Abstract
We previously reported a pilot study of thalidomide monotherapy for Japanese patients with refractory or relapsed multiple myeloma. In the present work, we have extended this clinical trial to a single‐institute phase 2 study with a larger number of patients and longer follow‐up time. New information on the optimal dose and prognostic factors as well as the correlation of toxicities with treatment schedule was obtained. Fifteen of 56 (27%) patients achieved a partial response, including three cases with near‐complete remission. Most patients suffered toxicities at a dose of 400 mg per day, but there was no clear dose–response relationship. Thus, a lower dose such as 200 mg per day or less is considered optimal. Multivariate analyses identified only lack of response to therapy as an adverse prognostic factor for progression‐free survival. Chromosomal abnormality, C‐reactive protein >10 mg/L, and more than six previous courses of chemotherapy were significantly associated with shorter overall survival. Grade 3 or 4 neutropenia and thrombocytopenia were observed in 23 and 11% of patients, respectively. Grade 4 interstitial pneumonia and grade 5 pulmonary hypertension were observed; however, no patient suffered deep vein thrombosis, which has frequently been observed in other studies. Duration of therapy was closely related to the development of peripheral neuropathy. The efficacy and prognostic factors of this treatment were confirmed in long‐term observation. However, special attention should be paid to toxicities such as hematological and pulmonary complications as well as peripheral neuropathy in long‐term users. (Cancer Sci 2008; 99: 1243–1250)
Multiple myeloma (MM) is a hematological malignancy that occurs predominantly in the elderly, with most patients being diagnosed in their early sixties. Although several chemotherapeutic regimens and high‐dose therapies combined with autologous hematopoietic stem cell transplantation have improved survival, most patients relapse and become resistant to therapy. In theory, allogeneic transplantation eradicates the disease via the graft‐versus‐myeloma effect. However, few patients are appropriate candidates for this treatment, and there is a high frequency of therapy‐related early death. Therefore, new potential therapies should be explored extensively.
Thalidomide is a glutamic acid derivative that was used in the late 1950s as a therapy for pregnancy‐related morning sickness. In 1961, when the teratogenicity of this drug was recognized, thalidomide was taken off the market worldwide.( 1 , 2 ) However, in the 1990s, thalidomide reemerged as an antineoplastic drug with antiangiogenic activity.( 3 ) Recent studies have shown that thalidomide is effective for the treatment of relapsed and refractory MM.( 4 , 5 ) For example, a large‐scale phase 2 trial including 169 patients revealed that 30% of the patients achieved a reduction of more than 50% in M protein with 9% progression‐free survival (PFS) and 25% overall survival (OS) at 4 years.( 6 ) The deletion of chromosome 13, high β2 microglobulin (β2M), a high plasma cell labeling index, and the use of less than 42 g thalidomide in 3 months were associated with shorter OS.( 6 ) A systematic review of phase 2 trials of thalidomide monotherapy in patients with relapsed or refractory MM revealed a 29% response rate and median OS at 14 months.( 5 ) Grade 3 or 4 toxicities included somnolence, constipation, peripheral neuropathy, skin rash, and deep vein thrombosis (DVT).( 5 ) Recently, the combination of thalidomide and dexamethasone has shown a synergistic effect with a higher response rate compared with thalidomide alone,( 7 ) and very recently the use of thalidomide with melphalan plus prednisone (MP) in untreated patients has provided a survival benefit over MP alone.( 8 , 9 )
We previously reported the results of thalidomide monotherapy for patients with refractory or relapsed MM.( 10 ) We extended the clinical trial as a single‐institute phase 2 study with a larger number of patients and longer follow‐up time. Namely, 56 patients including 24 cases reported in the previous report( 10 ) were observed with a median follow‐up time of approximately 4 years. Here we report on the clinical outcome of this more extensive study, including prognostic factors and optimal dose. We also demonstrated that the duration but not the daily dose of thalidomide correlated with the occurrence of peripheral neuropathy, a side‐effect that frequently hampers the continuation of thalidomide therapy.
Patients and Methods
Patients. A total of 61 patients with MM started thalidomide monotherapy at Keio University Hospital between November 1998 and October 2005, and the closeout day was 31 December 2006. All 61 patients were included in the evaluation of adverse events. Five patients were excluded from the evaluation of efficacy and prognosis because of a lack of disease marker in one patient with non‐secretory MM, early discontinuance of thalidomide due to grade 4 neutropenia in three patients, and interstitial pneumonitis in one patient, leaving a total of 56 patients. Patient eligibility was defined as follows: (1) patients with MM relapsed after hematopoietic stem cell transplantation or those who were resistant to at least two different chemotherapeutic regimens; (2) at least 4 weeks since the last chemotherapy or radiotherapy; and (3) Karnofsky performance status greater than 60. Women who might have become pregnant during thalidomide treatment were strictly excluded. Patients were also excluded if they had moderate to severe organ failure, if they had a serum transaminase or γ‐glutamyl transpeptidase level higher than three times the upper limit of normal subjects or a serum creatinine level over 30 mh/L, if they had a severe neurological or psychological disturbance, or if they had cardiac failure or arrhythmia requiring medical intervention. Patients with active infections or who were positive for hepatitis B or C virus, human immunodeficiency virus, or human T‐cell leukemia virus type I were also excluded. Written informed consent was obtained from all patients at the time of enrollment. This study was approved by the ethical committee of the Keio University School of Medicine. Beginning in December 2004, this study was conducted according to the guidelines for thalidomide treatment of MM established by the Japanese Society of Clinical Hematology.
