.
Discussion
The classification of high‐grade B‐cell lymphoma has undergone changes over the last several decades because distinction between BL, BLL, and DLBCL based on histopathological features is often difficult. A number of investigators have demonstrated that the results of immunohistochemical and genetic analyses could provide valuable information with regard to the pathological diagnosis of BL, BLL, and DLBCL.( 4 , 5 , 8 , 18 , 19 ) The new WHO classification defines cases with BL and BLL based on the presence of the c‐myc translocation. Therefore, BL/BLL without the c‐myc translocation, corresponding to the 20 ‘mBL‐like’ patients in the present study (including the nine patients who did not exhibit the typical immunohistochemical findings or possess the c‐myc translocation) may be categorized and subsequently treated as DLBCL. However, in the present study, the BL/BLL patients who underwent short‐term aggressive chemotherapy as is prescribed for BL showed better overall survival than the cases who received CHOP or CHOP‐like chemotherapy, regardless of the presence of the c‐myc translocation.
Furthermore, we analyzed the overall survival of the group of BL/BLL patients in whom we were uncertain regarding the presence of the c‐myc translocation (n = 16; overall survival, 35%). Even in this group, better overall survival was noted in the patients who received short‐term aggressive chemotherapy than in those who received CHOP or CHOP‐like chemotherapy (data not shown). This suggests the possibility that confirmation of the presence of the c‐myc translocation is not essential for the treatment of BL/BLL.
The c‐myc oncogene plays a central role in the transcriptional regulation of a set of downstream genes that control diverse cellular processes, including cell cycle progression and programmed cell death (apoptosis).( 20 ) Therefore, translocation of c‐myc is considered to induce rapid cell proliferation leading to a highly aggressive clinical course. Au et al. studied 87 cases with non‐Hodgkin's lymphoma (BL, BLL, follicular lymphoma, DLBCL, and mantle cell lymphoma) and concluded that in all histological groups, the presence of the 8q24 aberration had a significant influence on poor prognosis.( 21 ) However, we did not detect a significant relationship between the overall survival of BL/BLL and the presence of the c‐myc translocation.
Hummel et al.( 10 ) and Dave et al.( 22 ) proposed the concept of ‘molecular Burkitt's lymphoma’ in their literature; in such cases, the molecular signature of BL can be identified by gene expression profiling. They described that molecular BL patients treated with intensive regimens had a better prognosis than those treated with CHOP‐like regimens.( 22 ) Hummel et al. indicated the existence of patients with molecular BL but without the c‐myc translocation. There is a possibility that the ‘mBL‐like’ patients in our study could belong to this category.( 10 )
In general, the outcomes of childhood BL and BLL have improved following the introduction of highly aggressive short‐term chemotherapy. In contrast, adults and elderly patients cannot tolerate the adverse events of aggressive chemotherapy, such as drug toxicities and severe infections. However, several studies have recently reported high cure rates following the use of similar chemotherapeutic protocols in adults (particularly those less than 60 years of age) with BL or Burkitt's leukemia.( 23 , 24 ) Our results also showed improved overall survival after short‐term aggressive chemotherapy in adults (>15 years of age) with BL/BLL.
Our results showed that compared to DLBCL, a higher proportion of BL/BLL cases had a high MIB‐1 index and were negative for bcl‐2, as reported previously.( 4 , 5 , 8 , 18 ) These results suggest that both BL and BLL had enhanced cell proliferation and apoptosis and are compatible with the typical ‘starry sky’ appearance in these cases. High‐dose MTX is one of the key drugs used in the short‐term aggressive chemotherapeutic regimen. MTX is a folate antagonist and competitively inhibits dihydrofolate reductase; thus, it is known to interfere with several biosynthetic pathways, such as the synthesis of DNA, RNA, and proteins, which require the presence of reduced folate cofactors.( 25 ) MTX functions specifically in the S phase of cell kinetics and is known to induce cell death through apoptosis.( 26 ) Thus, the use of aggressive short‐term chemotherapy is effective against tumors that show rapid cell proliferation because it induces apoptosis of the tumor cells in concordance with tumor cell growth, particularly in tumors with enhanced apoptosis. In contrast, in DLBCL, which exhibits a sluggish growth rate and a slow cell cycle, low‐intensity chemotherapy based on the CHOP or CHOP‐like regimen could be effective when used over a longer duration. Braziel et al. have suggested that BLL represents a high‐grade lymphoma, which is much more similar to BL than to DLBCL.( 4 ) The ‘starry sky’ appearance, which is detected in both BL and BLL, reflects both rapid growth kinetics and the high rate of apoptotic tumor‐cell death.
