Abstract
This prospective study used antibody suspension bead arrays to identify biomarkers capable of predicting post‐operative recurrence with distal metastasis in patients with colorectal cancer. One hundred colorectal cancer patients who underwent surgery were enrolled in this study. The median follow‐up period was 3.9 years. The pre‐operative plasma concentrations of 24 angiogenesis‐related molecules were analyzed with regard to the TNM stage and the development of post‐operative recurrence. The concentrations of half of the examined molecules (13/24) increased significantly according to the TNM stage (P < 0.05). Meanwhile, a multivariate logistic regression analysis revealed that the concentrations of vascular cell adhesion molecule 1 (VCAM‐1) and plasminogen activator inhibitor‐1 (PAI‐1) were significantly higher in the post‐operative recurrence group. The VCAM‐1 and PAI‐1 model discriminated post‐operative recurrence with an area under the curve of 0.82, a sensitivity of 0.75, and a specificity of 0.73. A leave‐one‐out cross‐validation was applied to the model to assess the prediction performance, and the result indicated that the cross‐validated error rate was 12.5% (12/96). In conclusion, our results demonstrate that antibody suspension bead arrays are a powerful tool to screen biomarkers in the clinical setting, and the plasma levels of VCAM‐1 and PAI‐1 together may be a promising biomarker for predicting post‐operative recurrence in patients with colorectal cancer. (Cancer Sci 2010)
Colorectal cancer (CRC) is one of the leading causes of death in Japan (http://ganjoho.ncc.go.jp/public/statistics/index.html) and Western countries.( 1 ) Despite recent advances in adjuvant chemotherapy and surgical techniques, 20–40% of patients die because of metastasis after curative surgery.( 2 ) Tumor‐node‐metastasis (TNM) staging is well established and the most reliable system for predicting the outcome of CRC. In particular, the TNM staging system works very well for predicting the outcome of early stage I cancers and advanced stage IV cancers. However, the 5‐year survival rate varies from 44% to 83% within TNM stage III, indicating that a wide variation in outcomes exists within each stage as a result of biological heterogeneity.( 3 ) Thus, highly accurate predictors of post‐operative recurrence are needed for patients with CRC who undergo curative surgery, as such predictors would likely contribute to the further improvement of the 5‐year survival rate by justifying the addition of intensive adjuvant chemotherapy to the therapeutic regimens of subgroups with a high risk of post‐operative recurrence. Therefore, the prediction of post‐operative recurrence is regarded as one of the most important research themes in clinical settings and has been extensively studied, with particular attention given to the investigation of various molecular prognostic factors.
In addition to the TNM stage, the carcinoembryonic antigen (CEA) level is routinely used to monitor recurrence in patients with CRC.( 4 ) A large clinical study demonstrated that pre‐operative CEA levels provide prognostic information in addition to that provided by the TNM staging system and determined that the pre‐operative CEA level was an independent predictor of survival and recurrence.( 5 ) However, the study concluded that although an elevated pre‐operative CEA level (>5 mg/mL) may be correlated with a poor prognosis, the available data was insufficient to support the use of the CEA level for determining whether a patient should undergo adjuvant therapy.( 4 ) Other molecular markers, including the K‐Ras mutation status,( 6 , 7 ) microsatellite instability,( 8 ) the loss of heterogeneity at 18q,( 9 ) and p53,( 10 ) have been examined with regard to predicting the outcome of subgroups; unfortunately, none of these molecular markers are suitable for routine clinical use. Thus, further investigations of novel molecular markers are eagerly awaited.
The Bio‐Plex suspension array system (Bio‐Rad Laboratories, Hercules, CA, USA) utilizes a series of color‐coded beads, each of which is coupled to a unique antibody specific for a biochemical marker. This assay is capable of measuring the levels of multiple targets in a single well of a 96‐well microplate using as little as 12.5 μL of serum, plasma, or other matrix. In the present study, 24 angiogenesis‐related markers from this assay panel were used to evaluate plasma proteins and their potential associations with disease progression and the recurrence of CRC.
Materials and Methods
Patient selection. Patients with histologically confirmed colorectal cancer who were between the ages of 20 and 80 years and who were scheduled to undergo surgery were eligible for enrollment in this study. Additional inclusion criteria included an Eastern Cooperative Oncology Group performance status of 0–2. All the patients in this series underwent surgery. This prospective study was approved by the Institutional Review Board of the National Cancer Center Hospital and written informed consent was obtained from all the patients.
Clinical and pathologic features. Clinical features including age, sex, primary site of tumor, histologic type of tumor, TNM stage, and post‐operative recurrence were recorded. A pathologist reviewed the microscopic slides. Post‐operative recurrence was defined when distant metastasis was observed. The median follow‐up period of post‐operative recurrence was 3.9 years.
Preparation of plasma samples. Two milliliters of whole blood were collected into EDTA‐containing tubes before surgery (within 2 weeks) and were centrifuged at 1500 g for 10 min to obtain the plasma samples. The samples were stored at −80°C until further use.
