Abstract
The retinoblastoma protein‐interacting zinc finger gene, RIZ1, is thought to be a tumor suppressor gene. RIZ1 is inactivated by mutation, deletion and DNA methylation in several human cancers. In the present study, the relationship between DNA methylation of RIZ1 and mutation of p53 was investigated in prostate cancer (PCa). In total, 47 cases of node‐negative PCa (stages I–III) were analyzed. DNA methylation of the RIZ1 gene was detected in 20 (42.6%) of the 47 PCa tissues by methylation‐specific polymerase chain reaction. DNA methylation of the RIZ1 gene was not associated with clinicopathological features. DNA methylation of RIZ1 tended to be present more frequently in PCa specimens with a high Gleason score (16/30, 53.3%) than in those with a low Gleason score (4/17, 23.5%); however, this tendency was not statistically significant (P = 0.0675). Nuclear accumulation of p53 was observed in four (8.5%) of 47 PCa specimens by immunostaining. All four PCa specimens with nuclear accumulation of p53 were stage III disease and showed DNA methylation of RIZ1. However, of the remaining 43 cancers without nuclear accumulation of p53, DNA methylation of RIZ1 was observed in only 16 (37.2%) specimens (P = 0.0272). Of the three PCa cell lines, only the PC3 cell line showed loss of RIZ1 mRNA due to DNA methylation, and this loss was rectified by treatment with a demethylating agent, 5‐Aza‐2′‐deoxycytidine. These results suggest that transcriptional inactivation of RIZ1 by aberrant DNA methylation may contribute to prostate carcinogenesis. Genetic alterations are likely associated with epigenetic alterations in PCa. (Cancer Sci 2007; 98: 32–36)
Prostate cancer (PCa) is one of the most common cancers and the second leading cause of cancer death in men in the USA.( 1 ) An understanding of the genetic and epigenetic pathways involved in the pathogenesis of PCa is essential for development of improved diagnostic and treatment modalities. A variety of genetic and epigenetic alterations are associated with PCa.( 2 , 3 ) Epigenetic changes, such as DNA methylation of CpG islands, are detected commonly in human cancers. Hypermethylation of CpG islands is associated with silencing of many genes, especially defective tumor‐related genes, and has been proposed as an alternative way to inactivate tumor‐related genes in human cancers.( 4 , 5 ) Identification of methylated genes may be useful in the diagnosis and treatment of PCa and may provide insight into prostate carcinogenesis. Prior studies have shown that DNA hypermethylation is a crucial mechanism in transcriptional silencing of tumor‐related genes in PCa.( 6 , 7 )
The retinoblastoma protein‐interacting zinc finger gene, RIZ1, was isolated with a functional screen for retinoblastoma (Rb)‐binding proteins.( 8 ) Domain analysis suggested that RIZ1 is a histone methyltransferase (HMT) specific for the lysine 9 residue of histone H3, an activity known to be linked with transcriptional repression.( 9 ) RIZ1 is considered to be a tumor suppressor gene because it can induce G2–M arrest and apoptosis of several types of cancer cells.( 10 , 11 ) RIZ1 plays an important role in human cancers, as evidenced by genetic mutations.( 12 , 13 , 14 ) The RIZ1 gene is located on human chromosome 1p36, a region deleted in many human cancers,( 15 ) and chromosome 1p36 is a potential hereditary PCa susceptibility locus.( 16 ) In addition to genetic alterations, DNA methylation of RIZ1 has been shown to be a common mechanism for inactivation of RIZ1 expression in human cancers.( 17 , 18 ) In PCa, DNA methylation of RIZ1 is present in 31% of tumor tissues.( 19 )
A knockout study showed that RIZ1 is a tumor susceptibility gene in mice.( 14 ) RIZ1 and p53 deficiencies likely cooperate in tumor formation in mice and are expected to occur in human cancers as well.( 14 ) In fact, many sporadic human cancers carry both p53 mutations and a silenced RIZ1 gene.( 10 , 14 ) The p53 gene is involved in the tumorigenesis of many human cancers,( 20 ) including PCa.( 21 ) p53 functions as a transcriptional regulator involved in G1 phase growth arrest of cells in response to DNA damage. p53 also has roles in regulation of the spindle checkpoint, centrosome homeostasis and G2–M phase transition.( 22 ) Several lines of evidence suggest associations between genetic and epigenetic alterations. p53 mutations have been found frequently in colorectal and gastric cancers without DNA methylation.( 23 , 24 ) However, the association between genetic and epigenetic alterations has not been investigated in PCa.
