Abstract
The unique characteristics of stem cells, specifically pluripotency and self‐renewal, are critical for sustaining the lifelong functionality of organs. Stem cells reside in a special microenvironment called the niche. Stem cells interact with the niche via adhesion molecules and exchange molecular signals that maintain the specific features of stem cells. A better understanding of the nature of stem cells and their niches is expected to provide an alternative approach to the treatment of various serious diseases, including cancer, in clinical practice. It has been suggested that tumor tissue contains a type of stem cell referred to as a cancer stem cell. Interestingly, there are a number of molecules that are commonly expressed in normal and cancer stem cells that lead to different phenomena depending on the local conditions. In this review, the hematopoietic system is used as an example to show how stem cells interact with different niches. The regulatory mechanisms of two kinds of bone marrow niche, osteoblastic and vascular, are covered in this review. Furthermore, the involvement of the niche in cancer stem cell regulation, tumor invasion and metastasis, and its response to oxidative stress is described, and novel therapeutic approaches involving the interactions between cancer stem cells and their niches are addressed. (Cancer Sci 2009; 100: 1166–1172)
Stem cells possess two unique characteristics: pluripotency, which allows mature cells to compose specific organs or tissue, and self‐renewal, which supplies an organ with an adequate number of cells to maintain the organ's function. Stem cells tend to be found in specific areas of an organ where a special microenvironment called the niche maintains stem cell functions. Stem cells and niche cells interact with each other via adhesion molecules and paracrine factors. They exchange molecular signals that maintain the unique characteristics of the stem cells.
Stem cells do not only exist in normal organs or tissues. Recent findings suggest that a group of cells in tumors behave in a similar manner to normal stem cells.( 1 ) Such cells are called cancer stem cells and are considered to be critically important for tumor proliferation, invasion, and metastasis. Cancer stem cells include not only the stem cells with malignancy, but also the cancer‐initiating cells that are possibly derived from normal progenitor cells and reacquire self‐renewal capacity. Cancer cells are blocked from further differentiation (differentiation block) and proliferate aggressively (Fig. 1).
Figure 1.
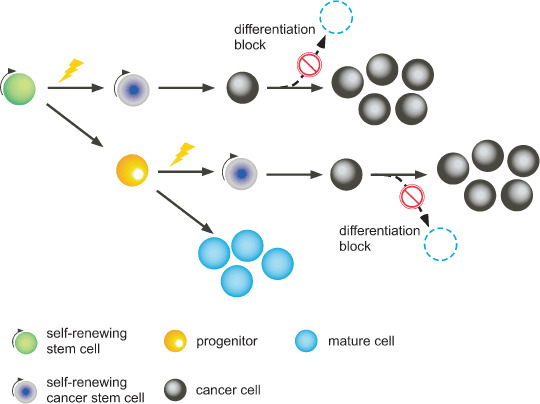
Extracellular perturbations affect the stem cell and/or progenitor in which genetic mutations have accumulated. Under these conditions, transformed stem cells show sustained self‐renewal, and the progenitors that have been affected acquire the capacity for self‐renewal. Both types of cells are called cancer stem cells. At a certain point, cancer stem cells give rise to cancer cells, which are more differentiated than stem cells, but not fully mature as observed in chronic myeloid leukemia. At some point after this stage, further differentiation is blocked and cancer cells aggressively proliferate as is typically observed in acute myeloid leukemia.
To date, the most advanced studies in stem cell niche research have examined the hematopoietic system. In the present paper, the interaction between hematopoietic stem cells (HSC) and their niches will be discussed. Furthermore, the involvement of the niche in cancer stem cell regulation, tumor invasion, and metastasis will be described, and novel therapeutic approaches involving the interactions between cancer stem cells and their niches will be addressed.
Stem cells and niches
Stem cell research into the maintenance of pluripotency and the capacity for self‐renewal was greatly advanced by experiments using Drosophila and Caenorhabditis elegans. In 1997, the research group of Deng and Lin demonstrated that the female fly has cells named ‘cap cells’ at the tip of the ovary to which germ line stem cells adhere.( 2 ) During mitosis, daughter stem cells, which divided in such a way that they no longer adhered to the cap cells, started to differentiate and grew into cystoblasts. In contrast, daughter stem cells that stayed attached to the cap cells after cell division remained germ line stem cells. These results suggested that the cap cells provide a special environment for the germ line stem cell and play a role as a stem cell niche in the fly ovary.
In 2000, Xie and Spradling demonstrated that cells that were newly introduced to the fly ovary remained germ line stem cells as long as they adhered to the cap cells, thus proving that the cap cells function as a true stem cell niche.( 3 ) They showed that germ line stem cells required a signal mediated by decapentaplegic (dpp), a member of the transforming growth factor (TGF)‐β super family, in order to remain adhered to the cap cells (i.e. in order to maintain the stem cell) and to control the frequency of cell division. Additional signals, such as hedgehog, wingless, or armadillo, were also suggested to be involved in the regulation of germ line stem cells.
