Abstract
Thymidylate synthase (TS) plays a major role in the response to 5‐fluorouracil (5‐FU) by binding directly to the 5‐FU metabolite, 5‐fluoro‐dUMP (FdUMP). The change in the TS expression levels after 5‐FU administration was examined in parallel to 5‐FU responsiveness in six human gastric adenocarcinoma cell lines to elucidate the source of variability of 5‐FU sensitivity. MKN‐1, SH‐10‐TC and MKN‐74 cells were more resistant to 5‐FU than MKN‐28, KATO III and MKN‐45 cells. Western blotting analysis revealed that the 5‐FU sensitivity of these cells did not correlate with the basal TS expression levels but did correlate with rapid detection of the TS‐FdUMP complex after exposure to 5‐FU. In 5‐FU‐resistant cells, very low levels of the TS‐FdUMP complex early after 5‐FU exposure were elevated by pretreatment with calpain inhibitors such as benzyloxycarbonyl‐leucyl‐leucinal (ZLLH), benzyloxycarbonyl‐leucyl‐leucyl‐leucinal (ZLLLH) and ALLN, but not by other protease inhibitors. In contrast, ONO‐3403, which causes calpain activation, stimulated downregulation of the TS‐FdUMP complex in 5‐FU‐sensitive cells. The expression levels of calpastatin, an endogenous calpain inhibitor, were higher in 5‐FU‐sensitive cells than in 5‐FU‐resistant cells. ZLLH increased the 5‐FU sensitivity of 5‐FU‐resistant cells, whereas ONO‐3403 decreased the sensitivity of 5‐FU‐sensitive cells. In addition, knockdown of m‐calpain by siRNA increased the 5‐FU sensitivity in 5‐FU‐resistant cells, while knockdown of calpastatin reduced the sensitivity in 5‐FU‐sensitive cells. These results suggest that calpain might reduce the chemosensitivity of human gastric cancer cells to 5‐FU possibly by rapid degradation of the TS‐FdUMP complex, a finding that is considered to have novel therapeutic implications. (Cancer Sci 2011; 102: 1509–1515)
Gastric cancer is one of the most common neoplasms worldwide.( 1 , 2 ) Although surgery remains the mainstay of potentially curative treatment, the prognoses even for patients who undergo a complete resection are not satisfactory. Postoperative adjuvant chemotherapy with S‐1, which includes tegafur as a prodrug of 5‐FU, has recently been reported to be effective for curatively resected stage II/III gastric cancer.( 3 ) At present, 5‐FU is still a key drug for gastric cancer in both the adjuvant and primary settings, while numerous multidrug regimens have been developed for this treatment.( 4 , 5 ) However, chemotherapeutic management is further complicated by the observation that, even among gastric cancers that share identical clinicopathological features, there are significant differences among individuals in the sensitivity to the same regimen.( 6 , 7 , 8 , 9 ) Therefore, the clinical response to 5‐FU‐based chemotherapy is patient dependent, and it is important to select patients in whom the treatment is likely to be effective, namely, to predict the sensitivity of gastric cancer cells to 5‐FU.( 5 , 7 , 8 , 9 ) Gastric adenocarcinoma cells that have a mutant p53 are likely to be more resistant to the growth‐inhibitory effect of 5‐FU than cells with a wild‐type p53. ( 6 ) This fact( 6 ) suggests a mechanism in which p53 protein contributes to the cellular response to chemotherapy. However, other possible molecular mechanisms responsible for this variation in the response to 5‐FU are still unclear.
Sensitivity to 5‐FU is significantly associated with the levels or activities of intratumoral 5‐FU‐metabolizing enzymes such as TS, DPD or OPRT before 5‐FU treatment in vitro, in vivo and in clinical model systems.( 7 , 8 , 9 , 10 , 11 , 12 , 13 ) In particular, TS (EC 2.1.1.45), an essential DNA synthetic enzyme that catalyzes the methylation of dUMP to dTMP, is inactivated by the formation of a covalent ternary complex with a 5‐FU metabolite, FdUMP and methylenetetrahydrofolate.( 14 ) Thymidylate synthase is a key chemotherapeutic target of 5‐FU or antifolate analogs( 14 , 15 ) and the basal expression level of TS gene, TS protein or TS enzymatic activity in tumors is a good predictor of response to 5‐FU.( 7 , 9 , 10 , 11 ) Furthermore, 5‐FU treatment causes increases in TS gene expression, TS levels and enzymatic activity in cancer cells,( 14 , 15 , 16 ) and in human colon carcinoma cells, such enhanced TS expression by 5‐FU exposure is less in 5‐FU‐sensitive cells.( 17 ) Although the 5‐FU‐mediated TS induction might be a crucial response for resistance to 5‐FU, the possible changes in TS protein levels after 5‐FU treatment have yet to be documented in human gastric cancer cells, and they might be related to the variability of 5‐FU sensitivity.
In the present study, we have focused on the TS level as a major site of action and have compared the changes in TS levels during 5‐FU treatment in two sets of human gastric adenocarcinoma cell lines that differ in their chemosensitivity toward 5‐FU. The results showed a difference in TS‐FdUMP complex levels between 5‐FU‐resistant and 5‐FU‐sensitive cells. Furthermore, this seems to be caused by a difference in the intrinsic calpain activity. The possible implications of calpain in regard to the chemotherapy with 5‐FU in human gastric cancer cells are also discussed.