In the present study, we investigated the relationship between RIZ1 methylation status and p53 mutation status in 47 PCa tissues. To determine whether transcriptional silencing of the RIZ1 gene is caused by DNA hypermethylation, we compared the methylation status with expression of RIZ1 mRNA in PCa cell lines.
Materials and Methods
Tissue samples. Subjects were 47 patients with PCa who were referred to the Department of Urology, Hiroshima University Hospital (Hiroshima, Japan). Forty‐seven PCa tissues from these 47 patients were analyzed for DNA methylation of RIZ1 and localization of p53. PCa samples were obtained by radical prostatectomy, and all PCa cases were confirmed to be node negative by pathological examination. None of the 47 patients with PCa received preoperative treatment. All 47 specimens were archival, formalin‐fixed, paraffin‐embedded tissues. It was confirmed microscopically that the tumor specimens consisted mainly (>50%) of cancer cells. Tumor staging was according to the TNM classification system.( 25 ) In the present study, PCa were graded by the reporting pathologists on the radical surgery specimen, according to the system of the Gleason score.( 26 ) After prostatectomy, the serum prostate‐specific antigen (PSA) level was measured by E‐test Tosoh II Assay (Tosoh, Tokyo, Japan). Patients were followed up by PSA measurement monthly during the first 6 months after prostatectomy and then every 3 months thereafter. Biochemical relapse was defined as a PSA level of 0.2 ng/mL or greater. Because written informed consent was not obtained, for strict privacy protection, identifying information for all samples was removed before analysis. This procedure was in accordance with the Ethical Guidelines for Human Genome/Gene Research of the Japanese Government.
Cell lines and drug treatment. LNCaP, PC3 and DU145 PCa cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). All cell lines were maintained in RPMI‐1640 (Nissui Pharmaceutical, Tokyo, Japan) containing 10% fetal bovine serum (Whittaker, Walkersville, MD, USA) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Cells were treated with a final concentration of 1 µM 5‐aza‐2′‐deoxycytidine (Aza‐dC; Sigma Chemical, St Louis, MO, USA) for 5 days before they were harvested for DNA or RNA extraction.
Genomic DNA extraction and methylation‐specific polymerase chain reaction. For extraction of DNA from the archival, formalin‐fixed, paraffin‐embedded tissue samples, PCa samples were dissected manually from different sets of 10 serial, 10 µm‐thick, formalin‐fixed, paraffin‐embedded tissue sections with a fine needle. The dissected samples were lysed by incubation in 200 mg/mL proteinase K at 55°C for 3 days. Genomic DNA was purified by three rounds of phenol–chloroform extraction followed by ethanol precipitation. For DNA extraction from cell lines, genomic DNA was extracted with a Genomic DNA Purification Kit (Promega, Madison, WI, USA). To examine the DNA methylation pattern, genomic DNA was treated with 3 M sodium bisulfite, as described previously.( 27 ) For analysis of DNA methylation of the RIZ1 gene, methylation‐specific polymerase chain reaction (MSP) was carried out as described previously.( 17 ) Polymerase chain reaction (PCR) products (15 µL) were loaded onto 8% non‐denaturing polyacrylamide gels, stained with ethidium bromide, and visualized under ultraviolet light.
Immunohistochemistry. Formalin‐fixed, paraffin‐embedded samples were sectioned, deparaffinized, and stained with hematoxylin–eosin to ensure that the sectioned block contained tumor cells. Adjacent sections were then stained immunohisto‐schemically. For immunostaining of p53, a Dako LSAB Kit (Dako, Carpinteria, CA, USA) was used in accordance with the manufacturer's recommendations. In brief, sections were pretreated by microwaving in citrate buffer for 30 min to retrieve antigenicity. After peroxidase activity was blocked with 3% H2O2–methanol for 10 min, sections were incubated with normal goat serum (Dako) for 20 min to block non‐specific antibody binding sites. Anti‐p53 antibody (DO7, 1 : 100; Novocastra, Newcastle, UK) was incubated with tissue samples for 60 min at room temperature followed by incubations with biotinylated antimouse IgG and peroxidase‐labeled streptavidin for 10 min each. Staining was completed with a 10‐min incubation with the substrate–chromogen solution. The sections were counterstained with 0.1% hematoxylin. p53 staining was classified according to the percentage of stained cancer cells. When more than 10% of cancer cells were stained, the immunostaining was considered positive.