Interactions between hematopoietic stem cells and their niches
Osteoblastic niche. In 2003, two independent research groups led by Li and Scadden demonstrated that during hematopoiesis in bone marrow (BM), osteoblasts serve as the microenvironment or osteoblastic niche for HSC. HSC and osteoblasts bind each other via adhesion molecules such as N‐cadherin. Both groups approached the issue of how the number of the HSC is controlled from two different directions. Li's group reported that the bone morphogenetic protein (BMP) signaling pathway, which acts through BMP receptor type IA expressed in osteoblasts, controls the number of HSC by regulating the size of the niche.( 4 ) Scadden and his colleagues showed that an increase in the number of osteoblastic cells, stimulated by activated parathyroid hormone/parathyroid hormone‐related peptide receptors, produced high levels of the Notch ligand jagged 1 and increased the number of HSC.( 5 )
It is widely known that in general stem cells are in a quiescent state (G0 phase in the cell cycle) and that this quiescence prevents the stem cells from entering into the cell cycle and undergoing differentiation. We demonstrated that angiopoietin‐1 (Ang1) in osteoblasts interacts with Tie‐2, a type of receptor tyrosine kinase that is expressed in HSC in BM, and that this Tie‐2–Ang1 interaction activates β1‐integrin and N‐cadherin. This enhanced adhesion between the niche cell and the stem cell contributes to the maintenance of stem cell quiescence (Fig. 2).( 6 )
Figure 2.
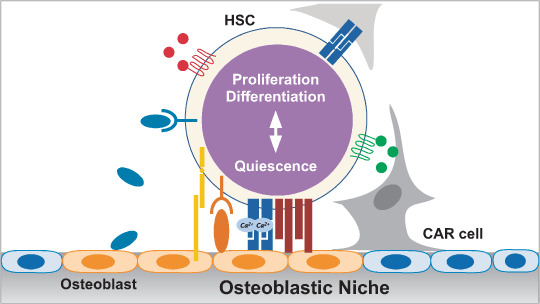
Schematic model of the molecular mechanism regulating a hematopoietic stem cell (HSC) in an osteoblastic niche in bone marrow. Molecular interactions between HSC and osteoblasts, factors secreted by osteoblasts, extracellular matrix, or CXCL12‐abundant reticular (CAR) cells as a whole determine HSC behavior and fate, including the balance between quiescence and proliferation or differentiation. For a detailed list of molecules, see Table 2.
Thrombopoietin (THPO) and its ligand Mpl, the regulator of megakaryopoiesis, were shown to be critical regulators of HSC maintenance in the osteoblastic niche by two independent research groups.( 7 , 8 ) We demonstrated that the majority of Mpl+Lineage−Sca‐1+c‐Kit+ (LSK) cells were found in a side‐population (SP), the most concentrated fraction of quiescent HSC. Mpl+ HSC were observed to be in close contact with THPO‐producing osteoblastic cells at the endosteal surface in the trabecular bone area. On the other hand, Qian et al. using adult Thpo−/– mice, demonstrated that THPO–Mpl signaling is required for postnatal HSC expansion, but is not required during the prenatal phase. In the knockout mice, the number of HSC was reduced 150‐fold. Meanwhile, we found that a CDK inhibitor p57kip2 was upregulated by stimulation with THPO and downregulated by AMM2, an anti‐Mpl neutralizing antibody. These data consistently indicate that Mpl–THPO signaling regulates the cell cycle of adult quiescent HSC to maintain the number of HSC at a physiologically reasonable level.
Osteopontin is expressed by osteoblasts and is also reported to be a key component of the HSC niche. Osteopontin supports the adhesion of HSC to the osteoblastic niche and negatively regulates HSC proliferation, contributing to the maintenance of stem cell quiescence.( 9 )
Two recent studies successfully detected HSC in the BM of live animals using state‐of‐the‐art, real‐time imaging technology. Li's group showed that transplanted HSC tend to home to the endosteum in BM, which normally maintains HSC and promotes their expansion.( 10 ) Scadden's group showed that after transplantation, an HSC‐enriched population was observed in the area closest to the endosteum and that as differentiation progressed from multipotent progenitors to committed progenitors, they homed into distinct locations that were farther away from the endosteum. They also observed that BM stem and progenitor cells migrated to areas adjacent to bone and osteoblasts when engraftment or expansion was needed.( 11 )
Vascular niche. In addition to the osteoblastic niche, another kind of niche existing along the endothelial cells of the sinusoidal vessels in BM or spleen has recently been described. This microenvironment is called the vascular niche. This new type of niche was identified in a recent study designed to distinguish stem cells from progenitor cells in BM using the signaling lymphocyte activation molecule (SLAM) family of receptors. The most purified HSC were fractioned as CD150+CD244−CD48− cells, and the majority of these cells were found to be associated with the sinusoidal endothelium.( 12 )
Sugiyama et al. showed that HSC were specifically located adjacent to cells expressing a high level of CXCL12 (also called stromal cell‐derived factor‐1, or SDF‐1), which surrounded the sinusoidal endothelial cells. They named these cells CXCL12‐abundant reticular (CAR) cells (Fig. 2). They also demonstrated that a depletion of CXCR4 led to a reduction of the HSC pool and to a poor survival rate after 5‐fluorouracil‐induced BM suppression, suggesting that CXCL12–CXCR4 chemokine signaling plays an essential role in maintaining the quiescent HSC pool.( 13 ) The connection between CAR cells and HSC has also been observed at the endosteum. It appears to be the universal component of the HSC niche.
