Abstract
We have previously reported that NM23 genes are overexpressed in various hematological malignancies and that serum NM23‐H1 protein levels are useful for predicting patient outcomes. In this study we assessed the clinical implications of serum NM23‐H1 protein on neuroblastoma. We examined serum NM23‐H1 protein levels in 217 patients with neuroblastoma, including 131 found by mass‐screening and 86 found clinically by an enzyme‐linked immunosorbent assay, and determined the association between levels of this protein, and known prognostic factors or the clinical outcome. The serum NM23‐H1 protein level was higher in neuroblastoma patients than in control children (P < 0.0001). Patients with MYCN amplification had higher serum NM23‐H1 levels than those with a single copy of MYCN. Overall survival was assessed in the 86 patients found clinically, and was found to be worse in patients with higher serum MN23‐H1 levels (≥ 250 ng/mL) than in those with lower levels (< 250 ng/mL; P = 0.034). The higher level of NM23‐H1 was correlated with a worse outcome in patients with a single MYCN copy, or in those younger than 12 months of age. Serum NM23‐H1 protein levels may contribute to predictions of clinical outcome in patients with neuroblastoma. (Cancer Sci 2005; 96: 653 – 660)
The NM23 gene was identified by differential hybridization of a cDNA library with total RNA extracted from slightly and highly metastatic melanoma cell lines.( 1 ) The NM23 gene has been identified as a family of genes encoding different isoforms of nucleoside diphosphate kinase (NDPK).( 2 ) NM23 genes play critical roles in cellular proliferation, differentiation, oncogenesis, and tumor metastasis.( 1 , 3 ) The mechanisms for these pleiotropic effects are not well understood. Eight isotypes of the human NM23 gene (NM23‐H1, NM23‐H2, NM23‐H3/DR‐NM23, NM23‐H4, NM23‐H5, NM23‐H6, NM23‐H7, and NM23‐H8) have been identified.( 2 ) Among these, only NM23‐H1 and NM23‐H2 have been studied extensively in human cancers.
The level of NM23‐H1 expression is inversely correlated with the tumor's metastatic potential in experimental rodent cells and in human tumors such as breast, ovarian, cervical and gastric cancer, hepatocellular carcinoma, and melanomas.( 4 ) Therefore, NM23‐H1 is implicated in the regulation of metastasis in a variety of human cancers. However, overexpression of the NM23‐H1 gene has been reported in various neoplasms including neuroblastoma, hematological malignancies, and pancreatic, lung, ovarian and gastric cancers.( 5 , 6 , 7 , 8 ) Overexpression of NM23‐H1 is indicative of a poor patient prognosis for patients with neuroblastoma, acute myelogenous leukemia (AML), or non‐Hodgkin's lymphoma (NHL).( 8 , 9 , 10 )
In neuroblastoma, a gain of 17q is the most frequent genetic abnormality, followed by 1p deletion and MYCN amplification, both of which correlate closely with 17q gain. The three genetic events are strong predictors of unfavorable prognosis.( 11 , 12 ) The NM23 genes are located at the edge of the common chromosomal region of 17q gain. Godfrid et al. identified genes that are activated in the MYCN downstream pathway using SAGE libraries of MYCN‐transfected and control neuroblastoma cell lines.( 13 ) The NM23‐H1 and NM23‐H2 genes are strongly induced in MYCN‐expressing cells. Neuroblastoma tumor and cell line panels reveal a striking correlation between MYCN amplification and mRNA or protein expression of both NM23 genes. These findings suggest that NM23‐H1 and NM23‐H2 expression may be increased by 17q gain in neuroblastoma, and can be further upregulated by MYCN overexpression. These observations suggest a role of NM23‐H1 and NM23‐H2 in the tumorigenesis of an unfavorable type of neuroblastoma.
We previously established an enzyme‐linked immunosorbent assay (ELISA) technique for determining the serum level of NM23‐H1 protein.( 14 ) Serum levels of NM23‐H1 in patients with NHL and AML are significantly higher than those in controls, and elevated NM23‐H1 levels correlate with poor prognosis in these patients.( 10 , 15 ) It has been strongly suggested that serum NM23‐H1 protein is produced directly by tumor cells and its level depends on the total mass of malignant cells overexpressing NM23‐H1.( 14 , 16 ) These results indicate that the serum level of NM23‐H1 protein may be clinically useful as a prognostic factor in NHL and AML. The present study assessed the clinical implications of serum NM23‐H1 protein levels in patients with neuroblastoma, in whom tumor samples were used to determine the biological prognostic factors.