Materials and Methods
Cell lines and culture. Six human gastric adenocarcinoma cell lines( 6 , 18 , 19 ) were used. MKN‐1 (JCRB No. 0252), MKN‐28 (JCRB No. 0253), MKN‐45 (JCRB No. 0254), MKN‐74 (JCRB No. 0255) and KATO III (JCRB No. 0611) cells were obtained from Health Science Research Resources Bank/Japanese Collection of Research Bioresources (Osaka, Japan) and SH‐10‐TC (RCB No. 1940) was obtained from Riken Bioresource Center Cell Bank (Tsukuba, Japan). The mutational status of the p53 gene in the five cell lines has been reported,( 6 , 18 , 19 ) while DNA single‐strand conformation polymorphism analysis and direct DNA sequencing confirmed that MKN‐45 and SH‐10‐TC possess a wild‐type p53.( 6 ) MKN‐1 has a point mutation (T to C transition) at codon 143 in exon 5 in the p53 gene.( 18 , 19 ) MKN‐28 has a point mutation (A to C transversion) at codon 251 in exon 7.( 18 , 19 ) The MKN‐74 strain used in the present study has the same point mutation as that in MKN‐28,( 19 ) although no mutation was reportedly detected in other MKN‐74 strains.( 6 , 18 ) Entire deletion of the p53 gene was confirmed in KATO III as previously reported.( 18 , 19 )
The gastric cancer cells were maintained in DMEM supplemented with 1% non‐essential amino acids, 100 U/mL penicillin, 100 U/mL streptomycin and 10% heat‐inactivated fetal bovine serum in a 5% humidified CO2 incubator at 37°C. Mouse NIH3T3 fibroblasts transformed by activated Ha‐ras (ras‐NIH3T3)( 20 ) were cultured in DMEM supplemented with 5% bovine serum and 100 μg/mL of kanamycin. The culture medium including 5‐FU was prepared just before each experiment by the addition of the drug directly to the medium, as described previously.( 6 ) The cells in the logarithmic phase of growth were used for all experiments.
Chemicals. DMEM, 5‐FU, PMSF and MTT were purchased from Sigma (St Louis, MO, USA). DMSO was obtained from Wako Pure Chemicals (Kyoto, Japan). Anti‐TS,( 21 ) anti‐OPRT( 22 ) and anti‐DPD polyclonal antibodies were generous gifts from Taiho Pharmaceutical Co., Ltd (Tokyo, Japan). Anti‐μ‐calpain, anti‐m‐calpain, anti‐calpastatin( 23 , 24 ) and anti‐β‐actin polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Clasto‐lactacystin β‐lactone (lactacystin)( 24 , 25 ) was from Biomol Research Laboratories (Plymouth Meeting, PA, USA). N‐Acetyl‐leucyl‐leucyl‐norleucinal (ALLN)( 26 ) was purchased from Nacalai Tesque (Kyoto, Japan). ZLLH,( 27 ) ZLLLH,( 27 ) which is also known as MG132,( 15 ) and pepstatin A were purchased from Peptide Institute (Osaka, Japan). MMP inhibitor was purchased from Merck Biosciences (Darmstadt, Germany). Serine protease inhibitors, ONO‐3403( 28 , 29 , 30 ) and ONO‐5046, were provided by Ono Pharmaceuticals (Kyoto, Japan). All other chemicals used were of the highest standard grade commercially available.
Evaluation of the cytotoxicity of 5‐FU. The in vivo chemosensitivity was evaluated using the MTT colorimetric assay( 31 ) as previously described.( 24 , 32 ) Briefly, 5 × 103 cells per well were plated in 96‐well plates at various concentrations of 5‐FU and cultured for 3 days. Cell viability was quantified by measuring the absorbance at 570 nm with a reference wavelength of 655 nm after incubation with 0.5 mg/mL MTT for 4 h. Absorbance reflects the viable cell number and the results are shown as percentages of the control results.
When indicated, gastric cancer cells were pretreated with several protease inhibitors or the solvent (DMSO) as a control for 1 h and then the chemosensitivity to 5‐FU was examined using the MTT assay in the presence of these compounds.
Western blotting analysis. Cells were incubated with or without 5 μM 5‐FU for the indicated periods. From each sample, 30 μg total protein of the cytoplasmic fraction was separated on 11% polyacrylamide gels containing sodium dodecylsulfate (SDS) by electrophoresis (SDS‐PAGE), and Western blotting was performed as described previously,( 6 , 24 ) followed by treatment with a secondary antibody and detection using ImmunoStar Reagents (Wako Pure Chemicals) according to the manufacturer’s instructions.
When indicated, the cells were incubated in the presence of protease inhibitors or the solvent DMSO for 3 h just before harvest and were also exposed to 5 μM 5‐FU for the last 2 h.
Transfection of siRNA and cDNA. The siRNA used to knockdown m‐calpain (Sigma) were siCAPN2 (gene name of m‐calpain)‐1 (5′rCrGrCUrAUUrCrArArGrAUrAUUUrArATT and 5′UUrArArAUrAUrCUUrGrArAUrArGrCrGTT), siCAPN2‐2 (5′GrArArArCUrGrAUrCrCrGrCrAUrCrCrGrATT and 5′UrCrGrGrAUrGrCrGrGrAUrCrArGUUUrCTT) and siCAPN2‐3 (5′rCUUrAUrCUrCUUrGrGrCUrCUrGUUTT and 5′rArArCrArGrArGrCrCrArArGrArGrAUrArArGTT). The siRNA for calpastatin (Sigma) were siCAST (gene name of calpastain)‐1 (5′rCrArGrArArCrArCrArGrCrArArArGrGrAUrATT and 5′UrAUrCrCUUUrGrCUrGUrGUUrCUrGTT) and siCAST‐2 (5′CrArGrCrArArArGrGrAUrArArGUrGrCrArATT and 5′UUrGrCrArCUUrAUrCrCUUUrGrCUrGTT). MISSION siRNA Universal Negative Control SIC‐001 (Sigma) was used as a control siRNA. MKN‐1 or MKN‐45 cells (1 × 106 cells) were transfected with a total of 500 pmol of siRNA by electroporation using Neon Transfection System (Life Technologies, Carlsbad, CA, USA). Cells were cultured for 2 days and replated for the MTT assay and Western blotting analysis.