Treatment schedule. Thalidomide was supplied by the Sociedade Farmaceutic Brasifa (Rio de Janeiro, Brazil) or by the Penn Pharmaceutical Service (Tredegar, UK) in 100‐mg tablets. Thirteen patients were treated with thalidomide provided from the former supplier in Brazil, and six of them (46%) responded to therapy. No notable differences in toxicity pattern were observed between the 13 patients and the rest of the cases. A dose of 200 mg was administered at night to all patients, and unless intolerable adverse effects were observed within 1 week, the dose was increased to 400 mg, which was then continued as a maintenance dose. In cases in which the dose escalation was difficult because of adverse effects, the affected patients continued to receive the lower dose of thalidomide. During thalidomide treatment, the patients were not permitted to receive any other chemotherapy or radiotherapy. When disease progression was observed, different treatment plans, including combination therapy consisting of thalidomide with dexamethasone, were considered. Dexamethasone was used for 4 consecutive days, usually on a schedule of every second week, at a standard dose of 20 mg per day. Based on tolerability and disease activity, the dose was modified from 10 to 40 mg per day. Supportive therapies including blood transfusion, granulocyte‐colony stimulating factor (G‐CSF), and supplemental gamma globulin were allowed. Bisphosphonates were permitted only if they had been used prior to the commencement of thalidomide. The patients’ symptoms and laboratory examination results, including complete blood cell counts, serum transaminase, serum creatinine, serum calcium, C‐reactive protein (CRP), M protein level, and β2M were examined at least once per month. Chromosomal abnormalities were recorded based on the data from 20 Giemsa‐banded metaphases.
Assessment of response. Response to thalidomide was evaluated as described previously.( 10 ) Briefly, all responses, including stable disease, had to be maintained for at least 4 weeks in order to be counted as a response. The responses were classified as complete remission (CR), partial remission (PR), minimal response (MR), no change (NC), or progression of disease (PD). CR was defined as: (1) the disappearance of monoclonal protein and urine Bence–Jones protein determined by immunoelectrophoresis; (2) the disappearance of plasmacytoma; (3) fewer than 5% plasma cells in bone marrow; (4) no progression of bone lesions; and (5) normalization of anemia and hypercalcemia. PR was defined as: (1) a decrease of more than 50% in serum M protein levels or daily urine Bence–Jones protein; (2) a decrease in bone marrow plasma cells; (3) no progression of plasmacytoma or lytic bone lesions; and (4) normalization of serum calcium levels. If the decrease in M protein was between 25 and 50%, the response was considered to be minimal. NC was defined as both of the following: (1) a decrease of less than 25% in M protein levels; and (2) no progression of plasmacytoma or bone lesions. PD was defined as one or both of the following: (1) an increase in M protein or bone marrow plasma cells; or (2) progression of plasmacytoma or bone lesions. Patients who achieved CR, PR, or MR were considered to be responders, and those classified as NC or PD were considered non‐responders. In responders, an increase in serum or urine M protein of more than 25% above the minimum value on at least two consecutive occasions was considered to constitute a relapse.
Assessment of adverse effects. The severity of adverse effects was graded according to the National Cancer Institute Common Adverse Events version 3.0. The dose of thalidomide was adjusted according to the adverse effects as follows. If a grade 3 non‐hematological toxicity occurred, the drug was withheld. In the case of a grade 2 non‐hematological toxicity, the dose was reduced to tolerance level; that is, the toxicity was improved to at least grade 1. If the patient's white blood cell count was below 2 × 109/L or his or her neutrophil count was less than 1 × 109/L, the dose of thalidomide was reduced by one‐half, and G‐CSF was used if necessary. If the white blood cell count was below 1 × 109/L or the neutrophil count was less than 0.5 × 109/L, thalidomide was discontinued. If any other grade 3 or higher hematological toxicity was observed, the drug was withheld. Thalidomide was restarted at 50% of the original maintenance dose when the toxicity was alleviated and the patient had recovered to the pretreatment level.
Plasma concentration of thalidomide. Plasma samples were collected 1 month after the start of therapy, when plasma concentrations had stabilized. The measurement of plasma concentrations at 12 ± 2 h after the last thalidomide administration was described in our previous work.( 10 ) The lower limit of detection was 0.1 µg/mL. Our examination of the pharmacokinetics of a single dose of 200 or 400 mg thalidomide in patients with MM showed that the maximum concentration was obtained in 4–6 h, and that the concentration then stabilized at 10–14 h after each dose. Thalidomide was given nightly, and plasma concentrations were examined the next morning at 12 ± 2 h after the last dose. To examine correlations between plasma concentration and treatment efficacy, if M protein was reduced by more than 25% at plasma sampling, the patient was considered to be a responder.
Plasma concentrations of growth factors. Plasma samples were collected within 2 h of blood sampling. Plasma concentrations of basic fibroblast growth factor (FGF)‐2, vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) were determined using a sandwich enzyme‐linked immunosorbent assay technique (R & D Research Corporation, Minneapolis, MN, USA) as described previously.( 11 ) Tests at all time points were carried out in triplicate. The plasma concentrations of normal subjects were: FGF‐2, 2.19 ± 1.87 ng/L (range 1.0–7.67 ng/L); VEGF, 17.7 ± 5.4 ng/L (range 15.6–38.3 ng/L); and HGF, <0.3 µg/L.( 11 ) When concentrations were higher than the maximum of normal subjects, that is, over 7.67 ng/L for FGF‐2, over 38.3 ng/L for VEGF, and over 0.3 µg/L for HGF, they were considered to have increased.