Materials and Methods
Patients and controls
Serum NM23‐H1 protein was measured in 217 untreated neuroblastoma patients who were admitted to various institutions in Japan and underwent biopsy or surgery between 2000 and 2002. The 217 patients included 131 who were found by a mass‐screening (MS) program for infants at 6 months of age by measuring urinary catecholamine metabolites and 86 who were found clinically.( 17 ) Of the 86 patients, 29 who were younger than 12 months old were mostly found before MS, and 57 who were 12 months old or older underwent MS with a negative result, or did not undergo MS. Patients were staged according to the International Neuroblastoma Staging System (INSS).( 18 ) Patients of any age with stage 1 or 2 disease, and those younger than 12 months of age with stage 3 disease were treated by surgery or surgery and chemotherapy consisting of cyclophosphamide and vincristine; patients 12 months or older with stage 3 or stage 4 disease and those younger than 12 months of age with stage 4 disease were treated according to the protocol published by the Japanese Neuroblastoma Study Group.( 19 ) Serum samples from 23 children consisting of 22 with inguinal hernias and one with an edematous scrotum before surgery were analyzed for comparison. The median age of the children was 23 months (range: 3–49 months). Informed consent was obtained from patients and/or their parents, and the ethics committee of Saitama Cancer Center approved the study design.
Venous blood samples
Peripheral venous blood samples were collected in sterile test tubes with heparin and placed on ice. The samples were centrifuged at 2000 g for 15 min at 4°C, and stored at −20°C. As a marker of hemolysis, free serum hemoglobin (Hb) was determined according to the method of Testa et al.( 20 )
ELISA for human NM23‐H1 protein
NM23‐H1 protein levels in serum were determined using a sandwich ELISA assay, as described previously.( 14 , 15 ) Recombinant NM23‐H1‐GST protein was used as a standard.
Examination of MYCN copy number, TRKA expression and ploidy
DNA preparation, digestion, and Southern blot analysis using the MYCN probe were carried out as described previously.( 12 ) The presence of more than three copies of the MYCN gene per haploid genome was considered to indicate amplification.( 21 ) TRKA expression was examined by northern blotting as reported previously.( 22 ) DNA index was analyzed on a Becton‐Dickinson FACScan flow cytometer by DNA cell‐cycle analysis software (version C).
Statistical analysis
The significance of differences in various clinical and biological aspects of the disease among the patient groups was examined by using the Mann–Whitney U or Kruskal–Wallis test (non‐parametric analysis). Spearman's correlation coefficient (rs) by ranks was used to evaluate the correlation between paired values. Survival analysis was performed according to the Kaplan–Meier method, and the significance of differences in survival was determined by using the generalized Wilcoxon's and log‐rank tests. A multivariate analysis of prognostic factors was performed using Cox's proportional‐hazards regression model. All statistical analyses were performed with Excell Statcel and Stat Flex software (version 5.0, Artech Co. Ltd, Osaka, Japan), and P < 0.05 was taken to indicate significance.
Results
Examination of serum NM23‐H1 protein levels in neuroblastoma patients and control children
The serum level of NM23‐H1 was examined in 217 neuroblastoma patients and 23 control children. The serum levels of NM23‐H1 were significantly higher in patients with neuroblastoma (n = 217, mean ± SD 176 ± 280 ng/mL) than in the control children (n = 23, 27 ± 41 ng/mL, P < 0.0001; Fig. 1a). The serum NM23‐H1 levels of the control children were higher than those of the healthy adults (data not shown). The serum NM23‐H1 levels in patients with neuroblastoma were significantly higher than those in patients with various hematological malignancies (data not shown). Next, the relationship between serum levels of NM23‐H1 and Hb was examined in 217 neuroblastoma patients and 23 control children, because the NM23‐H1 protein leaked from red blood cells by hemolysis may have elevated the serum NM23‐H1 levels.( 23 ) The results showed a weak correlation (rs = 0.3958, P = 7.5356 × 10−10, Spearman's correlation coefficient by ranks), although some patients had a higher Hb level but a lower NM23‐H1 level, or a lower Hb level but a higher NM23‐H1 level (Fig. 1b). When we chose samples from 156 patients and 20 control children with serum Hb less than 500 µg/mL, the correlation between serum NM23‐H1 and Hb levels was negligible (rs = 0.2351, P = 0.0035). Even in these patients, the serum levels of NM23‐H1 were significantly higher (n = 156, 113 ± 184 ng/mL) than in the control children (n = 20, 20 ± 35 ng/mL, P < 0.0001; Fig. 1c).
Figure 1.