cDNAs of μ‐calpain and m‐calpain large subunits (CAPN1 and CAPN2) were provided by Dr Koichi Suzuki (Toray Industries, Tokyo, Japan).( 33 , 34 ) Human calpastatin cDNA was provided by Dr Masatoshi Maki (Nagoya University, Aichi, Japan).( 35 ) Human DAN cDNA was provided by Dr Shigeru Sakiyama (Chiba Red Cross Blood Center, Chiba, Japan).( 36 ) Expression vectors containing the cDNA were transfected into ras‐NIH3T3 cells, and G418‐resistant stable transfectants were isolated as described previously.( 24 , 32 )
Results
Sensitivity of gastric cancer cells to 5‐FU. By MTT assay, MKN‐1, SH‐10‐TC and MKN‐74 cells were found to be relatively resistant to 5‐FU compared with MKN‐28, KATO III and MKN‐45 cells (Fig. 1). The half‐maximal inhibition concentrations of MKN‐1, SH‐10‐TC and MKN‐74 cells were higher than 40 μM, whereas those of MKN‐28, KATO III and MKN‐45 cells were 10–20 μM. Based on these results, MKN‐1, SH‐10‐TC and MKN‐74 cells were designated as the 5‐FU‐resistant group and MKN‐28, KATO III and MKN‐45 cells were the 5‐FU‐sensitive group, and 5 μM was selected as the concentration of 5‐FU in the following experiments. The p53 gene is mutated in MKN‐1, MKN‐74, MKN‐28 and KATO III cells but not in MKN‐45 and SH‐10‐TC cells, thus suggesting that the chemosensitivity to 5‐FU does not simply indicate the p53 status.
Figure 1.
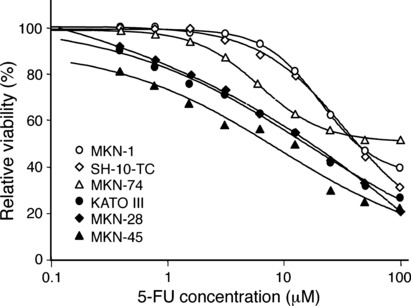
Different sensitivities to 5‐fluorouracil (5‐FU) among the human gastric cancer cell lines. MKN‐1, SH‐10‐TC, MKN‐74, MKN‐28, KATO III and MIN‐45 cells were cultured in the presence of varying concentrations of 5‐FU for 3 days. Cell viabilities were measured by MTT assay and are shown as percentages of the control results.
Effects of 5‐FU on TS protein levels in gastric cancer cells. By Western blotting analysis, the basal state of the TS protein appeared as a band at approximately 36 kDa, the expression levels of which showed no relationship with the sensitivity to 5‐FU (Fig. 2). The 5‐FU treatment at 5 μM for 1 day (24 h) or 2 days (48 h) resulted in the induction of another distinct band of 38 kDa (indicated as TS*) in all strains (Fig. 2). This higher molecular mass band can never be found in the untreated cells and can be detected just after 5‐FU exposure; it represents TS protein bound in a covalent complex (TS‐FdUMP complex) to a 5‐FU metabolite, FdUMP and methylenetetrahydrofolate.( 15 , 17 )
Figure 2.
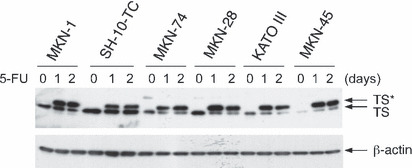
Varying expression levels of thymidylate synthase (TS) protein and the TS–5‐fluoro‐dUMP (FdUMP) complex in the human gastric cancer cell lines before or after 5‐fluorouracil (5‐FU) treatment. MKN‐1, SH‐10‐TC, MKN‐74, MKN‐28, KATO III and MIN‐45 cells were treated with 5 μM 5‐FU for 0 (no treatment), 1 day (24 h) or 2 days (48 h). Extracts of control (0) and 5‐FU‐treated cells were analyzed by Western blotting using anti‐TS antibody (upper panel) or anti‐β‐actin antibody (lower panel). TS* represents the TS‐FdUMP complex.
Alterations in TS protein levels after exposure to 5‐FU. To determine whether there was any direct effect of 5‐FU on the TS levels, changes in the levels of TS protein were analyzed using Western blotting in the six gastric adenocarcinoma cell lines after treatment with 5 μM 5‐FU for 0–24 h, based on the results shown in Figure 2. The 38 kDa TS‐FdUMP complex was not apparent until 4–6 h after the addition of 5‐FU in 5‐FU‐resistant cells such as MKN‐1, SH‐10‐TC and MKN‐74 (Fig. 3A–C). In contrast, the complex appeared as early as 2 h after 5‐FU addition in 5‐FU‐sensitive MKN‐28, KATO‐III and MKN‐45 cells (Fig. 3D–F). The levels of FdUMP‐free TS protein of 36 kDa were relatively constant after 5‐FU addition in 5‐FU‐resistant cells, whereas they rapidly decreased after 5‐FU addition in 5‐FU‐sensitive cells. As a result, the ratios of the TS‐FdUMP complex versus free TS were much higher in 5‐FU‐sensitive cells 2–6 h after 5‐FU addition than those in 5‐FU‐resistant cells. The levels of OPRT as well as those of the loading control β‐actin did not change throughout the treatment period (Fig. 3). In addition, no apparent alteration in the expression levels of DPD was confirmed (data not shown).