PCR–single‐strand conformation polymorphism analysis. Exons 5–8 of the p53 gene were examined for mutations by PCR–single‐strand conformation polymorphism (SSCP) analysis with 10 sets of primers, as described previously.( 28 ) Each target sequence was amplified in a 20‐µL reaction volume containing 10–20 ng genomic DNA, 0.2 µM dNTP, 10 mM Tris‐HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 0.3 µM of each primer and 0.2 µL of Ampli Taq Gold (Applied Biosystems, Foster City, CA, USA). PCR amplification consisted of 35 cycles of 94°C for 30 s, 60°C or 55°C for 30 s, and 72°C for 30 s after the initial activation step of 94°C for 10 min. PCR products were diluted 10‐fold with formamide dye solution, denatured at 85°C for 10 min, and separated by electrophoresis on 6% polyacrylamide gels. Gels were stained, and bands were visualized with a Silver Staining II kit (WAKO, Osaka, Japan).
Reverse transcription–polymerase chain reaction. Expression of RIZ1 mRNA was analyzed by reverse transcription (RT)‐PCR. Total RNA was extracted with an RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and 1 µg of total RNA was converted to cDNA with a First‐Strand cDNA Synthesis Kit (Amersham Biosciences, Piscataway, NJ, USA). Primer sequences and amplification conditions were as described previously.( 18 ) RT‐PCR products were subjected to 1.5% agarose gel electrophoresis, stained with ethidium bromide, and examined under ultraviolet light. ACTB‐specific PCR products were amplified from the same RNA samples and served as internal controls.
Statistical methods. Associations between clinicopathological parameters and DNA methylation of RIZ1 were analyzed by Fisher's exact test. A P‐value of less than 0.05 was considered statistically significant.
Results
DNA methylation of RIZ1 and p53 mutation status in PCa tissues. DNA methylation status of the RIZ1 gene was examined in a total of 47 PCa tissue specimens from 47 patients. DNA methylation of RIZ1 was detected in 20 (42.6%) of 47 PCa tissues. Representative results of MSP for RIZ1 are shown in Fig. 1A. No association was detected between the methylation status of RIZ1 and age (P = 1.000), T grade (P = 0.1425), stage (P = 0.1425), preoperative PSA concentration (P = 0.7674), or relapse (P = 0.3917) (Table 1). DNA methylation of RIZ1 tended to occur more frequently in PCa cases with a high Gleason score (16/30, 53.3%) than in those with a low Gleason score (4/17, 23.5%); however, the difference was not statistically significant (P = 0.0675; Table 1). We next investigated the nuclear accumulation of p53 in the 47 PCa tissues by immunostaining. Nuclear accumulation of p53 typically indicates the presence of p53 gene mutations.( 29 ) Immunostaining revealed nuclear accumulation of p53 in four (8.5%) of 47 PCa tissues (Fig. 1B). We also carried out PCR‐SSCP analysis of p53. Representative results are shown in Fig. 1C. Of the four PCa specimens with nuclear accumulation of p53, two (50.0%) exhibited a p53 mutation. No mutation was found in the 43 PCa specimens without nuclear accumulation of p53. All PCa specimens with nuclear accumulation of p53 showed DNA methylation of RIZ1 whereas only 16 (37.2%) of 43 PCa specimens without nuclear accumulation of p53 showed DNA methylation of RIZ1 (P = 0.0272, Fisher's exact test; Table 1). We found no association between the methylation status of the RIZ1 and p53 mutations determined by PCR‐SSCP analysis (P = 0.1758, Fisher's exact test; Table 1).
Figure 1.