The functional difference between the osteoblastic and vascular niche has yet to be elucidated; however, it might be reasonable to approach this issue from a physiological perspective. One of the major differences between these two microenvironments is the oxygen level. Oxygen availability would be expected to be higher in the vascular niche than in the osteoblastic niche. In such a microenvironment of high oxygen, the cell cycle of the stem cell would resume.( 14 ) Thus, a model of the dynamics of hematopoiesis can be hypothesized: HSC in a G0 state located at the osteoblastic niche under regional hypoxia would move to the vascular niche at a certain time and undergo differentiation to provide the peripheral blood stream with the necessary supply of mature cells.( 15 ) When the supply of mature cells is no longer needed, HSC in the vascular niche would move back to the osteoblastic niche where they would be maintained in the G0 state once again. The logistics of shuttling HSC between the two types of niches might be the key to well‐balanced hematopoiesis.
Is there a niche for cancer stem cells?
It is likely that there is a functional microenvironment to support cancer stem cells as well. This should also be considered a niche and is thus called the cancer stem cell niche. A representative example is observed in acute myeloid leukemia (AML) and its niche in BM. Dick and colleagues showed that anti‐CD44 antibody‐treated non‐obese diabetic/severe combined immunodeficient (NOD/SCID) mice transplanted with AML cells exhibited a significantly lower rate of disease onset.( 16 ) Also, Van Etten and colleagues showed that there was impaired induction of chronic myeloid leukemia (CML)‐like myeloproliferative disease among recipient mice that received transplanted BCR–ABL1‐transduced CML progenitors from CD44‐mutant donors.( 17 ) These results indicate that for both AML and CML, CD44 is essential for the homing and engraftment of the cancer stem cells to the niche. In other words, CD44‐expressing leukemic stem cells adhere to the niche and bind to hyaluronic acid expressed by cells on the surface of sinusoidal endothelium or endosteum in BM; this binding is crucial for the niche's maintenance of the stem cells. Interestingly, this molecular mechanism resembles that of the interaction between normal HSC and the vascular niche described earlier. Cancer and normal stem cells have much in common with regard to the maintenance system within their niches.
In contrast to the hematopoietic system, stem cell research in the solid cancer field is relatively new. A study of stem cells in brain tumors has made significant progress in this area. Gilbertson and colleagues showed that brain tumor cells coexpressing Nestin and CD133, the fraction believed to contain the cancer stem cell, were found near the capillaries in the brain tumor.( 18 ) When these cells were cocultured, the cancer stem cells selectively adhered to the endothelial cells. This suggested that the endothelial cells secreted factors necessary to maintain the cancer stem cells. Furthermore, the CD133‐positive cells derived from human medulloblastoma developed brain tumors only when xenografted to the brain of a recipient nude mouse with endothelial cells. Collectively, these data suggest that cancer stem cells of brain tumors rely on endothelial cells, which form a vascular niche that maintains the capacity of the cancer stem cells for self‐renewal, differentiation, and proliferation.
In addition to its role in brain tumors, CD133 has been extensively studied in other kinds of cancer, such as colon, prostate, and pancreatic cancers, and is now considered to be a tumor marker for those cancers (Table 1). Despite the growing interest in CD133, the functional role of CD133 itself remains unclear. CD133 is a cholesterol‐binding pentaspan membrane glycoprotein and is associated with a membrane microdomain. The microdomain in the stem cells or progenitors has been proposed to be a carrier of important molecular factors necessary for the maintenance of stem cell properties. Therefore, it is hypothesized that the localized distribution of CD133 during cell division might reflect the localized distribution of the microdomains that determine the daughter cell's fate, that is, whether it remains as a stem cell or undergoes differentiation.( 27 ) Future studies are likely to reveal the molecular mechanism underlying the relationship between CD133 and stem cell characteristics.
Table 1.
Cancer stem cell markers identified for various cancers
Roles of the niche in the development, maintenance, and proliferation of cancer
In a recent dynamic, real‐time imaging study, it was reported that leukemic cells outcompete normal stem cells for normal niche occupancy in BM. The normal stem cells were directed by stem cell factor (SCF) secreted by leukemic cells into an abnormal niche created by leukemic cells in an atypical region, where the supportive functions of the stem cells’ capacity for self‐renewal, proliferation, and differentiation were highly defective.( 28 ) Thus, the niche can control the pool, function, and even the fate of stem cells. It is indeed an important aspect of the niche to maintain the stem cells in a quiescent state. For the purpose of maintaining organs, the niche is also responsible for simultaneously driving an adequate number of stem cells into the proliferation and differentiation pathways. This two‐way signaling of the stem cells by the niche must be carefully regulated (Fig. 2). For example, for HSC in the osteoblastic niche in BM, Tie‐2–Ang1 signaling or BMP is involved in maintaining quiescence and proliferation suppression, whereas Wnt or Notch signaling promotes self‐renewal, proliferation, and differentiation (Table 2).