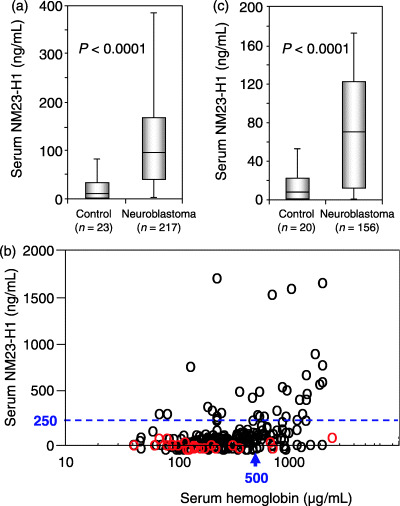
Serum NM23‐H1 levels in patients with neuroblastoma and in control children. (a) Box plots of NM23‐H1 serum levels for 217 patients with neuroblastoma and 23 control children with any serum hemoglobin levels. (b) Relationship between the serum levels of NM23‐H1 and hemoglobin in all samples examined (black circles, neuroblastoma patients [n = 217]; red circles, control children [n = 23]). (c) Box plots of NM23‐H1 serum levels for 156 patients with neuroblastoma and 20 control children with serum hemoglobin levels less than 500 µg/mL.
Relationship between serum NM23‐H1 protein levels and clinicopathological features in neuroblastoma
The relationship between serum NM23‐H1 levels and various clinical and biological features in the 217 patients is shown in Table 1. The serum NM23‐H1 levels tended to be higher in patients found clinically than in those found by MS (P = 0.0595), and were significantly higher in patients with amplified MYCN copies than in those with a single MYCN copy (P = 0.0006; Table 1). There was a correlation between MYCN amplification and the elevated serum NM23‐H1 level (≥ 250 ng/mL) in all 217 patients (rs = 0.6970, P = 0.0005). However, serum Hb concentrations did not correlate with MYCN amplification (P = 0.6320), or other factors (data not shown). There was no significant difference in the serum NM23‐H1 levels between two groups of patients classified by age of the patients, stage of the disease, expression levels of TRKA, or tumor cell ploidy (Table 1).
Table 1.
Relationship between serum NM23‐H1 protein levels and clinicopathological findings in 217 patients with neuroblastoma and 23 control children
| Clinicopathological findings | Number of patients (mean ± SD) | Serum NM23‐H1 (ng/mL) | P‐value (analysis) |
|---|---|---|---|
| Control children | 23 | 27 ± 41 | |
| All patients | 217 | 176 ± 280 | < 0.0001 (MW) |
| Method of detection | |||
| Mass‐screening | 131 | 135 ± 206 | |
| Found clinically | 86 † | 239 ± 357 | 0.0595 (MW) |
| Age of patients | |||
| < 12 months | 134 | 168 ± 292 | |
| ≥ 12 months | 83 | 190 ± 260 | 0.2427 (MW) |
| Stage of the disease | |||
| 1 + 2 + 4s | 122 | 136 ± 159 | |
| 3 + 4 | 95 | 227 ± 378 | 0.8088 (MW) |
| Primary site | |||
| Mediastinum | 31 | 145 ± 212 | |
| Adrenal | 101 | 187 ± 290 | |
| Abdomen | 78 | 184 ± 302 | 0.3393 (KW) |
| Others | 7 | 74 ± 82 | |
| MYCN copy number | |||
| 1 | 186 | 143 ± 204 | |
| > 3 | 31 | 378 ± 519 | 0.0006 (MW) |
| TRKA expression | 173 | ||
| Medium or high | 125 | 150 ± 209 | |
| None or low | 48 | 238 ± 373 | 0.4629 (MW) |
| Ploidy | 168 | ||
| Diploid | 69 | 188 ± 273 | |
| Hyperdiploid | 99 | 185 ± 284 | 0.9012 (MW) |
| Others | 7 | 112 ± 126 | |
MW, Mann–Whitney U‐test; KW, Kruskal–Wallis test. † Table 2.