In the present study, BL/BLL patients who received aggressive short‐term chemotherapy showed better overall survival than those who received CHOP or CHOP‐like chemotherapy, regardless of the genetic and immunohistochemical findings; moreover, by multivariate analysis, we confirmed that the type of chemotherapeutic regimen is an independent and significant prognostic factor. To verify our result, a prospective study is needed in the future.
We conclude that patients with BL/BLL should be treated with the same chemotherapeutic regimen that is used for BL, irrespective of their age, IPI, or the presence of the c‐myc translocation, provided their general condition permits aggressive chemotherapy.
Acknowledgments
This study was supported in part by a Grant‐in‐Aid for Exploratory Research, Japan.
References
- 1. Harris NL, Jaffe ES, Stein H et al . A revised European–American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994; 84: 1361–92. [PubMed] [Google Scholar]
- 2. The Non‐Hodgkin's Lymphoma Pathologic Classification Project . National Cancer Institute sponsored study of classifications of non‐Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. Cancer 1982; 49: 2112–35. [DOI] [PubMed] [Google Scholar]
- 3. Diebold JE, Raphael M, Warnke RA. Burkitt lymphoma. In: Jaffe ESHN, Stein H, Vardiman JW, eds. Pathology and Genetics of Tumours Haematopoietic and Lymphoid Tissues: World Health Organization Classification of Tumours. Lyon: IARC Press, 2001. [Google Scholar]
- 4. Braziel RM, Arber DA, Slovak ML et al . The Burkitt‐like lymphomas: a Southwest Oncology Group study delineating phenotypic, genotypic, and clinical features. Blood 2001; 97: 3713–20. [DOI] [PubMed] [Google Scholar]
- 5. McClure RF, Remstein ED, Macon WR et al . Adult B‐cell lymphomas with Burkitt‐like morphology are phenotypically and genotypically heterogeneous with aggressive clinical behavior. Am J Surg Pathol 2005; 29: 1652–60. [DOI] [PubMed] [Google Scholar]
- 6. Macpherson N, Lesack D, Klasa R et al . Small noncleaved, non‐Burkitt's (Burkitt‐like) lymphoma: cytogenetics predict outcome and reflect clinical presentation. J Clin Oncol 1999; 17: 1558–67. [DOI] [PubMed] [Google Scholar]
- 7. Yano T, Van Krieken JH, Magrath IT, Longo DL, Jaffe ES, Raffeld M. Histogenetic correlations between subcategories of small noncleaved cell lymphomas. Blood 1992; 79: 1282–90. [PubMed] [Google Scholar]
- 8. Nakamura N, Nakamine H, Tamaru J et al . The distinction between Burkitt lymphoma and diffuse large B‐cell lymphoma with c‐myc rearrangement. Mod Pathol 2002; 15: 771s6. [DOI] [PubMed] [Google Scholar]
- 9. Spina D, Leoncini L, Megha T et al . Cellular kinetic and phenotypic heterogeneity in and among Burkitt's and Burkitt‐like lymphomas. J Pathol 1997; 182: 145–50. [DOI] [PubMed] [Google Scholar]
- 10. Hummel M, Bentink S, Berger H et al . A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med 2006; 354: 2419–30. [DOI] [PubMed] [Google Scholar]
- 11. Cairo MS, Sposto R, Perkins SL et al . Burkitt's and Burkitt‐like lymphoma in children and adolescents: a review of the Children's Cancer Group experience. Br J Haematol 2003; 120: 660–70. [DOI] [PubMed] [Google Scholar]