(A) Methylation‐specific polymerase chain reaction (PCR) of RIZ1 in prostate cancer (PCa). Methylated RIZ1 was detected in two cases (cases 2 and 3) of PCa. U, unmethylated PCR product; M, methylated PCR product. (B) Immunostaining of p53 in PCa. Nuclear accumulation of p53 was observed in PCa cells. Original magnification, ×400. (C) PCR–single‐strand conformation polymorphism analysis of p53. A p53 mutation was detected in one case (case 10).
Table 1.
Association between RIZ1 methylation status and clinicopathological features and nuclear accumulation of p53 in prostate cancer
| Feature | RIZ1 methylation status | P‐value | ||
|---|---|---|---|---|
| Methylated | n% | Unmethylated | ||
| Age (years) | ||||
| >70 | 10 | 43.5 | 13 | 1.0000 |
| ≤70 | 10 | 41.7 | 14 | |
| Tumor grade | ||||
| T1/2 | 7 | 30.4 | 16 | 0.1425 |
| T3 | 13 | 54.2 | 11 | |
| Stage † | ||||
| I/II | 7 | 30.4 | 16 | 0.1425 |
| III | 13 | 54.2 | 11 | |
| Gleason score ‡ | ||||
| 2–6 | 4 | 23.5 | 13 | 0.0675 |
| 7–10 | 16 | 53.3 | 14 | |
| Preoperative PSA (ng/mL) § | ||||
| <10 | 13 | 44.8 | 16 | 0.7674 |
| >10 | 7 | 38.9 | 11 | |
| Relapse ¶ | ||||
| Positive | 7 | 35.0 | 13 | 0.3917 |
| Negative | 13 | 48.1 | 14 | |
| Nuclear accumulation of p53 | ||||
| Positive | 4 | 100.0 | 0 | 0.0272 |
| Negative | 16 | 37.2 | 27 | |
| p53 mutation determined by PCR‐SSCP | ||||
| Mutant‐type | 2 | 100.0 | 0 | 0.1758 |
| Wild‐type | 18 | 40.0 | 27 | |
Tumor stage according to TNM classification.
‡ Tumor grade according to Gleason criteria.
§ Prostate‐specific antigen (PSA) concentration was determined as described in the Materials and Methods.
Relapse was defined as serum PSA concentration of 0.2 ng/mL or higher. PCR‐SSCP, polymerase chain reaction–single‐strand conformation polymorphism.
DNA methylation status and expression of RIZ1 in PCa cell lines. To determine whether DNA hypermethylation of RIZ1 inactivates transcription of the gene, DNA methylation and expression of RIZ1 were investigated in three PCa cell lines (Fig. 2A). MSP revealed DNA hypermethylation of RIZ1 in PC3 cells, whereas hypermethylation of RIZ1 was not detected in LNCaP or DU145 cells. To study the relationship between DNA methylation status and RIZ1 expression levels, we carried out RT‐PCR of mRNA from PC3 cells. Transcriptional inactivation of RIZ1 was observed in PC3 cells with DNA hypermethylation (Fig. 2B). LNCaP and DU145 cells expressed RIZ1. To investigate whether transcriptional inactivation of RIZ1 was caused by DNA methylation in PC3 cells, we treated PC3 cells and LNCaP cells (unmethylated control) with Aza‐dC and carried out MSP (Fig. 2C) and RT‐PCR (Fig. 2D) analyses. Unmethylated RIZ1 was detected in PC3 cells after Aza‐dC treatment. Expression of RIZ1 was restored in PC3 cells after treatment with Aza‐dC. RIZ1 expression in LNCaP cells was not changed significantly by Aza‐dC treatment.
Figure 2.