Figure 3.
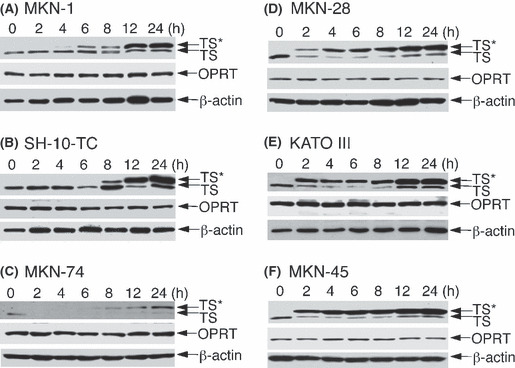
Time course of changes in expression levels of thymidylate synthase (TS) and the TS–5‐fluoro‐dUMP (FdUMP) complex (TS*) in 5‐fluorouracil (5‐FU)‐resistant (A‐C) and 5‐FU‐sensitive (D‐F) gastric cancer cell lines after treatment with 5 μM 5‐FU for the periods indicated. The cell extracts were analyzed by Western blotting using anti‐TS, anti‐orotate phosphoribosyltransferase (OPRT) and anti‐β‐actin antibodies.
Influences of protease inhibitors on the TS level and chemosensitivity to 5‐FU. The difference in the TS expression levels between 5‐FU‐resistant and 5‐FU‐sensitive cells might be caused by the difference in the synthesis and/or degradation of TS protein. A large difference in synthesis rates was unlikely because treatment with cycloheximide, a protein synthesis inhibitor, for 2 h led to no detectable decrease in TS protein (data not shown). The influence of proteolysis on the levels of TS protein expression was investigated using protease inhibitors. The 5‐FU‐resistant MKN‐1 cells pretreated with protease inhibitors for 1 h were then treated with 5‐FU in the presence of these compounds for 2 h, and then analyzed by Western blotting. The level of the TS‐FdUMP complex increased after pretreatment with calpain inhibitor ZLLH and was moderately raised by proteasome/calpain inhibitor ZLLLH( 27 ) (Fig. 4A). The dose of either ZLLH or ZLLLH used is considered enough to inhibit calpain activity.( 27 ) Protease inhibitors such as lactacystin,( 24 , 25 ) PMSF, pepstatin A, MMP inhibitor and elastase inhibitor ONO‐5046 caused no apparent change in the TS levels, whereas the calpain/proteasome inhibitor ALLN increased the levels of the TS‐FdUMP complex (data not shown). In turn, the TS‐FdUMP complex, which was rapidly detectable in 5‐FU‐sensitive KATO‐III cells (Fig. 3E), remarkably decreased by pretreatment with calpain stimulator ONO‐3403( 28 , 29 , 30 ) for 1 h followed by 5‐FU treatment in the presence of ONO‐3403 for 2 h (Fig. 4B).
Figure 4.
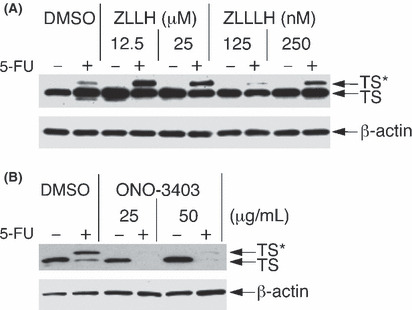
Effects of calpain inhibitor/activator on expression levels of thymidylate synthase (TS) and the TS–5‐fluoro‐dUMP (FdUMP) complex (TS*). MKN‐1 cells were pretreated with calpain inhibitors such as benzyloxycarbonyl‐leucyl‐leucinal (ZLLH) and benzyloxycarbonyl‐leucyl‐leucyl‐leucinal (ZLLLH) (A) and KATO III cells were pretreated with calpain activator ONO‐3403 (B) at concentrations indicated or the solvent DMSO (0.1%) for 3 h just before harvest, and were also exposed to 5 μM 5‐fluorouracil (5‐FU) for the last 2 h. Extracts of control (−) and 5‐FU‐treated (+) cells were analyzed by Western blotting using anti‐TS and anti‐β‐actin antibodies.
Different calpain/calpastatin levels between 5‐FU‐resistant and 5‐FU‐sensitive cells. Two types of calpain, μ‐calpain and m‐calpain, are ubiquitously expressed in most cells.( 24 , 37 ) Their proteolytic activity is activated by Ca2+ and is inhibited by the endogenous inhibitor calpastatin.( 23 , 24 ) Although the expression levels of μ‐calpain were similar, the expression of m‐calpain was relatively lower in 5‐FU‐sensitive MKN‐28, KATO III and MKN‐45 cells than in 5‐FU‐resistant MKN‐1, SH‐10‐TC and MKN‐74 cells (Fig. 5). Furthermore, the expression of calpastatin was exclusively detectable only in the three 5‐FU‐sensitive cells (Fig. 5).
Figure 5.
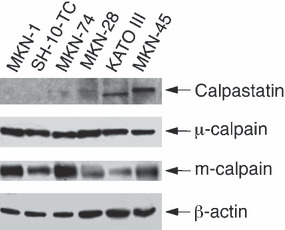
Comparison of the expression levels of calpain and calpastatin among human gastric cancer cells by Western blotting analysis using anti‐μ‐calpain, anti‐m‐calpain, anti‐calpastatin and anti‐β‐actin antibodies.