Serum NM23‐H1 levels and overall survival
Of the 217 patients, the 86 patients who were found clinically were included and the 131 patients found by MS were excluded from survival analysis, because all the 131 patients were alive at the last follow‐up (18–51 months), and the clinical and biological features are different for the patients found by MS and those found clinically.( 12 ) The relationship between serum NM23‐H1 levels and various clinical and biological features in the 86 patients was similar to that found for all 217 patients (1, 2). The 86 patients were divided into two groups according to various cut‐off points over 100 ng/mL, which was the upper limit in control serum (mean + 2 × SD = 20 + 2 × 35 = 90). The cut‐off points used here were 100 ng/mL (< 100, n = 39, vs≥ 100, n = 47), 150 ng/mL (< 150, n = 54, vs ≥ 150, n = 32), 200 ng/mL (< 200, n = 60, vs≥ 200, n = 26) and 250 ng/mL (< 250, n = 64, vs ≥ 250, n = 22). The cut‐off value of greater than 250 ng/mL showed the most significant prognostic effects with generalized Wilcoxon's and log‐rank test analysis (data not shown). Therefore, we used 250 ng/mL of serum NM23‐H1 as a cut‐off value. As shown in Figure 2a, patients with the higher serum NM23‐H1 levels had worse overall survival than those with the lower levels (P = 0.0219 according to the generalized Wilcoxon test, P = 0.0340 according to the log‐rank test). Overall survival was significantly worse for patients who were 12 months or older than for those younger than 12 months of age (P = 0.0364 and P = 0.0158), for patients at stages 3 and 4 than for those at stages 1, 2 and 4S (P = 0.0157 and P = 0.0082), and for patients with MYCN amplification than for those with a single copy of MYCN (P = 0.0195 and P = 0.0054; Fig. 2b,c,d). These results indicate that the serum NM23‐H1 level serves as a useful prognostic factor for neuroblastoma, as well as the other well‐known prognostic factors.
Table 2.
Relationship between serum NM23‐H1 protein levels and clinicopathological findings in 86 patients with neuroblastoma found clinically
| Characteristics | No. of patients (mean ± SD) | Serum NM23‐H1 (ng/mL) | P‐value (analysis) |
|---|---|---|---|
| All patients | 86 | 239 ± 357 | |
| Age | |||
| < 12 months | 27 | 282 ± 471 | |
| ≥ 12 months | 59 | 219 ± 294 | 0.7694 (MW) |
| Stage | |||
| 1 + 2 + 4s | 21 | 154 ± 187 | |
| 3 + 4 | 65 | 266 ± 394 | 0.3900 (MW) |
| Primary site | |||
| Mediastinal | 11 | 124 ± 207 | |
| Adrenal | 46 | 285 ± 383 | |
| Abdominal | 26 | 220 ± 375 | 0.0982 (KW) |
| Others | 3 | ||
| MYCN copy number | |||
| 1 | 59 | 157 ± 193 | |
| > 3 | 27 | 418 ± 534 | 0.0028 (MW) |
| TrkA expression | 63 | ||
| Medium + high | 28 | 154 ± 189 | |
| 0 + low | 35 | 296 ± 422 | 0.1865 (MW) |
| Ploidy | 66 | ||
| Diploid | 37 | 255 ± 436 | |
| Hyperdiploid | 27 | 234 ± 352 | 0.4304 (MW) |
MW, Mann–Whitney U‐test; KW, Kruskal–Wallis test.
Figure 2.
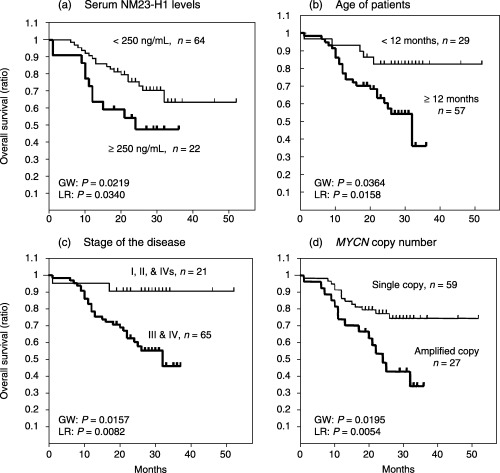
Overall survival curves for 86 patients with neuroblastoma who were found clinically. (a) Overall survival curves for 22 patients with a serum NM23‐H1 level ≥ 250 ng/mL, and for 64 patients with a level < 250 ng/mL. (b) Overall survival curves for 57 patients 12 months of age or older, and for 29 patients younger than 12 months. (c) Overall survival curves for 65 patients at stages 3 and 4 of the disease, and for 21 patients at stages 1, 2 and 4s. (d) Overall survival curves for 27 patients with MYCN amplification, and for 59 patients with a single copy of MYCN. GW, generalized Wilcoxon's test; LR, log‐rank test.