Table 2.
Molecular interaction between hematopoietic stem cells (HSCs) and their niche in bone marrow
| Niche | Molecule in niche | Molecule in HSC | Function | Reference |
|---|---|---|---|---|
| Wnt | Frizzled | Proliferation of HSC | 29 | |
| Osteoblastic | BMPR1A | BMP | Regulation of number of HSC | 4 |
| Osteoblastic | Jagged1 | Notch | Proliferation of HSC | 5 |
| Osteoblastic | Ang1 | Tie‐2 | Maintenance of quiescence | 6, 30 |
| Osteoblastic | Thpo | c‐Mpl | Maintenance of quiescence | 7, 8 |
| Osteoblastic | Osteopontin | β1 integrin | Maintenance of quiescence | 9 |
| Hyaluronic acid | CD44 | Homing and lodgement | 31, 32 | |
| Calcium | CaR | Lodgement | 33 | |
| Vascular | CXCL12 | CXCR4 | Maintenance of HSC pool | 13 |
| Vascular | VCAM‐1 | VLA‐4 | Homing and lodgement | 34 |
Different sets of molecules play crucial roles in HSC maintenance and regulation. The molecules without a specific indicated niche location are primarily expressed in the extracellular matrix.
It is well known that cancer is a highly proliferative disease. Does the stem cell regulatory system in the niche for cancer support self‐renewal more than differentiation? Li and Neaves approached this question by studying the dependence of stem cells on their niches. They hypothesized that the behaviors of cancer stem cells and normal stem cells are regulated by the niche to different degrees.( 35 ) The cancer stem cell is engendered by an intrinsic mutation that leads to its high proliferation. This highly proliferative state itself can alter the signaling balance between the niche and stem cells. Namely, the characteristics of the niche that function to maintain quiescence become relatively ineffective, and the characteristics of the niche that function to support proliferation and differentiation become more dominant. This model is supported by some clinical symptoms, one of which is the blast crisis of CML. The Wnt/β‐catenin signal, a causative pathway for self‐renewal and differentiation, is enhanced in the mutated nucleus of granulocyte‐macrophage progenitors in CML patients due to the higher concentration of β‐catenin and irregularly activated lymphoid enhancer factor/T‐cell factor (Lef/Tcf) transcription activity.( 36 ) The blast crisis is thought to be triggered by this irregular Lef/Tcf activity, leading to excess transcription of Wnt target gene products. Similarly, irregular Wnt–β‐catenin activation has been reported in colon cancer and melanoma, in which β‐catenin is mutated. It is important to note that many signaling pathways involved in the interaction between normal stem cells and their niches are also involved in the interactions between cancer stem cells and their niches, and can play a role as promoters of tumorigenesis and cancer proliferation. An identical set of proteins under slightly different conditions can deliver totally different results.
Mechanism of cancer metastasis is regulated by niche
The purpose of the niche is not only the cradling of existing cancer stem cells, but also the cradling of future incoming cancer stem cells. The niche is constantly sending passive signals of invitation to remote cancer stem cells. Matrix metalloproteinases (MMP) are well‐known factors, not only for their contribution to the repair of inflammation and wounds, but also for their involvement in cancer invasion and metastasis. A model of the molecular mechanism for remote metastasis and invasion in association with MMP has been proposed for the lung.( 37 ) In this model, vascular endothelial growth factor secreted by primary cancer cells induces specific MMP9 expression in lung endothelial cells and macrophages via vascular endothelial growth factor receptor (VEGFR) tyrosine kinase, resulting in the formation of the cancer stem cell niche. This means that the cancer cells can produce their own favorable microenvironment, the future cancer stem cell niche, from a distance by secreting factors that influence the protein composition at that site.
This niche formation mechanism by cancer cells has also been verified for various other kinds of metastases; for example, bone metastasis of prostate cancer has been shown to be supported by urokinase‐type plasminogen activator or prostate‐specific antigen secreted by prostate cancer cells through alteration of the growth factors in the bone microenvironment, thus enhancing the proliferation of the osteoblasts that serve as the cancer stem cell niche.( 38 ) Lung metastasis of breast cancer via secreted protein acidic and rich in cysteine osteonectin or MMP2 has also been found to be based on this mechanism.( 39 )
Factors existing in the extracellular matrix can also influence the development of cancer. The oncogene mesenchymal‐epithelial transition factor (MET) is upregulated in cancer stem cells undergoing hypoxia. MET binds to its ligand hepatocyte growth factor in the extracellular matrix and enhances the transcription of plasminogen activator inhibitor type 1 and cyclooxygenase 2. This leads to enhanced blood coagulation and fibrin deposition (fibrin nest). The fibrin nest induces vasculogenesis in the surrounding area, supporting the homing of cancer stem cells and their proliferation, and thus serving as the cancer stem cell niche. The migration of the vascular vessel triggered by the fibrin nest also facilitates metastasis to other sites.( 40 )
Lyden and his colleagues recently reported the existence of a new type of cancer stem cell niche created by a third party in lethally irradiated mice that had been transplanted with BM‐derived cells (BMDC) that later developed into lung cancer or melanoma cells. The transplanted BMDC expressing VEGFR1 gathered at a site, such as the lung, creating a group of cells and a microenvironment that was highly receptive for cancer metastasis; this has been referred to as the ‘premetastatic niche’.( 41 ) Premetastatic niche formation is associated with breast, lung, and gastrointestinal cancer in humans. The BMDC that create the premetastatic niche are considered to be a type of hematopoietic progenitor expressing VLA‐4 (integrin α4β1) and/or inhibitor of DNA binding 3. Primary cancer cells secrete factors (such as cytokines) that positively regulate the expression of fibronectin (a ligand of VLA‐4) by fibroblasts located at the sites of future premetastatic niches, which induces the homing of VEGFR1‐expressing BMDC. The mice transplanted with VEGFR1‐depleted BMDC or treated with an anti‐VEGFR1 antibody did not exhibit premetastatic niches or metastasis, proving that this new metastatic mechanism induced by cancer cells involves a third party.