Statistical analysis. Time to cessation and time to dose reduction were evaluated and described by the Kaplan–Meier method. The prognostic factors studied prospectively for response to therapy, PFS, and OS were age (>55 years vs younger), sex, Durie–Salmon stage (stage 3 vs stage 1 or 2), International Staging System (stage 3 vs stage 2 vs stage 1), M protein type (IgG, Bence‐Jones, IgA, or IgD vs other types), previous history of hematopoietic stem cell transplantation, chromosomal abnormality detected by Giemsa‐banding, serum albumin (<30 g/L vs 30 g/L or higher), serum β2M (3.5 mg/L or higher vs <3.5 mg/L), lactate dehydrogenase (LDH) (>200 IU/L vs 200 IU/L or lower), hemoglobin (85 g/L or higher vs <85 g/dL), CRP (10 mg/L or higher vs <10 mg/L), serum creatinine (>15 mg/L vs 15 mg/L or lower), corrected calcium level (>102 mg/L vs 102 mg/L or lower), bone marrow plasma cell percentage (>30% vs 30% or less), response to the last treatment (CR, PR, or MR vs NC or PD), frequency of previous chemotherapy (>6 courses vs 6 courses or fewer), primary refractory case or not, mean daily dose (>200 mg per day vs 200 mg or less), and total dose (>20 g vs 20 g or less). Associations between clinical characteristics and response to thalidomide were examined using Fisher's exact test and the x 2‐test.( 2 ) The correlation of plasma concentrations of angiogenic growth factors with response to therapy was evaluated using the Mann–Whitney U‐test. PFS was measured from the date of initiation of thalidomide treatment to the date of progression or death for any reason. OS was measured from the date of initiation of thalidomide treatment to the date of death. For PFS and OS, response to thalidomide therapy (CR, PR, and MR vs NC and PD), total dose of thalidomide and pretreatment plasma concentrations of angiogenic growth factors including HGF (>0.3 µg/L vs 0.3 µg/L or less), FGF‐2 (>7.67 ng/L vs 7.67 ng/L or less), and VEGF (>38.3 ng/L vs 38.3 ng/L or less) were also included. The Kaplan–Meier method was used to evaluate the prognostic factors of PFS and OS in univariate analysis, censoring PFS and OS on 31 December 2006. For multivariate analysis, those variables with a value of P < 0.1 in univariate analyses were subsequently included in a Cox proportional hazards regression analysis by backward stepwise selection.( 12 ) The association of seven frequent toxicities with mean daily dose, plasma concentrations of thalidomide, total dose, and duration of treatment of thalidomide was evaluated using the Mann–Whitney U‐test. The software package SPSS version 15 (SPSS, Chicago, IL, USA) was used for statistical analysis. P‐values <0.05 were considered statistically significant.
Results
Patients’ characteristics and treatment. The clinical backgrounds of the 56 evaluable patients prior to thalidomide therapy are shown in Table 1. The median age was 57 years (range 30–70 years). Ninety‐one percent of the patients were diagnosed as having Durie–Salmon stage 3, and 20% were classified as having stage 3 by the International Staging System. Sixty‐nine percent had received more than six cycles of chemotherapy. Twenty‐one patients received autologous hematopoietic stem cell transplantation, and five relapsed after allogeneic stem cell transplantation. Thus, most patients were considered to have far‐advanced disease and had been heavily treated prior to thalidomide therapy. Chromosomal abnormality was observed in 14 patients including 10 cases with deletion of chromosome 13. The median follow‐up time from the start of thalidomide to the closeout day was 219 weeks (range 65–426 weeks).
Table 1.
Patients’ characteristics
| Variable | n/N (%) |
|---|---|
| Age > 55 years old | 35/56 (63) |
| Male | 36/56 (64) |
| Durie–Salmon stage 3 | 51/56 (91) |
| International Staging System | |
| Stage 1 | 28/56 (50) |
| Stage 2 | 17/56 (30) |
| Stage 3 | 11/56 (20) |
| M protein | |
| IgG | 28/56 (50) |
| BJP | 12/56 (21) |
| IgA | 14/56 (25) |
| IgD | 2/56 (4) |
| kappa light chain | 34/56 (61) |
| λαµβαλιγητχηαιν | 22/56 (39) |
| Stem cell transplantation | 26/56 (46) |
| Chromosomal abnormality | 14/52 (27) |
| Albumin <30 g/L | 17/56 (30) |
| β2 microglobulin >3.5 mg/L | 24/56 (43) |
| Lactate dehydrogenase > 200 IU/L | 21/56 (38) |
| Hb <85 g/L | 17/56 (30) |
| C‐reactive protein > 10 mg/L | 7/56 (13) |
| Serum Cr >15 mg/L | 6/56 (11) |
| Corrected calcium >10.2 mg/dL | 9/55 (16) |
| Bone marrow plasma cell >30% | 15/53 (28) |
| No response to the last therapy | 37/56 (66) |
| Plasma concentrations >2 mg/L | 25/43 (58) |
| Previous therapy >6 courses | 38/55 (69) |
| Primary refractory case | 14/56 (25) |
| Pretreatment growth factor level | |
| Hepatocyte growth factor >0.3 ng/L | 18/52 (35) |
| Fibroblast growth factor‐2 >7.67 ng/L | 38/52 (73) |
| Vascular endothelial growth factor >38.3 ng/L | 36/52 (69) |
BJP, Bence‐Jones protein; Cr, creatinine; Hb, hemoglobin; n, number of patients; N, number with valid data for each factor.
Time to cessation of thalidomide is shown in Figure 1a. The median duration of thalidomide monotherapy was 14 weeks. At the end of 2006, only one patient was still receiving thalidomide monotherapy. The reasons for the cessation of thalidomide therapy in the remaining 55 patients were progression of disease in 41 cases, adverse events in 12 cases, and preparation for autologous hematopoietic stem cell transplantation in two cases. Adverse events that led to the discontinuance of therapy included neutropenia in six patients, and pulmonary hypertension, interstitial pneumonitis, peripheral neuropathy, myopathy, fungal pneumonia, and hearing disturbance in one case each. In the present study, the patients received a daily dose of 200 mg thalidomide for 2 weeks as an initial dose and, unless significant toxicity was observed, the daily dose was then increased to 400 mg as a standard dose. The dose was reduced due to toxicities in 39 cases. The primary reasons for dose reduction within the first 3 months included somnolence in 10 cases, neutropenia in five, peripheral neuropathy in four, infection in four, skin rash in three, dizziness in three, nausea and vomiting in three, constipation in two, and fever in one case. Dose reduction later than 3 months after the initiation of therapy occurred in four cases, all due to peripheral neuropathy. The time to dose reduction is shown in Figure 1b. The median time of dose reduction was 3 weeks after the initiation of therapy. Figure 1c shows changes in the daily thalidomide dose over time. Even though the standard dose was 400 mg per day in the present study, approximately half of the patients were unable to tolerate 400 mg for 1 month, and only 5 of 21 (24%) patients were able to continue this dose for 6 months. In contrast, a daily dose of 200 mg or lower was well tolerated in 14 (67%) cases at 6 months after the initiation of therapy and one patient was able to continue the lower dose for 45 months. Thus, a dose of 200 mg or less per day was considered preferable from the point of view of toxicity.
Figure 1.