Decrease in calpain activity sensitizes 5‐FU‐resistant gastric cancer cells to 5‐FU. The survival of 5‐FU‐resistant MKN‐1 cells after exposure to 5‐FU decreased in the presence of ZLLH but not in the presence of ZLLLH (Fig. 6A). Lactacystin caused a marginal increase in cell viability at high concentrations (20–100 μM) of 5‐FU (Fig. 6A), thus suggesting that the inhibition of proteasome activity might have an opposite effect on the sensitivity to 5‐FU. ZLLH did not further increase the sensitivity of 5‐FU‐sensitive cells (data not shown). When 5‐FU‐sensitive KATO III cells were pretreated with calpain activator ONO‐3403, the 5‐FU‐induced decrease in cell viability was efficiently suppressed (Fig. 6B). ONO‐3403 did not further decrease the sensitivity of 5‐FU‐resistant cells (data not shown). Consequently, the decrease in calpain activity might increase the sensitivity to 5‐FU.
Figure 6.
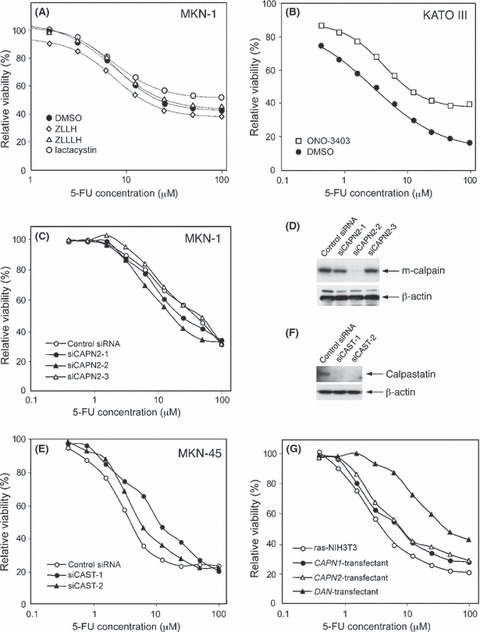
Effects of calpain inhibition/activation on the chemosensitivity to 5‐fluorouracil (5‐FU). The respective cell viability (shown in A–C, E and G) was determined by MTT assay as described in the legend of Figure 1. (A) MKN‐1 cells were treated for 3 days with varying concentrations of 5‐FU in the presence of 25 μM benzyloxycarbonyl‐leucyl‐leucinal (ZLLH), 250 nM benzyloxycarbonyl‐leucyl‐leucyl‐leucinal (ZLLLH), 2 μM lactacystin or the solvent DMSO (0.1%). (B) Chemosensitivity of KATO III cells to 5‐FU was examined in the presence of 25 μg/mL of ONO‐3403 or the solvent DMSO (0.1%). (C) Chemosensitivity of MKN‐1 cells cultured for 2 days after transfection with control siRNA, siCAPN2‐1, siCAPN2‐2 or siCAPN2‐3. (D) The protein expression levels of m‐calpain and β‐actin in siRNA‐transfected MKN‐1 cells were examined using Western blotting. (E) Chemosensitivity of MKN‐45 cells cultured for 2 days after transfection with control siRNA, siCAST‐1 or siCAST‐2. (F) The protein expression levels of calpastatin and β‐actin in siRNA‐transfectanted MKN‐45 cells were examined using Western blotting. (G) Chemosensitivity of stable transfectants of ras‐NIH3T3 to 5‐FU. ras‐NIH3T3 cells transfected with CAPN1 (μ‐calpain), CAPN2 (m‐calpain) or DAN cDNA and the parental cells were examined.
This was further supported by the results of siRNA transfection. In most 5‐FU‐resistant MKN‐1 cells, knockdown of m‐calpain by siCAPN2‐1 or siCAPN2‐2 increased the sensitivity to 5‐FU but not by siCAPN2‐3 (Fig. 6C). The effect of each siCAPN2 on 5‐FU sensitivity correlated well with the knockdown level of m‐calpain protein determined by Western blotting (Fig. 6D). Transfection with siRNA for μ‐calpain caused no apparent change in the 5‐FU sensitivity (data not shown). In most 5‐FU‐sensitive MKN‐45 cells, knockdown of calpastatin by siCAST‐1 or siCAST‐2 reduced the 5‐FU sensitivity (Fig. 6E). The effective knockdown of calpastatin protein by either siCAST was confirmed by Western blotting (Fig. 6F).
Similar effects of calpain on the sensitivity to 5‐FU was also observed in ras‐NIH3T3 cells that were stably transfected with expression vectors containing cDNA coding for CAPN1 (μ‐calpain large subunit), CAPN2 (m‐calpain large subunit) or DAN, a calpain‐activating gene.( 36 ) In comparison to parental ras‐NIH3T3 cells, CAPN1‐ and CAPN2‐transfected cells were more resistant to 5‐FU (Fig. 6G). Furthermore, DAN‐transfected cells were found to be much more resistant to 5‐FU than the other three cell types (Fig. 6G).
Discussion
The results of the present study clearly demonstrate that in human gastric adenocarcinoma cells the alteration of the TS‐FdUMP complex levels after exposure to 5‐FU is associated with chemosensitivity to 5‐FU and calpain regulates the TS‐FdUMP levels accompanied by the corresponding alteration in the 5‐FU‐sensitivity.