Are cancer stem cells rare or common?
The frequency of cancer stem cells is often assessed by their engraftment rate in immunocompromised mice. In a report by Dick et al. SCID mice were injected with human AML cells obtained from patients’ peripheral blood, and the estimated frequency of cancer stem cells was 0.0004%.( 42 ) As summarized in Table 3, cancer stem cell frequency in general is considered to be very low, far below 1% of unfractionated cancer cell populations.
Table 3.
Frequencies of cancer stem cells in various human cancers
| Human cancer | Recipient mice | Cancer stem cell frequency (%) | Reference |
|---|---|---|---|
| Acute myeloid leukemia | SCID | 0.0004 | 42 |
| NOD/SCID | 0.001 | 43 | |
| Pancreatic cancer | NOD/SCID | 0.2–0.8 | 44 |
| Colon cancer | NOD/SCID | 0.002 | 45 |
| Melanoma | NOD/SCID | 0.0001 | 46 |
| Melanoma | NOD/SCID | 0.0001 after 8 weeks | 47 |
| 0.0009 after 32 weeks | |||
| NOD/SCID Il2rg−/– | 0.0036, 0.15 | ||
| after 32 weeks | |||
| NOD/SCID Il2rg−/– | 25 | ||
| after 32 weeks injected with matrigel |
The frequencies were estimated from results with xenotransplantation assays using patient‐derived unfractionated cells in tumor and immunocompromised recipient mice. For acute myeloid leukemia xenotransplantation, patients’ peripheral blood cells were used.
The issue in frequency estimation of cancer stem cells using xenotransplantation is its possible dependence on some factors that can substantially affect the result. These factors may include the immune system of the recipient against the transplanted cells, observation cut‐off time until the detection of tumor, and physiological human–mouse non‐homology of the factors critical in homing or maintenance of cancer stem cells.
SCID or NOD/SCID mice are known for their severe immunodeficiency. However, even these mice produce natural killer cells and it is expected that the transplanted human cancer cells may not survive to the degree similar to the case of congenic transplantation. Due to this background, even more highly immunocompromised mice, NOD/SCID Il2rg−/– mice, have been developed. These mice do not produce natural killer cells, and therefore higher receptivity of xenotransplanted cells can be expected.
Cancer stem cells in some tumors may have lower proliferation rate than those in other kinds of tumors. In addition, not all cancer stem cells are necessarily heterogenous and some may develop detectable tumor sooner than others, and vice versa. Setting the observation cut‐off time too soon may underestimate the frequency of cancer stem cells.
A study by Morrison's group addressed some of these issues by comparing receptivities between NOD/SCID and NOD/SCID Il2rg−/– mice after prolonged observation periods.( 47 ) Human melanoma cells were obtained directly from patients and injected first into NOD/SCID mice. The result at 8 weeks post‐transplantation was consistent with earlier studies; however, more tumors developed to a detectable size during the period beyond this point. Consequently, the estimated cancer stem cell frequency from the result of 32 weeks observation was nine times higher than that of 8 weeks (Table 3). On the other hand, as shown in Table 3, the estimated frequency in NOD/SCID Il2rg−/– mice was 4–166 times higher than in NOD/SCID mice, approximately. Furthermore, when the melanoma cells were injected with matrigel, which is expected to enhance homing or maintenance of the transplanted cells, the estimated frequency of melanoma stem cells was as high as 25%. Kelly et al. tested the hypothesis that the frequency of cancer stem cells can best be tested by transfer of a titrated number of mouse cancer cells into non‐irradiated histocompatible recipient mice.( 48 ) They harvested murine pre‐B/B lymphoma cells from Eµ‐myc transgenic mice and injected them into non‐irradiated congenic mice. Even with as few as 10 cells injected, all recipient mice developed lymphoma, whereas only a small fraction (~2–5%) of the primary cells displayed the characteristic stem cell markers.