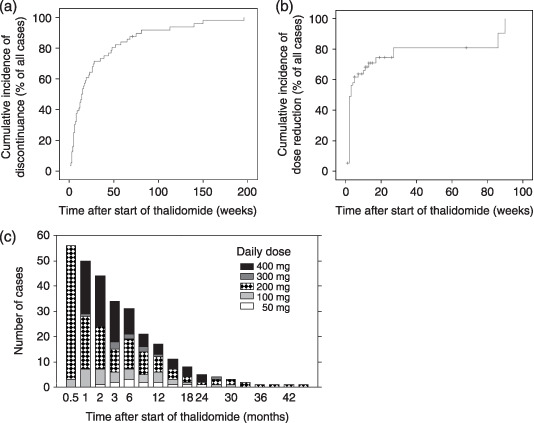
Dose reduction and discontinuance of thalidomide. Although 400 mg thalidomide daily was the standard dose for all patients, only a limited number of patients could tolerate this dose longer than 6 months because of the toxicities. (a) Time to cessation of thalidomide; (b) time to the first dose reduction; (c) time course change of the daily dose during thalidomide monotherapy. A daily dose of 200 mg or less is considered optimal for long‐term use.
Response to therapy. Twenty‐two of 56 patients showed a reduction of more than 25% in M protein during treatment with thalidomide alone, and the overall response rate was 39%. Among the 22 responders, 15 achieved PR (a reduction of more than 50% in M protein), and near‐CR (disappearance of the M peak by urinary or blood protein electrophoresis) was also observed in three cases (5.4%). In order to predict the response to thalidomide, monovariate analysis of the patients’ backgrounds was conducted. However, none of the following factors was significantly correlated with treatment response: age, sex, clinical stage, type of M protein, anemia, serum albumin level, renal function, β2M level, LDH level, CRP level, corrected serum calcium level, bone marrow plasma cell percentage, chromosomal abnormality, response to the last treatment, previous history of hematopoietic stem cell transplantation, or pretreatment plasma concentration of angiogenic growth factors (data not shown). Thus, we conclude that it is difficult to predict the response before the initiation of therapy. The median daily dose and plasma concentration of thalidomide at 1 month after the initiation of therapy were also compared between responders and non‐responders; however, neither was found to be significantly correlated with response. Specifically, the median daily doses for responders and non‐responders were 300 and 200 mg per day (P = 0.28), respectively, and plasma concentrations of thalidomide in responders and non‐responders were 24 and 21 mg/L, respectively (P = 0.61). Therefore, dose intensity is not an important factor for the response to thalidomide monotherapy.
Thirty out of 56 patients (54%), including 19 cases who were primarily refractory to thalidomide monotherapy and 11 cases who relapsed after first achieving a response, were treated with combination therapy of thalidomide with dexamethasone. Seven patients, including four refractory cases and three relapsed cases, responded to the combination therapy and achieved PR.
Progression‐free survival and overall survival. Figure 2 shows the Kaplan–Meier curves for the PFS and OS of all patients at 2 years, which were estimated to be 10.7 and 41.3%, respectively. The median time of PFS was 8 weeks, and the median time of OS was 83 weeks. In order to evaluate the aspects of each patient's background that affected survival, univariate analysis was conducted, and the results are summarized in Table 2. The Kaplan–Meier curves of statistically significant factors are also shown in Figure 2. The poor prognostic factors for PFS were a lack of response to therapy including NC and PD (P < 0.001) and Durie–Salmon stage 3 (P = 0.037). The poor prognostic factors for OS were lack of response to therapy (P = 0.009), chromosomal abnormalities (P < 0.001), high LDH level (over 200 IU/L; P = 0.001), elevation of CRP (over 10 mg/L; P = 0.002), more than six cycles of previous chemotherapies (P = 0.002), elevated pre treatment HGF level (P = 0.025), and lower pretreatment VEGF level (P = 0.044). Mean daily dose was not significantly correlated with PFS or OS. Thus, treatment with a higher dose did not always improve survival. Lack of response to therapy was a poor prognostic factor for PFS, and chromosomal abnormality, increased CRP level, and more than six cycles of previous chemotherapy were significant poor prognostic factors for OS (Table 3).
Figure 2.
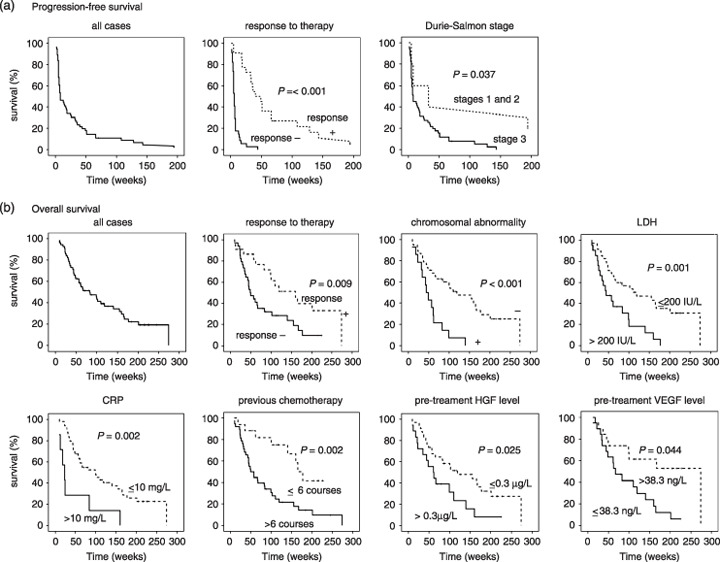
Probability of survival of the patients treated with thalidomide. (a) Kaplan–Meier curve of progression‐free survival (PFS); (b) Kaplan–Meier curve of overall survival (OS). The median time of PFS was 8 weeks. Lack of response to therapy and Durie–Salmon stage 3 were inversely correlated with PFS. The median time of OS was 83 weeks. Lack of response to therapy, chromosomal abnormality, a serum lactate dehydrogenase (LDH) level of 200 IU/L or higher, a C‐reactive protein (CRP) level of 10 mg/L or higher, more than six courses of previous chemotherapy, elevated pretreatment hepatocyte growth factor (HGF) levels (>0.3 µg/L), and low pretreatment vascular endothelial growth factor (VEGF) levels (≤38.3 ng/L) were associated with poor prognosis for OS.
Table 2.