Subsequently, we classified the 86 patients into two groups according to the age of the patients, stage of the disease, or copy numbers of MYCN, and evaluated the influence of the serum NM23‐H1 levels on the overall survival in each one of the six groups (Fig. 3). Of the 29 patients younger than 12 months of age, the seven patients with higher levels of NM23‐H1 had a worse outcome than the 22 patients with the lower levels (P = 0.0401 according to the generalized Wilcoxon test and P = 0.0273 according to the log‐rank test; Fig. 3a). The seven patients with higher levels of NM23‐H1 had the following attributes: stage 1 + 2 + 4S (n = 3); stage 3 + 4 (n = 4); with non‐amplified MYCN (n = 4); with more than three MYCN (n = 3). Likewise, of the 19 patients with a stage 3 tumor, four patients with higher levels had a worse outcome than the 15 patients with lower levels (P = 0.0005 and P < 0.0001; Fig. 3c). The four patients with higher levels of NM23‐H1 had the following attributes: < 12 months of age (n = 0); > 12 months of age (n = 4); with non‐amplified MYCN (n = 1); with more than three MYCN (n = 3). Of the 59 patients with a single copy of MYCN, the 11 patients with higher levels had a worse outcome than the 48 patients with lower levels of serum NM23‐H1 (P = 0.0301 and P < 0.0366; Fig. 3e). The 11 patients with higher levels of NM23‐H1 had the following attributes: < 12 months of age (n = 4); > 12 months of age (n = 7); stage 1 + 2 + 4S (n = 2); stage 3 + 4 (n = 9). In contrast, a higher serum NM23‐H1 level did not influence overall survival in the 57 patients 12 months old or older, in the 46 patients with stage 4 disease, or in the 27 patients with MYCN amplification (Fig. 3b,d,f).
Figure 3.
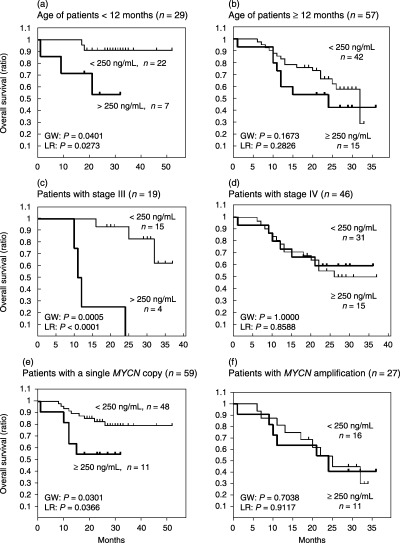
Clinical significance of the serum NM23‐H1 levels in the groups classified according to the age of the patients, or stage of the disease, or copy number of MYCN. (a) Survival curves for seven patients with a serum NM23‐H1 level of ≥ 250 ng/mL, and for 22 patients with a level < 250 ng/mL. Both groups of patients were younger than 12 months of age. (b) Survival curves for 15 patients with a serum NM23‐H1 level of ≥ 250 ng/mL, and for 42 patients with a level < 250 ng/mL. Both groups of patients were 12 months old or older. (c) Survival curves for four patients with a serum NM23‐H1 level of ≥ 250 ng/mL, and for 15 patients with a level < 250 ng/mL. Both groups of patients were at stage 3 of the disease. (d) Survival curves for 15 patients with the serum NM23‐H1 level ≥ 250 ng/mL, and for 31 patients with the level < 250 ng/mL. Both groups of patients were at stage 4 of the disease. (e) Survival curves for 11 patients with a serum NM23‐H1 level of ≥ 250 ng/mL, and for 48 patients with a level < 250 ng/mL. Both groups of patients had a single copy of MYCN. (f) Survival curves for 11 patients with a serum NM23‐H1 level of ≥ 250 ng/mL, and for 16 patients with a level < 250 ng/mL. Both groups of patients had MYCN amplification in the tumor. GW, generalized Wilcoxon's test; LR, log‐rank test.
Four prognostic factors, including the age of the patients, stage of the disease, MYCN copy number, and the serum NM23‐H1 level, were available for multivariate analysis in the 217 patients (Table 3a) and 86 patients (Table 3b). According to multivariate analysis, the serum NM23‐H1 level provided no significant influence on overall survival in either group of patients (Table 3).
Table 3.
Univariate and multivariate analysis for predictors of survival in neuroblastoma
| Prognostic factors | Univariate (χ2, log‐rank) | P‐value | Multivariate (relative risk & 95% CI) | P‐value |
|---|---|---|---|---|
| Patients found by mass‐screening or clinically (n = 217) | ||||
| Serum NM23‐H1(< 250/> 250 ng/mL) | 11.211 | 0.0008 | 1.7294 (0.7997–3.7398) | 0.1639 |
| Age (< 12/≥ 12 months) | 32.353 | < 0.00001 | 3.8979 (1.3818–10.996) | 0.0101 |
| Stage (1, 2, 4s/3, 4) | 33.142 | < 0.00001 | 8.2514 (1.8173–37.466) | 0.0063 |
| NMYC amplification (–/+) | 43.997 | < 0.00001 | 2.3253 (1.0541–5.1297) | 0.0366 |
| Patients found clinically (n = 86) | ||||
| Serum NM23‐H1(< 250/> 250 ng/mL) | 4.493 | 0.0340 | 1.6143 (0.7386–3.5282) | 0.2299 |
| Age (< 12/≥ 12 months) | 5.825 | 0.0158 | 1.4742 (0.4877–4.4563) | 0.4916 |
| Stage (1, 2, 4s/3, 4) | 6.994 | 0.0082 | 3.5721 (0.7158–17.826) | 0.1206 |
| NMYC amplification (–/+) | 7.749 | 0.0054 | 1.9682 (0.9016–4.2967) | 0.0892 |
CI, confidence interval.