These results indicate that the solid methodology for assessment of cancer stem cell frequency is yet to be established. Xenotransplantation assay may underestimate the population, at least in some cancers, or may drive the non‐cancer stem cells into cancer stem cells in a heterologous environment. It is also important to determine whether only the cells expressing specific combinations of cell surface markers can initiate tumor development. As discussed in the introduction, cancer stem cells can be derived from normal progenitor cells. If normal progenitor cells home to a cancer‐driving niche, they may conceivably turn to cancer stem cells. Whatever the case may be, understanding the nature of the cancer stem cells and their niche is the key to successful development of novel therapeutic protocols for eradication of cancer.
Novel cancer therapies targeting cancer stem cells and their niches
Most of the current anticancer drugs inhibit DNA synthesis or cell division of cancer cells, thereby targeting tumor growth. Consequently, cancer cells must be actively dividing and not be quiescent for the positive effect of chemotherapy to be visible. Cancer stem cells, as well as normal stem cells, are generally in a quiescent state. Thus, even if the size of a tumor is decreased following chemotherapy, it might be simply because the regular cell‐cycling cancer cells have been killed. The quiescent, tumorigenetic cancer stem cells would be expected to survive the chemotherapy.
A report by Graham et al. demonstrated that cancer stem cells are insensitive to chemotherapy by showing that growth of most of the BCR–ABL expressing blood cells of patients with CML were arrested after the addition of STI571 to the cell culture; however, cells in a quiescent state, namely the leukemic stem cells, survived.( 49 ) Similarly, prolonged treatment with gemcitabine enriched the CD133+ pancreatic cancer stem cell population both in vitro and in vivo,( 50 ) and CD133+ colon cancer stem cells remained viable after standard chemotherapeutic treatment with oxaliplatin or 5‐FU.( 51 ) These data suggest that destroying quiescent cancer stem cells is a very promising therapeutic approach for future treatments that aim to completely cure cancer. This concept has been tested by altering cancer stem cell characteristics, manipulating the niche environment to induce a resumption of the cell cycle, and inducing differentiation of the cancer stem cells. For example, it was revealed that CD133+ colon cancer cells produced and utilized interleukin (IL)‐4 in a paracrine manner to protect themselves from apoptosis, and a treatment blocking IL‐4 signaling significantly enhanced the sensitivity of CD133+ cells against chemotherapy.( 51 ) From the viewpoint of cancer stem cell control, complete inhibition of cancer stem cell division, even if it is only partially effective, might still have potential.
External oxidative stress and stem cells
Ito et al. investigated whether the treatment outcome of CML could be improved by enhancing the cycling of quiescent leukemia stem cells with arsenic trioxide (As2O3).( 52 ) As2O3 is known to induce oxidative stress. The number of quiescent leukemia stem cells significantly decreased after As2O3 treatment. More leukemia stem cells entered the cell cycle than normal stem cells following As2O3 treatment in vivo, and the addition of cytosine arabinoside (Ara‐C) following As2O3 treatment induced significant apoptosis of leukemia stem cells, leading to the complete remission of CML in recipient mice in a serial transplantation assay.
Parthenolide is a sesquiterpene lactone and a major component of the herbal medicine feverfew. Parthenolide has a number of unique effects on tumors, including the inhibition of DNA synthesis, cancer cell proliferation, and nuclear factor‐κB activation, as well as increasing intracellular reactive oxygen species (ROS).( 53 ) We have previously shown that oxidative stress can affect the long‐term HSC capability.( 54 ) Treatment of immature hematopoietic cells in the LSK fraction with buthionine sulfoximine, even at low levels, decreased the repopulation capacity of HSC after transplantation, but not the number of colonies formed in culture. This result indicated that elevation of ROS induced by buthionine sulfoximine did not affect the colony‐forming progenitors in the LSK fraction, but specifically led to defects in HSC function. The elevation of ROS resulted in a specific upregulation of tumor suppressors p16Ink4a and p19Arf in HSC and treatment with a p38 mitogen‐activated protein kinase (MAPK) inhibitor or the antioxidant N‐acetyl‐l‐cysteine blocked the ROS‐induced increase of p16Ink4a and p19Arf. These findings suggested that oxidative stress induces the HSC‐specific phosphorylation of p38 MAPK and that this activation of p38 MAPK leads to defects in the maintenance of HSC capacity for self‐renewal (Fig. 3).
Figure 3.
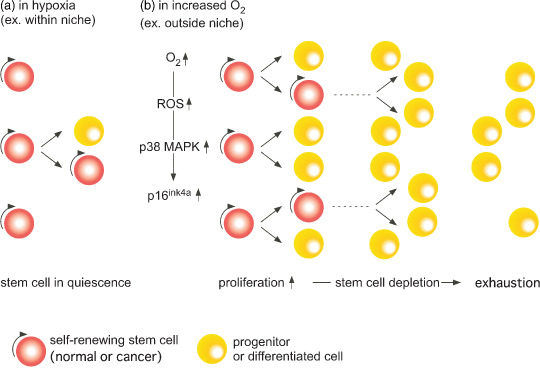
Stem cells, whether normal or cancer, in general reside in a hypoxic niche where self‐renewal and differentiation activity is balanced. With an increase in oxygen levels, proliferation becomes a dominant feature mediated by an increase in p38 MAPK and p16ink4a. This transiently leads to the expansion of the progenitors, which results in a long‐term decrease in the stem cell pool and its eventual exhaustion. ROS, reactive oxygen species.