Patient characteristics affecting progression‐free and overall survival in univariate analysis
| Characteristics | No. patients | Estimated median survival (weeks) | P‐value |
|---|---|---|---|
| Progression‐free survival | |||
| Response to therapy | |||
| No response | 34 | 6.00 ± 0.52 | |
| Response | 22 | 40.00 ± 7.51 | <0.001 |
| Durie–Salmon stage | |||
| Stages 1 and 2 | 5 | 33.00 ± 27.39 | |
| Stage 3 | 51 | 8.00 ± 3.11 | 0.037 |
| International staging system | |||
| Stages 1 and 2 | 45 | 13.00 ± 3.83 | |
| Stage 3 | 11 | 6.00 ± 0.89 | 0.131 |
| Chromosomal abnormality | |||
| – | 38 | 8.00 ± 6.94 | |
| + | 14 | 7.00 ± 0.46 | 0.184 |
| Plasma concentration of thalidomide | |||
| <2 mg/L | 18 | 13.00 ± 10.61 | |
| ≥2 mg/L | 25 | 14.00 ± 4.00 | 0.172 |
| Overall survival | |||
| Response to therapy | |||
| No response | 34 | 48.00 ± 9.48 | |
| Response | 22 | 160.00 ± 37.58 | 0.009 |
| Chromosomal abnormality | |||
| – | 38 | 119.00 ± 35.57 | |
| + | 14 | 44.00 ± 6.55 | <0.001 |
| Lactate dehydrogenase | |||
| ≤200 IU/L | 35 | 119.00 ± 45.10 | |
| >200 IU/L | 21 | 44.00 ± 14.71 | 0.001 |
| C‐reactive protein | |||
| ≤10 mg/L | 49 | 100.00 ± 23.08 | |
| >10 mg/L | 7 | 18.00 ± 7.86 | 0.001 |
| Previous therapy | |||
| ≤6 courses | 17 | 177.00 ± 14.08 | |
| >6 courses | 38 | 49.00 ± 9.25 | 0.002 |
| Pretreatment plasma hepatocyte growth factor level | |||
| ≤0.3 µg/L | 34 | 119.00 ± 44.39 | |
| >0.3 µg/L | 18 | 61.00 ± 14.85 | 0.025 |
| Pretreatment plasma vascular endothelial growth factor level | |||
| ≤38.3 ng/L | 16 | 49.00 ± 16.00 | |
| >38.3 ng/L | 36 | 119.00 ± 25.66 | 0.044 |
| Allogeneic stem cell transplantation | |||
| – | 51 | 99.00 ± 19.99 | |
| + | 5 | 37.00 ± 6.57 | 0.077 |
| International staging system | |||
| Stages 1 and 2 | 45 | 84.00 ± 26.28 | |
| Stage 3 | 11 | 57.00 ± 35.23 | 0.078 |
Table 3.
Significant prognostic factors in multivariate analysis
| Characteristic | Hazard ratio (95% confidence interval) |
|---|---|
| Progression‐free survival | |
| Response to therapy | |
| No response | 1 |
| Response | 0.054 (0.018–0.168) |
| Overall survival | |
| Chromosomal abnormality | |
| – | 1 |
| + | 5.631 (2.450–12.943) |
| C‐reactive protein | |
| ≤10 mg/L | 1 |
| >10 mg/L | 5.403 (2.044–14.283) |
| Previous therapy | |
| ≤6 courses | 1 |
| >6 courses | 2.905 (1.258–6.707) |
Adverse events during thalidomide monotherapy. During treatment with thalidomide, all 61 patients complained of at least one adverse event. Somnolence, constipation, peripheral neuropathy, dry mouth, and skin rash appeared in more than 50% of patients (Table 4). Severe toxicities of grade 3 or higher were observed in 31 patients (Table 4). Hematological toxicities were the most frequent serious adverse events, with neutropenia and thrombocytopenia being observed in 13 (21%) and 7 (11%) of our 61 patients, respectively. Grade 3 infection occurred in 11 patients (18%), and grade 3 or 4 lymphopenia in 3 patients (5%) during thalidomide monotherapy. Grade 3 peripheral neuropathy, dizziness, nausea, hearing disturbance, and wrist‐drop were also observed in one case each (2%) (Table 4). Pulmonary complications such as grade 4 interstitial pneumonia and grade 5 pulmonary hypertension were observed in one case each. A detailed case report of pulmonary hypertension was presented in our previous work.( 13 ) In the present case, there was no finding of pulmonary embolism in the postmortem autopsy, and it remains unknown whether the occurrence of pulmonary hypertension was related to thalidomide or was coincidental. Importantly, DVT was not observed during thalidomide therapy in the present study, even though it has frequently been reported in previous studies.( 6 , 14 )
Table 4.
Adverse events
| Adverse event | No. patients (%) n = 61 |
|---|---|
| All adverse events | |
| Somnolence | 48 (79%) |
| Constipation | 35 (57%) |
| Dry mouth | 34 (56%) |
| Peripheral neuropathy | 34 (56%) |
| Skin rash | 33 (54%) |
| Fever | 20 (33%) |
| Neutropenia | 15 (25%) |
| Finger tremor | 14 (23%) |
| Infection | 12 (20%) |
| Dizziness | 11 (18%) |
| Thrombocytopenia | 10 (16%) |
| Fatigue | 9 (15%) |
| Gastrointestinal symptoms | 6 (10%) |
| Headache | 6 (10%) |
| Grade ≥ 3 adverse events only | |
| Neutropenia | 13 (22%) |
| Infection | 11 (18%) |
| Thrombocytopenia | 7 (11%) |
| Lymphopenia | 3 (5%) |
| Peripheral neuropathy | 1 (2%) |
| Dizziness | 1 (2%) |
| Gastrointestinal symptoms | 1 (2%) |
| Interstitial pneumonia | 1 (2%) |
| Pulmonary hypertension | 1 (2%) |
| Hearing disturbance | 1 (2%) |
| Wrist‐drop | 1 (2%) |
The relationship between the frequently observed adverse events and the dose and duration of thalidomide therapy was examined and the results are shown in Table 5. None of the adverse events was significantly associated with mean daily dose or plasma concentration of thalidomide. Peripheral neuropathy, constipation, and dry mouth were significantly correlated with total dose and treatment time but not with daily dose or plasma concentration of thalidomide. Assuming that constipation and dry mouth are neurogenic toxicities, the time of exposure to thalidomide is likely to be an important factor for these adverse events. In order to clarify the correlation of adverse events with treatment time, the time course frequency of each adverse event was examined (Fig. 3). Somnolence, fever, and neutropenia frequently emerged in the first 2 weeks, and their frequency declined over time. In contrast, peripheral neuropathy, constipation, and dry mouth gradually increased in frequency with increasing duration of treatment. For example, peripheral neuropathy appeared in approximately half of the patients at 6 months after the start of therapy and in all patients at 1 year.