Discussion
The NM23‐H1 gene is overexpressed in various hematological malignancies and other neoplasms including neuroblastoma. Overexpression of NM23‐H1 mRNA is indicative of a poor prognosis in patients with neuroblastoma, and mutations and increased copy numbers of NM23‐H1 have been reported in advanced neuroblastoma.( 6 , 24 ) In the present study, we found that the serum NM23‐H1 level was significantly higher in patients with neuroblastoma than in the control children (Fig. 1), and that the serum NM23‐H1 level predicted a poor outcome for patients with tumors (Fig. 2a). Furthermore, the higher level of NM23‐H1 was correlated with a worse outcome in patients younger than 12 months of age, in those with stage 3 disease, or in those with a single MYCN copy (Fig. 3). In contrast, a higher serum NM23‐H1 level did not influence overall survival in patients who were 12 months old or older, in those with stage 4 disease, or in those with MYCN amplification (Fig. 3). These findings suggest that the NM23‐H1 level may be an important factor for predicting the outcome of patients in these low or intermediate risk groups (i.e. patients younger than 12 months of age, with stage 3 disease, or with a single copy of MYCN). In addition, the serum NM23‐H1 level may be a clinically useful prognostic factor, because the measurement of serum NM23‐H1 protein is easily and quickly carried out prior to treatment.
According to multivariate analysis, the serum NM23‐H1 level provided no significant influence on overall survival in either group of patients shown in Table 3. These results might be due to the short observation time, the small number of cases, or the strong correlation between MYCN amplification and the elevated serum NM23‐H1 level.
Although all the 131 patients found by MS were alive at the last follow‐up (18–51 months) and were excluded from survival analysis, they contained 15 patients (the last follow‐up: 19–37 months) with higher levels than 250 ng/mL of serum NM23‐H1. It might be interesting to follow up these patients to clarify the clinical significance of serum NM23‐H1 in the MS group.
Prognostic factors in neuroblastoma have been thoroughly investigated and include MYCN copy number, TRKA expression level, chromosomal ploidy, 1p loss, and 17q gain in tumor cells. Laborious and time‐consuming work is required to examine these biological factors in tumor tissues. Therefore, serum markers that are easily measurable and can predict a clinical outcome are desired. Serum levels of lactate dehydrogenase (LDH) and ferritin are high in advanced stage neuroblastomas, but both may reflect a rapid cellular turnover or a large tumor burden.( 25 , 26 ) Neuron‐specific enolase (NSE) is a cytoplasmic protein that is associated with neural cells, and serum NSE is a useful marker for patients with advanced neuroblastoma in whom the elevated levels are associated with a poor outcome.( 27 ) The disialoganglioside GD2 is found on the surface of most neuroblastoma cells, and elevated plasma levels have been found in patients.( 28 ) Nevertheless, none of these markers is used at present to predict clinical outcomes or to choose treatment protocols. Therefore, serum NM23‐H1 levels might be useful for clinical purposes.
The elevated serum level of NM23‐H1 was correlated with a poor prognostic feature, namely, MYCN amplification (Table 1). Godfrid et al. identified genes that are part of the MYCN downstream pathway using SAGE libraries of MYCN transfected and control neuroblastoma cell lines.( 13 ) The chromosome 17q genes NM23‐H1 and NM23‐H2 were strongly induced in MYCN‐expressing cells. A striking correlation between MYCN amplification and mRNA or protein expression of both NM23 genes was found in the cell lines. The present multivariate analysis showed no influence of serum NM23‐H1 level on overall survival, and this finding might be caused by the overlap of patients with MYCN amplification with those with a high serum level of NM23‐H1. However, within the group of patients with a single copy of MYCN, patients with a higher level of NM23‐H1 had a worse outcome (Fig. 3e). The findings suggest that NYCN amplification may influence serum NM23‐H1 levels as well as clinical outcome, and that neuroblastomas with a single copy of MYCN and a higher serum NM23‐H1 level may have had a mutation or an increased copy number of the NM23‐H1 gene.( 6 , 24 , 29 ) MYCN overexpression in some neuroblastomas with a single copy of MYCN may have resulted in higher serum NM23‐H1 levels and a poor outcome; however, a recent study showed that MYCN overexpression did not affect the prognosis of advanced‐stage neuroblastomas with a single MYCN copy.( 30 )
In patients with NHL and AML, it is thought that serum NM23‐H1 protein is produced directly by the tumor cells, and its serum level depends on the total mass of malignant cells overexpressing NM23‐H1.( 14 ) High concentrations of NM23 protein were found in the serum and body fluid of patients with lung cancer overexpressing the NM23 genes.( 31 ) Tumor cells may secrete this protein through some unknown mechanism, because there is no signal peptide sequence for secretion in the NM23 molecule. Serum NM23‐H1 in patients with neuroblastoma might be derived from tumor cells and might be induced by MYCN amplification/overexpression or by NM23‐H1 overexpression independent of MYCN.