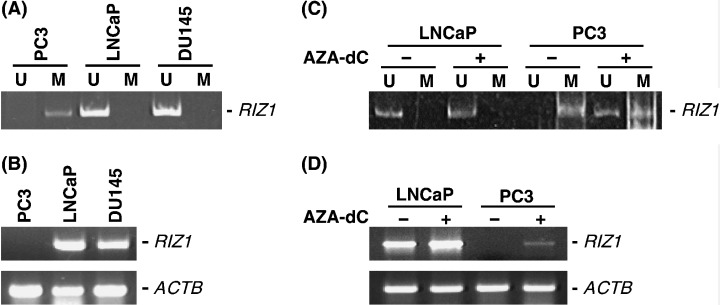
DNA methylation status and expression of RIZ1 in prostate cancer (PCa) cell lines. (A) Methylation‐specific polymerase chain reaction (MSP) of RIZ1. The methylated allele was detected only in the PC3 cell line. U, unmethylated polymerase chain reaction (PCR) product; M, methylated PCR product. (B) Reverse transcription–PCR of mRNA from PCa cell lines. RIZ1 is not expressed in the PC3 cell line. (C) Effect of 5‐Aza‐2′‐deoxycytidine (Aza‐dC) treatment. Aza‐dC‐treated LNCaP and PC3 cells and untreated LNCaP and PC3 cells were analyzed by MSP. The unmethylated allele was detected in Aza‐dC‐treated PC3 cells but not in untreated PC3 cells (D) Expression of RIZ1 was analyzed in Aza‐dC‐treated LNCaP and PC3 cells and untreated LNCaP and PC3 cells. RIZ1 mRNA is expressed in Aza‐dC‐treated PC3 cells but not in untreated PC3 cells.
Discussion
A variety of genetic and epigenetic alterations are associated with human cancers. Although there have been several reports regarding genetic and epigenetic changes in various genes in PCa, in most of these studies, the alteration was investigated for just a single gene. In the present study, the relationship between DNA methylation of RIZ1 and mutation of p53, as measured by nuclear accumulation of p53, was investigated, and we found that PCa tissues with nuclear accumulation of p53 also showed DNA methylation of the RIZ1 gene.
In the present study, DNA methylation of RIZ1 was found in 42.6% of PCa cases analyzed, a frequency slightly higher than that reported previously.( 19 ) In the PCa cell lines, DNA hypermethylation of RIZ1 was detected in PC3 cells, which expressed undetectable levels of the RIZ1 mRNA. After 5 days of Aza‐dC treatment, unmethylated RIZ1 was observed, and expression of RIZ1 mRNA followed. Thus, hypermethylation of RIZ1 plays an important role in inactivation of the RIZ1 gene. Previous studies have demonstrated that CpG island hypermethylation in the region examined was well correlated with epigenetic silencing of the RIZ1 gene.( 17 , 18 ) In the present study, DNA methylation of the RIZ1 gene was not associated with clinicopathological features. Because DNA methylation of RIZ1 is a rare event in non‐malignant prostate tissues,( 19 ) these results suggest that DNA methylation of the RIZ1 gene may be associated with prostate carcinogenesis.
It has been reported that p53 is mutated in late‐stage PCa.( 21 ) In the present study, nuclear accumulation of p53 was observed only in stage III PCa, and all PCa tissues with nuclear accumulation of p53 showed DNA methylation of RIZ1. In the present study, of the four PCa specimens with nuclear accumulation of p53, only two exhibited a p53 mutation. These inconsistent results between immunohistochemistry and PCR‐SSCP may be due to methodological differences. Because only exons 5–8 were examined by PCR‐SSCP in the present study, the mutation of p53 may be in the remaining exons. It is also possible that the sensitivity of PCR‐SSCP may not be sufficient to detect a small number of mutant alleles in a background of wild‐type alleles from stromal cells and normal epithelial cells. It has been reported that the correlation between nuclear accumulation of p53 and the presence of p53 gene mutations can vary.( 30 )
RIZ1 has HMT activity.( 9 ) HMT activity is thought to be important to the tumor suppression function of RIZ1, because this activity is reduced by RIZ1 mutations found in human cancers.( 9 ) Because histone modification is thought to affect chromatin structure directly,( 31 ) aberrant chromatin structure may induce mutation of the p53 gene. In fact, Suv39h (an HMT) ‐deficient mice display severely impaired viability and chromosomal instabilities.( 32 ) A subset of PCa cells with mutations in p53 may arise from PCa cells with DNA methylation of RIZ1. Therefore, DNA methylation of RIZ1 may predict development of p53 mutant PCa cells. In the PCa cell lines, p53 mutation status has been described previously (PC3, mutant‐type p53; LNCaP, wild‐type p53; DU145, mutant‐type p53).( 33 ) Because DNA hypermethylation of RIZ1 was detected in PC3 cells, mutation of the p53 gene may be induced by inactivation of RIZ1. In contrast, DNA hypermethylation of RIZ1 was not detected in DU145 cells despite the presence of p53 mutation. Whether silencing of RIZ1 induces p53 mutation should be verified experimentally in the near future.