The six human gastric carcinoma cell lines failed to show any correlation between the basal TS levels and the sensitivity to 5‐FU (1, 2), which is consistent with the findings of a previous report.( 16 ) In contrast, the basal level of TS/TS expression or TS activity was suggested to be a good predictor of response to 5‐FU:( 7 , 9 , 10 , 11 ) high TS/TS tended to be associated with 5‐FU resistance and vice versa, which might be explained by the fact that TS catalytic activity is inhibited by FdUMP, a 5‐FU metabolite.( 14 ) However, care must be taken concerning the methods used to measure the TS/TS levels when interpreting the correlation between TS levels and 5‐FU sensitivity or the clinical outcome, since previous studies with clinical samples showed discrepancies between the protein expression and either the mRNA expression or enzymatic activity of TS.( 8 , 38 )
The treatment of cancer cells with 5‐FU results in an increase of TS/TS expression levels.( 14 , 15 , 16 , 17 ) While the findings of 5‐FU‐mediated TS protein induction previously shown corresponds to the current observations, this phenomenon was attributed to relieving the translational inhibition caused by binding of TS to its cognate mRNA,( 14 ) and also decreasing the 26S‐proteasome‐induced degradation of TS protein.( 15 ) These reported mechanisms mainly focused on the free TS levels. In contrast, the current study noticed the importance of the TS‐FdUMP ternary complex rather than free TS in the resistance of gastric cancer to 5‐FU. Once formed, the TS‐FdUMP complex is not subject to dissociation even under denaturing conditions.( 17 ) However, the presence of calpain inhibitor ZLLH increased the 38 kDa TS‐FdUMP complex level with no apparent change in the level of free TS (Fig. 4A), and calpain activator ONO‐3403 stimulated the downregulation of the TS‐FdUMP complex (Fig. 4B). This implies that the TS‐FdUMP complex rather than free TS is preferentially degraded by calpain in human gastric adenocarcinoma cells within 24 h during 5‐FU treatment (2, 3). In 5‐FU‐resistant cells, the endogenous calpain inhibitor calpastatin is lacking (Fig. 5) and calpain might be readily activated by extracellular stress such as 5‐FU exposure, as previously observed in ultraviolet‐irradiated human fibroblasts.( 23 ) As a result, high calpain activity might degrade the TS‐FdUMP complex in 5‐FU‐resistant cells. However, it remains unclear whether such selective degradation of the TS‐FdUMP complex by calpain was caused by a conformational change, subcellular localization or other unknown mechanisms. In contrast, it is possible that 5‐FU‐sensitive gastric cancer cells have a sufficient quantity of calpastatin to inhibit calpain, as shown in Figure 5.
The possible involvement of calpain in the sensitivity to 5‐FU was further confirmed by siRNA transfection. Knockdown of m‐calpain by siRNA in most 5‐FU‐resistant MKN‐1 cells increased the 5‐FU sensitivity, whereas knockdown of calpastatin in most 5‐FU‐sensitive MKN‐45 cells reduced the sensitivity (Fig. 6C,E). As knockdown of μ‐calpain did not affect the 5‐FU sensitivity, the proteolytic activity of m‐calpain might play a pivotal role in the regulation of 5‐FU sensitivity. Transient transfection with cDNA of CAPN1, CAPN2, calpastatin or calpain‐activating DAN failed to alter 5‐FU sensitivity, possibly because of transfection efficiency (data not shown). However, we previously showed that DAN‐transfected cells were the most resistant to 5‐FU among more than 110 stable transfectants examined for their chemosensitivity.( 32 ) The current study has also revealed by stable transfection into mouse fibroblasts that DAN‐transfected cells were much more resistant to 5‐FU than parental ras‐NIH3T3 cells (Fig. 6G). Since transfection and expression of DAN cDNA results in the activation of calpain,( 29 ) increased calpain activity might suppress the cytotoxic effects of 5‐FU. In comparison to DAN, transfection with cDNA of CAPN1 or CAPN2 had less effect on 5‐FU sensitivity. This implies that high expression of the calpain large subunit alone is insufficient to increase the calpain catalytic activity. In contrast, one might argue that similar effects could be obtained with other cell stress‐inducing agents unrelated to TS. However, calpain‐transfected cells were more resistant to 5‐FU than the parental line but not to other anticancer drugs such as cisplatin, ifosfamide, mitoxantrone, nitrosourea and taxol (data not shown). Accordingly, the intracellular calpain activity might affect the chemosensitivity specifically to 5‐FU, as shown in esophageal cancer cells to peplomycin.( 24 ) Transfection with calpain inhibitor calpastatin did not increase 5‐FU sensitivity (data not shown), possibly because the basal activity of calpain was sufficiently suppressed in the ras‐NIH3T3 cells.( 30 ) Transfection with calpain/calpastatin cDNA into human gastric adenocarcinoma cells failed to produce any significant difference in the sensitivity to 5‐FU presumably because of the much lower transfection efficiency in human cells compared with that in mouse fibroblasts (data not shown).
Although our results suggest a causal relationship between calpain activity and sensitivity to 5‐FU, the possibility that the variance in the early induction of the TS‐FdUMP complex resulted from differential calpain activation cannot be ruled out. In addition, it is not fully understood why the degradation of the TS‐FdUMP complex causes resistance to 5‐FU, because this is essentially an enzymatically dead complex. However, since the “ternary” complex includes methylenetetrahydrofolate, rapid degradation of this complex might release methylenetetrahydrofolate, which can be bound to free TS again, resulting in cell survival. Therefore, regulation of the TS‐FdUMP complex levels by calpain might play an important role in determining the sensitivity to 5‐FU, while some underlying mechanism has yet to be elucidated by in vivo models as well as in vitro experiments.