The serum level of NM23‐H1 protein is clinically useful as an important prognostic factor in NHL or AML, and the present study showed that the protein could be a factor predicting an outcome of patients with neuroblastoma. It would be interesting to examine whether the serum NM23‐H1 level generally predicts a poor outcome for patients with other tumors. The mechanisms by which the NM23‐H1 protein is secreted into the serum and how it affects patient outcome are unclear. We are now studying the possibility that a high concentration of serum NM23‐H1 may positively affect tumor cell growth or negatively affect normal cells.
Acknowledgments
We thank Ms K. Yagyu for secretarial assistance. We also appreciate the help of a number of physicians who provided clinical data, and the patients and control children who donated blood. This study was supported in part by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labor and Welfare of Japan for the Second Term Comprehensive 10‐year Strategy for Cancer Control.
References
- 1. Steeg PS, Bevilacqua G, Kopper L et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst 1988; 80: 200–4. [DOI] [PubMed] [Google Scholar]
- 2. Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO. The human nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr 2000; 32: 247–58. [DOI] [PubMed] [Google Scholar]
- 3. Lascu I, Gonin P. The catalytic mechanism of nucleoside diphosphate kinases. J Bioenerg Biomembr 2000; 32: 237–46. [DOI] [PubMed] [Google Scholar]
- 4. MacDonald NJ, Rosa ADL, Steeg PS. The potential roles of nm23 in cancer metastasis and cellular differentiation. Eur J Cancer 1995; 31A: 1096–100. [DOI] [PubMed] [Google Scholar]
- 5. Lacombe ML, Sastre‐Garau X, Lascu I et al. Overexpression of nucleoside diphosphate kinase (Nm23) in solid tumors. Eur J Cancer 1991; 27: 1302–7. [DOI] [PubMed] [Google Scholar]
- 6. Leone A, Seeger RC, Hong CM et al. Evidence for nm23 RNA overexpression, DNA amplification and mutation in aggressive childhood neuroblastoma. Oncogene 1993; 8: 855–65. [PubMed] [Google Scholar]
- 7. Chang C, Zhu X‐X, Thoraval D et al. nm23–H1 mutation in neuroblastoma. Nature (London) 1994; 370: 335–6. [DOI] [PubMed] [Google Scholar]
- 8. Yokoyama A, Okabe‐Kado J, Wakimoto N et al. Evaluation by multivariate analysis of the differentiation inhibitory factor nm23 as a prognostic factor in acute myelogenous leukemia and application to other hematological malignancies. Blood 1998; 91: 1845–51. [PubMed] [Google Scholar]
- 9. Hailat N, Keim DR, Melhem RF et al. High levels of p19/nm23 protein in neuroblastoma are associated with advanced stage disease and with N‐myc gene amplification. J Clin Invest 1991; 88: 341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Niitsu N, Okabe‐Kado J, Okamoto M et al. Serum nm23‐H1 protein as a prognostic factor in aggressive non‐Hodgkin's lymphoma. Blood 2001; 97: 1202–10. [DOI] [PubMed] [Google Scholar]
- 11. Bown N, Cotterill S, Lastowska M et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N Engl J Med 1999; 340: 1954–61. [DOI] [PubMed] [Google Scholar]
- 12. Kaneko Y, Kobayashi H, Maseki N, Nakagawara A, Sakurai M. Disomy 1 with terminal 1p deletion was frequent in mass screening‐negative/late‐presenting neuroblastomas in young children, but not in mass screening‐positive neuroblastomas in infants. Int J Cancer 1999; 80: 54–9. [DOI] [PubMed] [Google Scholar]
- 13. Godfried MB, Veenstra MV, Sluis P et al. The N‐myc and c‐myc downstream pathways include the chromosome 17q genes nm23‐H1 and nm23‐H2. Oncogene 2002; 21: 2097–101. [DOI] [PubMed] [Google Scholar]