Table 5.
Adverse events and treatment schedule of thalidomide
| Adverse event | No. patients (n = 61) | Mean daily dose (mg/day) | Plasma concentration (mg/L) † | Total dose (g) | Duration (months) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (range) | P‐value | Median (range) | P‐value | Median (range) | P‐value | Median (range) | P‐value | |||
| Peripheral neuropathy | + | 34 | 214.0 (29.6–393.2) | ns | 2.2 (0.7–5.7) | ns | 49.7 (2.0–394.4) | P < 0.001 | 27.5 (1–196) | P < 0.001 |
| – | 27 | 200.0 (76.2–378.8) | 2.1 (0.4–6.0) | 5.6 (0.4–96.4) | 5 (1–71) | |||||
| Somnolence | + | 48 | 207.9 (29.6–393.2) | ns | 2.2 (0.4–5.7) | ns | 22.7 (0.4–394.4) | ns | 14.5 (1–196) | ns |
| – | 13 | 193.1 (76.2–333.0) | 2.0 (0.5–6.0) | 10.6 (0.4–194.8) | 8 (1–140) | |||||
| Constipation | + | 35 | 204.9 (62.4–393.2) | ns | 2.4 (0.6–5.7) | ns | 39.3 (0.4–394.4) | P = 0.001 | 26 (1–196) | P < 0.001 |
| – | 26 | 206.5 (29.6–390.8) | 1.8 (0.4–6.0) | 7.7 (0.4–59.8) | 6 (1–75) | |||||
| Skin rash | + | 33 | 204.9 (62.4–393.2) | ns | 1.6 (0.4–5.7) | ns | 23.0 (3.0–394.4) | ns | 17 (3–196) | P = 0.041 |
| – | 28 | 213.5 (29.6–390.8) | 2.6 (0.6–6.0) | 11.8 (0.4–262.3) | 10 (1–113) | |||||
| Dry mouth | + | 34 | 219.9 (29.6–393.2) | ns | 2.3 (0.4–5.7) | ns | 32.6 (3.8–394.4) | P = 0.003 | 22.5 (3–196) | P < 0.001 |
| – | 27 | 200.0 (76.2–378.8) | 2.1 (0.5–6.0) | 11.2 (0.4–96.4) | 8 (1–71) | |||||
| Neutropenia | + | 15 | 206.3 (95.4–390.8) | ns | 1.4 (1.0–4.2) | ns | 28.0 (0.4–194.8) | ns | 14 (1–140) | ns |
| – | 46 | 205.8 (29.6–393.2) | 2.5 (0.4–6.0) | 16.0 (1.6–394.4) | 13.5 (1–96) | |||||
| Fever | + | 20 | 200.0 (29.6–331.6) | ns | 2.6 (0.5–6.0) | ns | 10.5 (0.4–394.4) | ns | 15 (1–196) | ns |
| – | 41 | 219.0 (62.4–393.2) | 2.2 (0.4–5.1) | 18.6 (0.4–208.8) | 14 (1–150) | |||||
The relationship between adverse events and treatment schedule was evaluated by the Mann–Whitney test. P < 0.05 was considered to be statistically significant. ns, not significant.
Plasma concentrations of thalidomide were available and examined in 47 cases.
Figure 3.
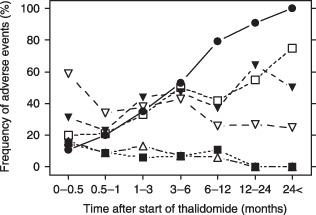
Time course change of the frequency of the primary adverse events during thalidomide monotherapy. Somnolence, fever, and neutropenia emerged in the first 2 weeks, and their frequency declined over time. In contrast, the frequency of peripheral neuropathy, constipation, and dry mouth increased gradually with increasing duration of treatment,  peripheral neuropathy;
peripheral neuropathy;  dry mouth;
dry mouth;  constipation;
constipation;  somnolence;
somnolence;  fever;
fever;  neutropenia.
neutropenia.
Discussion
A number of studies on single‐agent thalidomide therapy for refractory or relapsed MM have been reported and show a 30–60% response rate.( 5 , 6 , 15 , 16 , 17 , 18 , 19 ) In the present study, 39% of the patients responded to thalidomide monotherapy at a dose of 100–400 mg per day, and 27% achieved PR. Although the median daily dose in the present study was only 200 mg, which is lower than those reported in most previous studies, the present study demonstrated as good a response rate as the previous reports.( 5 , 6 , 15 , 16 , 17 , 18 , 19 ) Importantly, it was difficult for many of our patients to continue a daily dose of 400 mg thalidomide for a long time because of its toxicities (Fig. 1; Table 5). Thus, we consider 200 mg or less to be the optimal dose. Other studies also support the efficacy of low‐dose thalidomide therapy.( 20 , 21 ) As described in the Results section above, response to thalidomide therapy was not significantly associated with the median daily dose. A previous report by Barlogie et al. indicated a dose–response effect in that patients who were treated with more than 42 g thalidomide over a period of 3 months, corresponding to approximately 460 mg per day, showed a higher response rate and longer 2‐year OS.( 6 ) This discrepancy may have arisen from the longer follow‐up time and the lower daily dose in the present study. Our median follow‐up time was longer than 4 years. A daily dose of at least 400 mg for at least 3 months might be necessary to obtain an observable dose–response effect of thalidomide. However, such a high dose was unacceptable in our patients due to toxicity.