Treatment options for the early stages of PCa have been limited to local treatment. Treatments for more advanced disease rely on suppression of testosterone production, primarily with hormonal therapy. Until the introduction of luteinizing hormone‐releasing hormone agonist therapy, estrogen therapy was often used for hormonal manipulation of PCa.( 34 ) Because estradiol treatment produces a selective decrease in RIZ1 expression,( 35 ) it is interesting to investigate the RIZ1 expression in PCa tissues from patients who received preoperative hormone treatment.
In conclusion, our results suggest that genetic alterations are associated with epigenetic alterations and that these alterations are not random events in PCa. To better understand the development of PCa at the molecular level, molecular classification of PCa based on genetic and epigenetic alterations may be useful.
Acknowledgments
This work was supported, in part, by Grants‐in‐Aid for Cancer Research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan, and from the Ministry of Health, Labor, and Welfare of Japan. We thank Masayoshi Takatani and Masayuki Ikeda for excellent technical assistance and advice. This work was carried out with the kind cooperation of the Research Center for Molecular Medicine, Faculty of Medicine, Hiroshima University. We thank the Analysis Center of Life Science, Hiroshima University for the use of their facilities.
References
- 1. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin 2000; 50: 7–33. [DOI] [PubMed] [Google Scholar]
- 2. Isaacs W, De Marzo A, Nelson WG. Focus on prostate cancer. Cancer Cell 2002; 2: 113–16. [DOI] [PubMed] [Google Scholar]
- 3. Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J Natl Cancer Inst 2005; 97: 103–15. [DOI] [PubMed] [Google Scholar]
- 4. Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998; 72: 141–96. [PubMed] [Google Scholar]
- 5. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002; 3: 415–28. [DOI] [PubMed] [Google Scholar]
- 6. Jarrard DF, Kinoshita H, Shi Y et al. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res 1998; 58: 5310–14. [PubMed] [Google Scholar]
- 7. Shiina H, Breault JE, Basset WW et al. Functional loss of the gamma‐catenin gene through epigenetic and genetic pathways in human prostate cancer. Cancer Res 2005; 65: 2130–8. [DOI] [PubMed] [Google Scholar]
- 8. Buyse IM, Shao G, Huang S. The retinoblastoma protein binds to RIZ, a zinc‐finger protein that shares an epitope with the adenovirus E1A protein. Proc Natl Acad Sci USA 1995; 92: 4467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim KC, Geng L, Huang S. Inactivation of a histone methyltransferase by mutations in human cancers. Cancer Res 2003; 63: 7619–23. [PubMed] [Google Scholar]
- 10. He L, Yu JX, Liu L et al. RIZ1, but not the alternative RIZ2 product of the same gene, is underexpressed in breast cancer, and forced RIZ1 expression causes G2–M cell cycle arrest and/or apoptosis. Cancer Res 1998; 58: 4238–44. [PubMed] [Google Scholar]
- 11. Jiang GL, Huang S. Adenovirus expressing RIZ1 in tumor suppressor gene therapy of microsatellite‐unstable colorectal cancers. Cancer Res 2001; 61: 1796–8. [PubMed] [Google Scholar]
- 12. Jiang GL, Huang S. The yin‐yang of PR‐domain family genes in tumorigenesis. Histol Histopathol 2000; 15: 109–17. [DOI] [PubMed] [Google Scholar]
- 13. Piao Z, Fang W, Malkhosyan S et al. Frequent frameshift mutations of RIZ in sporadic gastrointestinal and endometrial carcinomas with microsatellite instability. Cancer Res 2000; 60: 4701–4. [PubMed] [Google Scholar]
- 14. Steele‐Perkins G, Fang W, Yang XH et al. Tumor formation and inactivation of RIZ1, an Rb‐binding member of a nuclear protein‐methyltransferase superfamily. Genes Dev 2001; 15: 2250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weith A, Brodeur GM, Bruns GA et al. Report of the second international workshop on human chromosome 1 mapping 1995. Cytogenet Cell Genet 1996; 72: 114–44. [PubMed] [Google Scholar]