Reactive oxygen species could affect the interaction between stem cells and their niches, as suggested by Hosokawa et al.( 55 ) 5‐FU treatment caused a shift of the LSK‐SP fraction in the BM to non‐SP, and supplementing with the antioxidant N‐acetyl‐l‐cysteine inhibited this transition and sustained the SP fraction for a longer period of time. These data indicate that ROS induce the HSC cell cycle, thereby allowing them to escape from the niche.
Summary
The stem cell, whether normal or cancer, and its niche influence each other, which maintains their capabilities and controls their fates. Stem cell regulation and maintenance is not a simple single‐factor phenomenon and a large part of the complicated molecular mechanism is yet to be elucidated. Understanding the regulatory system could lead to novel therapeutic approaches that directly target stem cells or niches. As described in this review, the latest drugs targeting cancer stem cells or their microenvironments use this state‐of‐the‐art strategy, and are expected to minimize complications and improve patient quality of life at the same time. From both scientific and clinical viewpoints, the biology of the stem cell and its niche is expected to be one of the most promising fields of research over the next few decades.
Acknowledgments
This research was partially supported by the Ministry of Education, Science, Sports, and Culture, Grant‐in‐Aid for Specially Promoted Research, 2008, and the Global COE Program ‘Education and Research Center for Stem Cell Medicine’.
References
- 1. Dick JE. Stem cell concepts renew cancer research. Blood 2008; 112: 4793–807. [DOI] [PubMed] [Google Scholar]
- 2. Deng W, Lin H. Spectrosomes and fusomes anchor mitotic spindles during asymmetric germ cell divisions and facilitate the formation of a polarized microtubule array for oocyte specification in Drosophila. Dev Biol 1997; 189: 79–94. [DOI] [PubMed] [Google Scholar]
- 3. Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science 2000; 290: 328–30. [DOI] [PubMed] [Google Scholar]
- 4. Zhang J, Niu C, Ye L et al . Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–41. [DOI] [PubMed] [Google Scholar]
- 5. Calvi LM, Adams GB, Weibrecht KW et al . Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425: 841–6. [DOI] [PubMed] [Google Scholar]
- 6. Arai F, Hirao A, Ohmura M et al . Tie2/angiopoietin‐1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118: 149–61. [DOI] [PubMed] [Google Scholar]
- 7. Yoshihara H, Arai F, Hosokawa K et al . Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007; 1: 685–97. [DOI] [PubMed] [Google Scholar]
- 8. Qian H, Buza‐Vidas N, Hyland CD et al . Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 2007; 1: 671–84. [DOI] [PubMed] [Google Scholar]
- 9. Nilsson SK, Johnston HM, Whitty GA et al . Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005; 15: 1232–9. [DOI] [PubMed] [Google Scholar]
- 10. Xie Y, Yin T, Wiegraebe W et al . Detection of functional haematopoietic stem cell niche using real‐time imaging. Nature 2009; 457: 97–101. [DOI] [PubMed] [Google Scholar]
- 11. Celso CL, Fleming HE, Wu JW et al . Live‐animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 2009; 457: 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121: 1109–21. [DOI] [PubMed] [Google Scholar]
- 13. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–88. [DOI] [PubMed] [Google Scholar]
- 14. Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 2007; 104: 5431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heissig B, Hattori K, Dias S et al . Recruitment of stem and progenitor cells from the bone marrow niche requires MMP‐9 mediated release of Kit‐ligand. Cell 2002; 109: 625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin L, Hope KJ, Zhai Q, Smadja‐Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nature Med 2006; 12: 1167–74. [DOI] [PubMed] [Google Scholar]
- 17. Krause DS, Lazarides K, Von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR‐ABL‐expressing leukemic stem cells. Nature Med 2006; 12: 1175–80. [DOI] [PubMed] [Google Scholar]
- 18. Calabrese C, Poppleton H, Kocak M et al . A perivascular niche for brain tumor stem cells. Cancer Cell 2007; 11: 69–82. [DOI] [PubMed] [Google Scholar]
- 19. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med 1997; 3: 730–7. [DOI] [PubMed] [Google Scholar]
- 20. Singh SK, Clarke ID, Terasaki M et al . Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63: 5821–8. [PubMed] [Google Scholar]
- 21. Singh SK, Hawkins C, Clarke ID et al . Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401. [DOI] [PubMed] [Google Scholar]
- 22. Al‐Hajj M, Wicha M, Benito‐Hernandez A et al . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collins AT, Berry PA, Hyde C et al . Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005; 65: 10946–51. [DOI] [PubMed] [Google Scholar]
- 24. Seigel GM, Campbell LM, Narayan M et al . Cancer stem cell characteristics in retinoblastoma. Mol Vis 2005; 11: 729–37. [PubMed] [Google Scholar]