Finally, the results of the present study suggest a mechanism in which calpain activity contributes to the chemoresistance of 5‐FU, leading to the proposal of a novel treatment strategy for chemotherapy to gastric cancer. First, it is possible that the chemosensitivity to 5‐FU can be predicted by the expression levels of calpain and calpastatin in gastric carcinoma cells. When the ratio of calpain versus calpastatin is high, the cells might be resistant to 5‐FU and vice versa. Second, it is also possible that introduction of calpain inhibitors might sensitize gastric cancer cells to 5‐FU. However, one should be cautious in the fact that inhibition of proteasome might desensitize cells to 5‐FU (Fig. 6A). Most, if not all, calpain inhibitors also inhibit proteasome as exemplified by ALLN and ZLLLH.( 26 , 27 ) Therefore, the development of more potent and specific calpain inhibitors is anticipated, and we believe that the present study presents significant information as a clue to enhance the chemotherapeutic efficacy of 5‐FU to human gastric adenocarcinomas.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- 5‐FU
5‐fluorouracil
- DPD
dihydropyrimidine dehydrogenase
- FdUMP
5‐fluoro‐dUMP
- OPRT
orotate phosphoribosyltransferase
- TP
thymidine phosphorylase
- TS
thymidylate synthase
- ZLLH
benzyloxycarbonyl‐leucyl‐leucinal
- ZLLLH
benzyloxycarbonyl‐leucyl‐leucyl‐leucinal
Acknowledgments
This work was supported in part by Grants‐in‐Aid for Scientific Research (C: #12671199 and #20591562) from the Japan Society for the Promotion of Science (JSPS), Japan. The authors are grateful to Taiho Pharmaceutical CO. Ltd. for kindly providing the anti‐TS, anti‐OPRT and anti‐DPD polyclonal antibodies. The authors also thank Drs Koichi Suzuki (Toray Industries), Shigeru Sakiyama (Chiba Red Cross Blood Center) and Masatoshi Maki (Nagoya University) for providing the plasmids.
References
- 1. Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer 2001; 37 (Suppl 8): S4–66. [DOI] [PubMed] [Google Scholar]
- 2. Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev 2000; 26: 243–55. [DOI] [PubMed] [Google Scholar]
- 3. Sakuramoto S, Sasako M, Yamaguchi T et al. ACTS‐GC Group. Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med 2007; 357: 1810–20. [DOI] [PubMed] [Google Scholar]
- 4. Kubota T. The role of S‐1 in the treatment of gastric cancer. Br J Cancer 2008; 98: 1301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Haller D, Ohtsu A. The role of chemotherapy in the current treatment of gastric cancer. Gastric Cancer 2002; 5 (Suppl 1): 17–22. [DOI] [PubMed] [Google Scholar]
- 6. Nabeya Y, Loganzo F Jr, Maslak P et al. The mutational status of p53 protein in gastric and esophageal adenocarcinoma cell lines predicts sensitivity to chemotherapeutic agents. Int J Cancer 1995; 64: 37–46. [DOI] [PubMed] [Google Scholar]
- 7. Fujiwara H, Terashima M, Irinoda T et al. Quantitative measurement of thymidylate synthase and dihydropyrimidine dehydrogenase mRNA level in gastric cancer by real‐time RT‐PCR. Jpn J Cancer Res 2002; 93: 1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishikawa Y, Kubota T, Otani Y et al. Thymidylate synthetase and dihydropyrimidine dehydrogenase levels in gastric cancer. Anticancer Res 1999; 19: 5635–40. [PubMed] [Google Scholar]
- 9. Johnston PG, Lenz HJ, Leichman CG et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5‐fluorouracil in human colorectal and gastric tumors. Cancer Res 1995; 55: 1407–12. [PubMed] [Google Scholar]
- 10. Habara K, Ajiki T, Kamigaki T, Nakamura T, Kuroda Y. High expression of thymidylate synthase leads to resistance to 5‐fluorouracil in biliary tract carcinoma in vitro . Jpn J Cancer Res 2001; 92: 1127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beck A, Etienne MC, Cheradame S et al. A role for dihydropyrimidine dehydrogenase and thymidylate synthase in tumour sensitivity to fluorouracil. Eur J Cancer 1994; 30A: 1517–22. [DOI] [PubMed] [Google Scholar]
- 12. Etienne MC, Cheradame S, Fischel JL et al. Response to fluorouracil therapy in cancer patients: the role of tumoral dihydropyrimidine dehydrogenase activity. J Clin Oncol 1995; 13: 1663–70. [DOI] [PubMed] [Google Scholar]
- 13. Ichikawa W, Uetake H, Shirota Y et al. Both gene expression for orotate phosphoribosyltransferase and its ratio to dihydropyrimidine dehydrogenase influence outcome following fluoropyrimidine‐based chemotherapy for metastatic colorectal cancer. Br J Cancer 2003; 89: 1486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, Schmitz JC, Lin X et al. Thymidylate synthase as a translational regulator of cellular gene expression. Biochim Biophys Acta 2002; 1587: 174–82. [DOI] [PubMed] [Google Scholar]
- 15. Forsthoefel AM, Pena MMO, Xing YY, Rafique Z, Berger FG. Structural determinants for the intracellular degradation of human thymidylate synthase. Biochemistry 2004; 43: 1972–9. [DOI] [PubMed] [Google Scholar]