- 12. Schwenn MR, Blattner SR, Lynch E, Weinstein HJ. HiC‐COM: a 2‐month intensive chemotherapy regimen for children with stage III and IV Burkitt's lymphoma and B‐cell acute lymphoblastic leukemia. J Clin Oncol 1991; 9: 133–8. [DOI] [PubMed] [Google Scholar]
- 13. Kawasaki C, Ohshim K, Suzumiya J et al . Rearrangements of bcl‐1, bcl‐2, bcl‐6, and c‐myc in diffuse large B‐cell lymphomas. Leuk Lymphoma 2001; 42: 1099–106. [DOI] [PubMed] [Google Scholar]
- 14. Ohshima K, Kawasaki C, Muta H et al . CD10 and bcl10 expression in diffuse large B‐cell lymphoma: CD10 is a marker of improved prognosis. Histopathology 2001; 39: 156–62. [DOI] [PubMed] [Google Scholar]
- 15. Zhang A, Ohshima K, Sato K et al . Prognostic clinicopathologic factors, including immunologic expression in diffuse large B‐cell lymphomas. Pathol Int 1999; 49: 1043–52. [DOI] [PubMed] [Google Scholar]
- 16. Li JY, Gaillard F, Moreau A et al . Detection of translocation t (11;14)(q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization. Am J Pathol 1999; 154: 1449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kodama T, Ohshima K, Nomura K et al . Lymphomatous polyposis of the gastrointestinal tract, including mantle cell lymphoma, follicular lymphoma and mucosa‐associated lymphoid tissue lymphoma. Histopathology 2005; 47: 467–78. [DOI] [PubMed] [Google Scholar]
- 18. Frost M, Newell J, Lones MA, Tripp SR, Cairo MS, Perkins SL. Comparative immunohistochemical analysis of pediatric Burkitt lymphoma and diffuse large B‐cell lymphoma. Am J Clin Pathol 2004; 121: 384–92. [DOI] [PubMed] [Google Scholar]
- 19. Haralambieva E, Boerma EJ, Van Imhoff GW et al . Clinical, immunophenotypic, and genetic analysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol 2005; 29: 1086–94. [PubMed] [Google Scholar]
- 20. Hecht JL, Aster JC. Molecular biology of Burkitt's lymphoma. J Clin Oncol 2000; 18: 3707–21. [DOI] [PubMed] [Google Scholar]
- 21. Au WY, Horsman DE, Gascoyne RD, Viswanatha DS, Klasa RJ, Connors JM. The spectrum of lymphoma with 8q24 aberrations: a clinical, pathological and cytogenetic study of 87 consecutive cases. Leuk Lymphoma 2004; 45: 519–28. [DOI] [PubMed] [Google Scholar]
- 22. Dave SS, Fu K, Wright GW et al . Molecular diagnosis of Burkitt's lymphoma. N Engl J Med 2006; 354: 2431–42. [DOI] [PubMed] [Google Scholar]
- 23. Todeschini G, Tecchio C, Degani D et al . Eighty‐one percent event‐free survival in advanced Burkitt's lymphoma/leukemia: no differences in outcome between pediatric and adult patients treated with the same intensive pediatric protocol. Ann Oncol 1997; 8 (Suppl 1): 77–81. [PubMed] [Google Scholar]
- 24. Adde M, Shad A, Venzon D et al . Additional chemotherapy agents improve treatment outcome for children and adults with advanced B‐cell lymphomas. Semin Oncol 1998; 25: 33–9. [PubMed] [Google Scholar]
- 25. Crom WR, Glynn‐Barnhart AM, Rodman JH et al . Pharmacokinetics of anticancer drugs in children. Clin Pharmacokinet 1987; 12: 168–213. [DOI] [PubMed] [Google Scholar]
- 26. Fairbanks LD, Ruckemann K, Qiu Y et al . Methotrexate inhibits the first committed step of purine biosynthesis in mitogen‐stimulated human T‐lymphocytes: a metabolic basis for efficacy in rheumatoid arthritis? Biochem J 1999; 342: 143–52. [PMC free article] [PubMed] [Google Scholar]