- 25. Kim CF, Jackson EL, Woolfenden AE et al . Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005; 121: 823–35. [DOI] [PubMed] [Google Scholar]
- 26. Ricci‐Vitiani L, Lombardi DG, Pilozzi E et al . Identification and expansion of human colon‐cancer‐initiating cells. Nature 2007; 445: 111–5. [DOI] [PubMed] [Google Scholar]
- 27. Bauer N, Fonseca AV, Florek M et al . New insights into the cell biology of hematopoietic progenitors by studying Prominin‐1 (CD133). Cells Tissues Organs, 2008; 188: 127–38. [DOI] [PubMed] [Google Scholar]
- 28. Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 2008; 322: 1861–5. [DOI] [PubMed] [Google Scholar]
- 29. Reya T, Duncan AW, Ailles L et al . A role for Wnt signalling in self‐renewal of haematopoietic stem cells. Nature 2003; 423: 409–14. [DOI] [PubMed] [Google Scholar]
- 30. Puri CM, Berstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci USA 2003; 100: 12753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matrosova VY, Orlovskaya IA, Serobyan N, Khaldoyanidi SK. Hyaluronic acid facilitates the recovery of hematopoiesis following 5‐Fluorouracil administration. Stem Cells 2004; 22: 544–5. [DOI] [PubMed] [Google Scholar]
- 32. Avigdor A, Goichberg P, Shivtiel S et al . CD44 and hyaluronic acid cooperate with SDF‐1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood 2004; 15: 2981–9. [DOI] [PubMed] [Google Scholar]
- 33. Adams GB, Chabner KT, Alley IR et al . Stem cell engraftment at the endosteal niche is specified by the calcium‐sensing receptor. Nature 2006; 439: 599–603. [DOI] [PubMed] [Google Scholar]
- 34. Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM‐1 adhesion pathway defines contrasting mechanism of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA 1995; 92: 9647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006; 66: 4553–7. [DOI] [PubMed] [Google Scholar]
- 36. Jamieson CH, Ailles LE, Dylla SJ et al . Granulocyte‐macrophage progenitors as candidate leukemic stem cells in blast‐crisis CML. N Engl J Med 2004; 351: 657–67. [DOI] [PubMed] [Google Scholar]
- 37. Hiratsuka S, Nakamura K, Iwai S et al . MMP9 induction by vascular endothelial growth factor receptor‐1 is involved in lung‐specific metastasis. Cancer Cell 2002; 2: 289–300. [DOI] [PubMed] [Google Scholar]
- 38. Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nature Rev 2005; 5: 21–8. [DOI] [PubMed] [Google Scholar]
- 39. Minn AJ, Gupta GP, Siegel PM et al . Genes that mediate breast cancer metastasis to lung. Nature 2005; 436: 518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boccaccio C, Sabatino G, Medico E et al . The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature 2005; 434: 396–400. [DOI] [PubMed] [Google Scholar]
- 41. Kaplan RN, Riba RD, Zacharoulis S et al . VEGFR1‐positive haematopoietic bone marrow progenitors initiate the pre‐metastatic niche. Nature 2005; 438: 820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lapidot T, Sirard C, Vormoor J et al . A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367: 645–8. [DOI] [PubMed] [Google Scholar]
- 43. Lumkul R, Gorin NC, Malehorn MT et al . Human AML cells in NOD/SCID mice: engraftment potential and gene expression. Leukemia 2002; 16: 1818–26. [DOI] [PubMed] [Google Scholar]
- 44. Li C, Heidt DG, Dalerba P et al . Identification of pancreatic cancer stem cells. Cancer Res 2007; 67: 1030–7. [DOI] [PubMed] [Google Scholar]
- 45. O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445: 106–10. [DOI] [PubMed] [Google Scholar]
- 46. Schatton T, Murphy GF, Frank NY et al . Identification of cells initiating human melanomas. Nature 2008; 451: 345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumor formation by single human melanoma cells. Nature 2008; 456: 593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science 2007; 317: 337. [DOI] [PubMed] [Google Scholar]
- 49. Graham SM, Jørgensen HG, Allan E et al . Primitive, quiescent, Philadelphia‐positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro . Blood 2002; 99: 319–25. [DOI] [PubMed] [Google Scholar]
- 50. Hermann PC, Huber SL, Herrler T et al . Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007; 1: 313–23. [DOI] [PubMed] [Google Scholar]
- 51. Todaro M, Alea MP, Di Stefano AB et al . Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin‐4. Cell Stem Cell 2007; 1: 389–402. [DOI] [PubMed] [Google Scholar]
- 52. Ito K, Bernardi R, Morotti A et al . PML targeting eradicates quiescent leukaemia‐initiating cells. Nature 2008; 453: 1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guzman ML, Rossi RM, Karnischky L et al . The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005; 105: 4163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ito K, Hirao A, Arai F et al . Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Med 2006; 12: 446–51. [DOI] [PubMed] [Google Scholar]
- 55. Hosokawa K, Arai F, Yoshihara H et al . Function of oxidative stress in the regulation of hematopoietic stem cell–niche interaction. Biochem Biophys Res Commun 2007; 363: 578–83. [DOI] [PubMed] [Google Scholar]