- 16. Chu E, Koeller DM, Johnston PG, Zinn S, Allegra CJ. Regulation of thymidylate synthase in human colon cancer cells treated with 5‐fluorouracil and interferon‐γ. Mol Pharmacol 1993; 43: 527–33. [PubMed] [Google Scholar]
- 17. Kirihara Y, Yamamoto W, Toge T, Nishiyama M. Dihydropyrimidine dehydrogenase, multidrug resistance‐associated protein, and thymidylate synthase gene expression levels can predict 5‐fluorouracil resistance in human gastrointestinal cancer cells. Int J Oncol 1999; 14: 551–6. [DOI] [PubMed] [Google Scholar]
- 18. Mattar R, Yokozaki H, Yasui W, Ito H, Tahara E. p53 gene mutations in gastric cancer cell lines. Oncol (Life Sci Adv) 1992; 11: 7–12. [Google Scholar]
- 19. Jia L‐Q, Osada M, Ishioka C et al. Screening the p53 status of human cell lines using a yeast functional assay. Mol Carcinog 1997; 19: 243–53. [DOI] [PubMed] [Google Scholar]
- 20. Sekiya T, Fushimi M, Hori H, Hirohashi S, Nishimura S, Sugimura T. Molecular cloning and the total nucleotide sequence of the human c‐Ha‐ras‐1 gene activated in a melanoma from a Japanese patient. Proc Natl Acad Sci USA 1984; 81: 4771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okabe H, Tsujimoto H, Fukushima M. Preparation of the antibodies against recombinant human thymidylate synthase for the detection of its intratumoral levels and the application to sensitivity‐study of 5‐fluorouracil. Oncol Rep 1997; 4: 685–90. [DOI] [PubMed] [Google Scholar]
- 22. Sakamoto K, Sugimoto Y, Miyadera K, Oka T, Fukushima T. Preparation of anti‐OPRT antibody for immunochemical detection (in Japanese with English abstract). Gan To Kagaku Ryoho (Jpn J Cancer Chemother) 2005; 32: 653–8. [PubMed] [Google Scholar]
- 23. Hiwasa T, Arase Y, Kikuno K et al. Increase in ultraviolet sensitivity by overexpression of calpastatin in ultraviolet‐resistant UVr‐1 cells derived from ultraviolet‐sensitive human RSa cells. Cell Death Differ 2000; 7: 531–7. [DOI] [PubMed] [Google Scholar]
- 24. Liu TL, Shimada H, Ochiai T et al. Enhancement of chemosensitivity toward peplomycin by calpastatin‐stabilized NF‐κB p65 in esophageal carcinoma cells: possible involvement of Fas/Fas‐L synergism. Apoptosis 2006; 11: 1025–37. [DOI] [PubMed] [Google Scholar]
- 25. Omura S, Fujimoto T, Otoguro K et al. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. J Antibiot 1991; 44: 113–6. [DOI] [PubMed] [Google Scholar]
- 26. Hiwasa T, Sawada T, Sakiyama S. Cysteine proteinase inhibitors and ras gene products share the same biological activities including transforming activity toward NIH3T3 mouse fibroblasts and the differentiation‐inducing activity toward PC12 rat pheochromocytoma cells. Carcinogenesis 1990; 11: 75–80. [DOI] [PubMed] [Google Scholar]
- 27. Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di‐leucine and tri‐leucine. J Biochem 1996; 119: 572–6. [DOI] [PubMed] [Google Scholar]
- 28. Hiwasa T. Induction of apoptosis by a calpain stimulator, ONO‐3403. Apoptosis 1996; 1: 75–80. [Google Scholar]
- 29. Hiwasa T, Nakamura Y, Ozaki T et al. Down‐regulation of protein kinase Cα and γ and TPA‐induced neurite formation in DAN‐transformed neuroblastoma cells. FEBS Lett 1998; 440: 25–8. [DOI] [PubMed] [Google Scholar]
- 30. Hiwasa T, Shimada H, Ochiai T et al. Decrease in growth factor receptors after treatment with serine protease inhibitor ONO‐3403. Int J Oncol 2002; 20: 797–802. [PubMed] [Google Scholar]
- 31. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 32. Hiwasa T, Shimada H, Sakaida T et al. Drug‐sensitivity pattern analysis for study of functional relationship between gene products. FEBS Lett 2003; 552: 177–83. [DOI] [PubMed] [Google Scholar]
- 33. Imajoh S, Aoki K, Ohno S et al. Molecular cloning of the cDNA for the large subunit of the high‐Ca2+‐requiring form of human Ca2+‐activated neutral protease. Biochemistry 1988; 27: 8122–8. [DOI] [PubMed] [Google Scholar]
- 34. Aoki K, Imajoh S, Ohno S et al. Complete amino acid sequence of human Ca2+‐activated neutral protease (μ‐CANP) deduced from its cDNA sequence. FEBS Lett 1986; 205: 313–7. [DOI] [PubMed] [Google Scholar]
- 35. Hitomi K, Yokoyama A, Maki M. Expression of biologically active human calpastatin in baculovirus‐infected insect cells and in Escherichia coli . Biosci Biotechnol Biochem 1998; 62: 136–41. [DOI] [PubMed] [Google Scholar]
- 36. Ozaki T, Sakiyama S. Molecular cloning and characterization of a cDNA showing negative regulation in v‐src‐transformed 3Y1 rat fibroblasts. Proc Natl Acad Sci USA 1993; 90: 2593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. Biochem J 1997; 328: 721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miyamoto S, Ochiai A, Boku N et al. Discrepancies between the gene expression, protein expression, and enzymatic activity of thymidylate synthase and dihydropyrimidine dehydrogenase in human gastrointestinal cancers and adjacent normal mucosa. Int J Oncol 2001; 18: 705–13. [DOI] [PubMed] [Google Scholar]