- 14. Okabe‐Kado J. Serum nm23‐H1 protein as a prognostic factor in hematological malignancies. Leuk Lymphoma 2002; 43: 859–67. [DOI] [PubMed] [Google Scholar]
- 15. Niitsu N, Okabe‐Kado J, Nakayama M et al. Plasma levels of the differentiation inhibitory factor nm23‐H1 protein and their clinical implication in acute myelogenous leukemia. Blood 2000; 96: 1080–6. [PubMed] [Google Scholar]
- 16. Niitsu N, Nakamine H, Okamoto M et al. Clinical significance of intracytoplasmic nm23‐H1 expression in diffuse large B‐cell lymphoma. Clin Cancer Res 2004; 10: 2482–90. [DOI] [PubMed] [Google Scholar]
- 17. Sawada T, Hirayama M, Nakata T et al. Mass screening for neuroblastoma in infants in Japan. Lancet 1984; 2: 271–3. [DOI] [PubMed] [Google Scholar]
- 18. Brodeur GM, Pritchard J, Berthold F et al. Revision of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clinic Oncol 1993; 11: 1466–77. [DOI] [PubMed] [Google Scholar]
- 19. Sawaguchi S, Kaneko M, Uchino J et al. Treatment of advanced neuroblastoma with emphasis on intensive induction chemotherapy. Cancer 1990; 66: 1879–87. [DOI] [PubMed] [Google Scholar]
- 20. Testa U, Thomopouls P, Vinci G et al. Transferrin binding to K562 cell line. Exp Cell Res 1982; 140: 251–60. [DOI] [PubMed] [Google Scholar]
- 21. Bowman LC, Castleberry RP, Cantor A et al. Genetic staging of unresectable or metastatic neuroblastoma in infants: a Pediatric Oncology Group Study. J Nat Cancer Inst 1997; 89: 373–80. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawara A, Arima‐Nakagawara M, Scavaruda NJ, Azar CG, Cantor AB, Brodeua GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med 1993; 328: 847–54. [DOI] [PubMed] [Google Scholar]
- 23. Willem R, Van Bockstaele DR, Lardon F et al. Decrease in nucleoside diphosphate kinase (NDPK/nm23) expression during hematopoietic maturation. J Biol Chem 1998; 273: 13663–8. [DOI] [PubMed] [Google Scholar]
- 24. Takeda O, Handa M, Uehara T et al. An increased NM23‐H1 copy number may be a poor prognostic factor independent of LOH on 1p in neuroblastomas. Br J Cancer 1996; 74: 1620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hann H‐WL, Evans AE, Siegel SE et al. Prognostic importance of serum ferritin in patients with stages III and IV neuroblastoma: The Children's Cancer Study Group Experience. Cancer Res 1985; 45: 2843–8. [PubMed] [Google Scholar]
- 26. Shuster JJ, McWilliams NB, Castleberry R et al. Serum lactate dehydrogenase in childhood neuroblastoma. A pediatric oncology group recursive partitioning study. Am J Clin Oncol 1992; 15: 295–303. [DOI] [PubMed] [Google Scholar]
- 27. Zeltzer PM, Marangos PJ, Evans AE, Schneider SL. Serum neuron‐specific enolase in children with neuroblastoma. Relationship to stage and disease course. Cancer 1986; 57: 1230–4. [DOI] [PubMed] [Google Scholar]
- 28. Landisch S, Wu Z‐L. Detection of a tumour‐associated ganglioside in plasma of patients with neuroblastoma. Lancet 1985; 1: 136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Almgren MAE, Henriksson KCE, Fujimoto J, Chang CL. Nucleoside diphosphate kinase A/nm23‐H1 promotes metastasis of NB69‐derived human neuroblastoma. Mol Cancer Res 2004; 2: 387–94. [PubMed] [Google Scholar]
- 30. Cohn SL, London WB, Huang D et al. MYCN expression is not prognostic of adverse outcome in advanced‐stage neuroblastoma with nonamplified MYCN . J Clin Oncol 2000; 18: 3604–13. [DOI] [PubMed] [Google Scholar]
- 31. Huwer H, Kalweit G, Engel M, Welter C, Dooley S, Gams E. Expression of the candidate tumor suppressor gene nm23 in the bronchial system of patients with squamous cell lung cancer. Eur J Cardiothorac Surg 1997; 11: 206–9. [DOI] [PubMed] [Google Scholar]