The prognostic factors for survival were then evaluated by monovariate and multivariate analyses. In previous reports, age and β2M were occasionally identified as significant prognostic factors.( 15 , 17 , 18 ) In the present study, however, the median age was only 57 years. The younger age of the patients in the present study might have had an effect on our failure to demonstrate the prognostic significance of age. In addition, patients with renal failure (creatinine > 3.0 g/L) who tended to show a significant elevation of β2M level were ineligible for the present study. Thus, β2M was also not identified as a prognostic marker. Of the fourteen patients, 10 patients demonstrated deletion of chromosome 13. Three out of the 10 responded well to thalidomide. Thus, patients with chromosomal abnormality revealed a response comparable to that of patients without this abnormality. However, those with deletion of chromosome 13 showed a significantly shorter OS. High serum LDH level was also a poor prognostic factor for OS in monovariate analysis. Previous studies have indicated that an elevated LDH level is associated with aggressive clinical course, drug resistance, and short survival.( 22 , 23 )
It is speculated that a decrease in the production of angiogenic growth factors by myeloma and stromal cells is one of the mechanisms of the antitumor effect of thalidomide. For this reason, we also examined the levels of angiogenic growth factors at 1 month after commencement of thalidomide therapy. As the data were available for only 38 patients, they were not considered conclusive, and were not shown above in the Results section. However, when the analysis was conducted with only these 38 patients, the elevation of post‐treatment HGF level (>0.3 µg/L) was significantly associated with poor response to therapy (P = 0.05) and shorter OS in univariate analysis (P = 0.01). When multivariate analysis of OS was conducted for these 38 patients, the post‐treatment HGF level was found to be statistically significant (hazard ratio, 3.31; 95% confidence interval, 1.22–8.97), indicating a possible association with prognosis. In addition, the post‐treatment HGF level was also associated with response to thalidomide. Thus, a decline in serum HGF level is implicated in the effect of therapy and is presumably important in improving survival. Previous reports have shown that serum HGF level is correlated with the efficacy of high‐dose therapy in MM patients and with their survival.( 24 , 25 ) In addition, molecular targeting of HGF by its specific inhibitor NK4 has been found to induce the regression of MM cell growth in vivo via direct as well as antiangiogenic effects.( 26 ) These results suggest that HGF is not merely a simple tumor marker but is an essential molecule for the pathogenesis of MM. Further investigation is needed to elucidate the prognostic and pathophysiological significance of HGF in MM.
As described in the Results section above, previously reported adverse events such as somnolence, constipation, peripheral neuropathy, and skin rash( 4 , 5 ) were observed frequently in the present study as well. However, unexpected patterns of toxicities were also observed. Hematological toxicities such as neutropenia, thrombocytopenia, and lymphopenia were observed frequently, and neutropenia was the most frequent grade 3 or 4 toxicity. Our previous observations revealed that patients with reduced bone marrow function deteriorated by previous treatment or a heavy tumor burden are at increased risk for neutropenia.( 27 ) Pulmonary complications such as pulmonary hypertension and interstitial pneumonia are other unexpected severe adverse events. To the best of our knowledge, a total of three cases of pulmonary hypertension, including the present patient, have been reported during thalidomide treatment.( 13 , 28 , 29 ) Further accumulation of data on the association of thalidomide with pulmonary hypertension is needed. Another unexpected pattern of toxicity in our study was the absence of DVT, which has been reported to be a serious side effect in less than 5% of patients under thalidomide monotherapy and in 10–20% of those under combination therapy with dexamethasone or other chemotherapeutic agents.( 6 , 14 , 30 ) The reasons for this may be the relatively low dose of thalidomide used (median, 200 mg per day), the fact that combination therapy with antineoplastic drugs other than dexamethasone was avoided, and the fact that the genetic background of Japanese patients is different from that of Western patients. For example, to the best of our knowledge, no Japanese patient with activated protein C resistance has ever been reported, even though this is an important risk factor for thalidomide‐induced DVT.( 14 ) The low frequency of DVT in Japanese patients has also been reported previously.( 21 )
In the present study, 11 patients were forced to reduce their dose, and one discontinued thalidomide therapy due to peripheral neuropathy. The duration of therapy and the total thalidomide dose were significantly associated with the occurrence of neuropathy (Table 5). Mileshkin et al. reported that the duration of thalidomide treatment is more important than the cumulative dose.( 31 ) Indeed, in the present study, 13 cases developed neuropathy even though their cumulative dose was less than 10 g. Importantly, in Mileshkin's study, 75% of patients received more than 400 mg per day of thalidomide.( 31 ) Even though the median dose was only 200 mg per day in the present study, we also found that the duration of thalidomide therapy played an important role in the development of thalidomide‐induced peripheral neuropathy. Similarly, dry mouth and constipation were also significantly associated with treatment time and total dose. Additionally, the cumulative dose until the development of these adverse events was not correlated with the occurrence of dry mouth and constipation. Thus, special attention must be paid to neurogenic adverse effects when thalidomide is used for a long period of time, even at lower doses.
In the present study, in which a relatively low dose of thalidomide was used, the drug was as effective as in previous reports in which doses of over 400 mg per day were used.( 6 , 15 , 16 , 17 , 18 , 19 ) Chromosomal abnormality, high CRP level, frequency of previous chemotherapy, and possibly post‐treatment HGF level were the factors associated with a poor prognosis. Special attention should be paid to the adverse events including hematological and pulmonary complications as well as peripheral neuropathy in long‐term users.
Acknowledgments
The authors thank Dr Kazuhiko Nakayama for his work as the pharmacist responsible for ensuring the safe use of thalidomide. This work was supported in part by an Aki Horinouchi Research Grant from the International Myeloma Foundation (YH), Keio Gijuku Academic Development Funds (YH) and a Grant‐in‐Aid from the Tokyo Biochemical Research Foundation (YH).