- 16. Gibbs M, Stanford JL, McIndoe RA et al. Evidence for a rare prostate cancer‐susceptibility locus at chromosome 1p36. Am J Hum Genet 1999; 64: 776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du Y, Carling T, Fang W, Piao Z, Sheu JC, Huang S. Hypermethylation in human cancers of the RIZ1 tumor suppressor gene, a member of a histone/protein methyltransferase superfamily. Cancer Res 2001; 61: 8094–9. [PubMed] [Google Scholar]
- 18. Oshimo Y, Oue N, Mitani Y et al. Frequent epigenetic inactivation of RIZ1 by promoter hypermethylation in human gastric carcinoma. Int J Cancer 2004; 110: 212–18. [DOI] [PubMed] [Google Scholar]
- 19. Suzuki M, Shigematsu H, Shivapurkar N et al. Methylation of apoptosis related genes in the pathogenesis and prognosis of prostate cancer. Cancer Lett 2006. Feb 1; [Epub ahead of print]. [DOI] [PubMed]
- 20. Kirsch DG, Kastan MB. Tumor‐suppressor p53: implications for tumor development and prognosis. J Clin Oncol 1998; 16: 3158–68. [DOI] [PubMed] [Google Scholar]
- 21. Bookstein R, MacGrogan D, Hilsenbeck SG, Sharkey F, Allred DC. p53 is mutated in a subset of advanced‐stage prostate cancers. Cancer Res 1993; 53: 3369–73. [PubMed] [Google Scholar]
- 22. Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem 1998; 273: 1–4. [DOI] [PubMed] [Google Scholar]
- 23. Oue N, Motoshita J, Yokozaki H et al. Distinct promoter hypermethylation of p16INK4a, CDH1, and RAR‐β in intestinal, diffuse‐adherent, and diffuse‐scattered type gastric carcinomas. J Pathol 2002; 198: 55–9. [DOI] [PubMed] [Google Scholar]
- 24. Toyota M, Ohe‐Toyota M, Ahuja N, Issa JP. Distinct genetic profiles in colorectal tumors with or without the CpG island methylator phenotype. Proc Natl Acad Sci USA 2000; 97: 710–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobin LH, Wittekind CH, eds. Prostate. TNM Classification of Malignant Tumors. New York: Wiley‐Liss, 2002; 184–7. [Google Scholar]
- 26. Gleason DF. Histologic grading and clinical staging of prostatic carcinoma. In: M Tannebaum, ed. Urologic Pathology: The Prostate. Philadelphia: Lea & Febiger, 1977: 171–97. [Google Scholar]
- 27. Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation‐specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996; 93: 9821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oue N, Shigeishi H, Kuniyasu H et al. Promoter hypermethylation of MGMT is associated with protein loss in gastric carcinoma. Int J Cancer 2001; 93: 805–9. [DOI] [PubMed] [Google Scholar]
- 29. Bartek J, Iggo R, Gannon J, Lane DP. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene 1990; 5: 893–9. [PubMed] [Google Scholar]
- 30. Casey G, Lopez ME, Ramos JC et al. DNA sequence analysis of exons 2 through 11 and immunohistochemical staining are required to detect all known p53 alterations in human malignancies. Oncogene 1996; 13: 1971–81. [PubMed] [Google Scholar]
- 31. Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev 2002; 12: 142–8. [DOI] [PubMed] [Google Scholar]
- 32. Peters AH, O’Carroll D, Scherthan H et al. Loss of the Suv39 h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 2001; 107: 323–37. [DOI] [PubMed] [Google Scholar]
- 33. Jackson P, Grimm MO, Kingsley EA et al. Relationship between expression of KAI1 metastasis suppressor gene, mRNA levels and p53 in human bladder and prostate cancer cell lines. Urol Oncol 2002; 7: 99–104. [DOI] [PubMed] [Google Scholar]
- 34. McLeod DG. Hormonal therapy: historical perspective to future directions. Urology 2003; 61: 3–7. [DOI] [PubMed] [Google Scholar]
- 35. Gazzerro P, Abbondanza C, D’Arcangelo A et al. Modulation of RIZ gene expression is associated to estradiol control of MCF‐7 breast cancer cell proliferation. Exp Cell Res 2006; 312: 340–9. [DOI] [PubMed] [Google Scholar]