References
- 1. McBride WG. Thalidomide and congenital abnormalities. Lancet 1961; II: 1358. [Google Scholar]
- 2. Lenz W. Thalidomide and congenital abnormalities. Lancet 1962; I: 45. [Google Scholar]
- 3. D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA 1994; 91: 4082–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singhal S, Mehta J, Desikan R et al . Anti‐tumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–71. [DOI] [PubMed] [Google Scholar]
- 5. Glasmacher A, Hahn C, Hoffmann F et al . A systematic review of phase‐II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol 2006; 132: 584–93. [DOI] [PubMed] [Google Scholar]
- 6. Barlogie B, Desikan R, Eddlemon P et al . Extended survival in advanced and refractory multiple myeloma after single‐agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood 2001; 98: 492–4. [DOI] [PubMed] [Google Scholar]
- 7. Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol 2003; 21: 16–19. [DOI] [PubMed] [Google Scholar]
- 8. Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 2006; 24: 431–6. [DOI] [PubMed] [Google Scholar]
- 9. Palumbo A, Bringhen S, Caravita T et al . Italian Multiple Myeloma Network, GIMEMA. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet 2006; 367: 825–31. [DOI] [PubMed] [Google Scholar]
- 10. Kakimoto T, Hattori Y, Okamoto S et al . Thalidomide for the treatment of refractory multiple myeloma: association of plasma concentrations of thalidomide and angiogenic growth factors with clinical outcome. Jpn J Cancer Res 2002; 93: 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sato N, Hattori Y, Wenlin D et al . Elevated level of plasma basic fibroblast growth factor in multiple myeloma correlates with increased disease activity. Jpn J Cancer Res 2002; 93: 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox D. Regression models and life tables. J R Stat SOC 1972; 43: 187–220. [Google Scholar]
- 13. Hattori Y, Shimoda M, Okamoto S, Satoh T, Kakimoto T, Ikeda Y. Pulmonary hypertension and thalidomide therapy in multiple myeloma. Br J Haematol 2005; 128: 885–7. [DOI] [PubMed] [Google Scholar]
- 14. Zangari M, Anaissie E, Barlogie B et al . Increased risk of deep‐vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood 2001; 98: 1614–15. [DOI] [PubMed] [Google Scholar]
- 15. Mileshkin L, Biagi JJ, Mitchell P et al . Multicenter phase 2 trial of thalidomide in relapsed/refractory multiple myeloma: adverse prognostic impact of advanced age. Blood 2003; 102: 69–77. [DOI] [PubMed] [Google Scholar]
- 16. Tosi P, Zamagni E, Cellini C et al . Salvage therapy with thalidomide in patients with advanced relapsed/refractory multiple myeloma. Haematologica 2002; 87: 408–14. [PubMed] [Google Scholar]
- 17. Neben K, Moehler T, Benner A et al . Dose‐dependent effect of thalidomide on overall survival in relapsed multiple myeloma. Clin Cancer Res 2002; 8: 3377–82. [PubMed] [Google Scholar]
- 18. Yakoub‐Agha I, Attal M, Dumontet C et al . Thalidomide in patients with advanced multiple myeloma: a study of 83 patients – report of the Intergroupe Francophone du Myelome (IFM). Hematol J 2002; 3: 185–92. [DOI] [PubMed] [Google Scholar]
- 19. Schey SA, Cavenagh J, Johnson R, Child JA, Oakervee H, Jones RW. A UK myeloma forum phase II study of thalidomide: long term follow‐up and recommendations for treatment. Leuk Res 2003; 27: 909–14. [DOI] [PubMed] [Google Scholar]
- 20. Durie BG. Low‐dose thalidomide in myeloma: efficacy and biologic significance. Semin Oncol 2002; 29 (6 Suppl. 17): 34–8. [DOI] [PubMed] [Google Scholar]
- 21. Murakami H, Handa H, Imai K et al . Thalidomide treatment of patients with refractory myeloma in the institutes participating in the Japan Myeloma Study Group. Jpn J Clin Haematol 2004; 45: 468–72. [PubMed] [Google Scholar]
- 22. Dimopoulos MA, Barlogie B, Smith TL, Alexanian R. High serum lactate dehydrogenase level as a marker for drug resistance and short survival in multiple myeloma. Ann Intern Med 1991; 115: 931–5. [DOI] [PubMed] [Google Scholar]
- 23. Anagnostopoulos A, Gika D, Hamilos G et al . Treatment of relapsed/refractory multiple myeloma with thalidomide‐based regimens: identification of prognostic factors. Leuk Lymphoma 2004; 45: 2275–9. [DOI] [PubMed] [Google Scholar]
- 24. Iwasaki T, Hamano T, Ogata A, Hashimoto N, Kitano M, Kakishita E. Clinical significance of vascular endothelial growth factor and hepatocyte growth factor in multiple myeloma. Br J Haematol 2002; 116: 796–802. [DOI] [PubMed] [Google Scholar]
- 25. Seidel C, Lenhoff S, Brabrand S et al . Hepatocyte growth factor in myeloma patients treated with high‐dose chemotherapy. Br J Haematol 2002; 119: 672–6. [DOI] [PubMed] [Google Scholar]
- 26. Du W, Hattori Y, Yamada T et al . NK4, an antagonist of hepatocyte growth factor (HGF), inhibits growth of multiple myeloma cells in vivo: molecular targeting of angiogenic growth factor. Blood 2007; 109: 3042–9. [DOI] [PubMed] [Google Scholar]
- 27. Hattori Y, Kakimoto T, Okamoto S, Sato N, Ikeda Y. Thalidomide‐induced severe neutropenia during treatment of multiple myeloma. Int J Hematol 2004; 79: 283–8. [DOI] [PubMed] [Google Scholar]
- 28. Younis TH, Alam A, Paplham P, Spangenthal E, McCarthy P. Reversible pulmonary hypertension and thalidomide therapy for multiple myeloma. Br J Haematol 2003; 121: 191–2. [DOI] [PubMed] [Google Scholar]
- 29. Antonioli E, Nozzoli C, Gianfaldoni G et al . Pulmonary hypertension related to thalidomide therapy in refractory multiple myeloma. Ann Oncol 2005; 16: 1849–50. [DOI] [PubMed] [Google Scholar]
- 30. Hattori Y, Iguchi T. Thalidomide for the treatment of multiple myeloma. Congenital Anomalies 2004; 44: 125–36. [DOI] [PubMed] [Google Scholar]
- 31. Mileshkin L, Stark R, Day B, Seymour JF, Zeldis JB, Prince HM. Development of neuropathy in patients with myeloma treated with thalidomide: patterns of occurrence and the role of electrophysiological monitoring. J Clin Oncol 2006; 24: 4507–14. [DOI] [PubMed] [Google Scholar]