Angiogenesis‐related molecules. The 24 plasma markers used in this study were as follows: interleukin 6 receptor (IL‐6R), matrix metallopeptidase 9 (MMP‐9), TIMP metallopeptidase inhibitor 1 (TIMP‐1), TIMP metallopeptidase inhibitor 2 (TIMP‐2), endostatin, P‐selectin, intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), Tie‐2, plasminogen activator inhibitor‐1 (PAI‐1), macrophage migration inhibitory factor (MIF), plasminogen activator urokinase receptor (uPAR), angiopoietin 2 (Ang‐2), follistatin, hepatocyte growth factor (HGF), interleukin 8 (IL‐8), colony stimulating factor 3 (G‐CSF), platelet‐derived growth factor beta polypeptide (PDGF‐BB), vascular endothelial growth factor (VEGF), leptin, platelet/endothelial cell adhesion molecule (PECAM‐1), interleukin 12 (IL‐12), fibroblast growth factor 2 (FGF‐basic), and tumor necrosis factor (TNF‐α). Ang‐2, Follistatin, The nine markers of HGF, IL‐8, PDGF‐BB, VEGF, Leptin, PECAM‐1, and G‐CSF are commercially available as the Human Premixed Angiogenesis (9) panel (Bio‐Rad Laboratories). The others are available for customization or in developing markers.
Antibody suspension bead arrays system. The plasma concentrations of each molecule were measured using a Bio‐Plex suspension array system (Bio‐Rad Laboratories), which permits the simultaneous measurement of multiple circulating proteins in a single well using only 12.5 μL of plasma. The assay was performed according to the manufacturer’s instructions and a previously described method.( 11 ) All plasma samples were diluted 1 in 4 with the appropriate diluents prior to assay. The samples were tested in duplicate.
Statistical analysis. The correlations between the plasma concentrations and the TNM stages were analyzed using a linear trend analysis and the proportional odds model. In the linear trend analysis, we used a one‐way anova model with a linear contrast, which consisted of the TMN stage scores. A t‐test was used to compare the post‐operative recurrence and the no recurrence groups. In the multivariate analysis, an analysis was performed for all the plasma markers but not for the clinical variables because too many explanatory variables for the sample size were included in this study. A logistic regression analysis was used to examine statistical differences according to post‐operative recurrence and a stepwise method was used to select the most useful explanatory parameters. Analyses were performed using SAS software version 9.1.3 (SAS Institute, Cary, NC, USA). A P‐value of <0.05 was considered statistically significant.
Results
Patient results. A total of 100 consecutive patients were enrolled in this study. The median age of the enrolled patients was 59 years (range, 31–79 years). Among them, 96 patients received curative operations and four patients had distal metastasis and received palliative operations. Eleven patients developed recurrences with distal metastasis during the follow‐up period. Table 1 summarizes the characteristics of the patients and their tumors.
Table 1.
Patient characteristics
| Characteristics | Curative ope. | Palliative ope. | Total | |
|---|---|---|---|---|
| Rec+ | Rec− | |||
| Age (years) | ||||
| ≥60 | 6 | 42 | 2 | 50 |
| <60 | 5 | 43 | 2 | 50 |
| Sex | ||||
| Male | 3 | 54 | 3 | 65 |
| Female | 8 | 31 | 1 | 35 |
| Primary site | ||||
| Colon | 8 | 40 | 1 | 49 |
| Rectum | 3 | 45 | 3 | 51 |
| Hist. type | ||||
| Well diff. | 8 | 59 | 3 | 70 |
| Others | 3 | 26 | 1 | 30 |
| TNM stage | ||||
| I | 1 | 26 | – | 27 |
| II | 2 | 22 | – | 24 |
| III | 8 | 37 | – | 45 |
| IV | – | – | 4 | 4 |
| Total | 11 | 85 | 4 | 100 |
Hist. type, histology of primary tumor; Rec+, post‐operative recurrence (+); Rec−, post‐operative recurrence (−).
Plasma concentrations of 24 angiogenesis‐related molecules. We measured the plasma concentrations of 24 angiogenesis‐related molecules: IL‐6R, MMP‐9, TIMP‐1, TIMP‐2, endostatin, P‐selectin, ICAM‐1, VCAM‐1, Tie‐2, PAI‐1, MIF, uPAR, Ang‐2, follistatin, HGF, IL‐8, G‐CSF, PDGF‐BB, VEGF, leptin, PECAM‐1, IL‐12, FGF‐basic, and TNF‐α (Table 2). Overall, 98.5% of the plasma samples were successfully quantified using a standard curve.
Table 2.
Plasma concentrations of 24 angiogenesis‐related molecules in 100 colorectal cancers
| Molecules | Range | Average (SD) | 25% | Percentile | |
|---|---|---|---|---|---|
| pg/mL | Median | 75% | |||
| IL‐6R | 23–149 | 54 (18) | 42 | 52 | 62 |
| MMP‐9 | 6–189 | 35 (27) | 20 | 27 | 41 |
| TIMP‐1 | 44–283 | 117 (35) | 96 | 118 | 135 |
| TIMP‐2 | 9–47 | 24 (5) | 21 | 24 | 28 |
| Endostatin | 90–456 | 187 (64) | 140 | 177 | 223 |
| P‐selectin | 0–164 | 64 (27) | 48 | 62 | 78 |
| ICAM‐1 | 103–605 | 282 (81) | 225 | 270 | 317 |
| VCAM‐1 | 76–333 | 138 (40) | 110 | 135 | 163 |
| Tie‐2 | 9–1070 | 143 (164) | 48 | 73 | 182 |
| PAI‐1 | 3–65 | 21 (12) | 14 | 19 | 26 |
| MIF | 0–120 | 53 (26) | 44 | 59 | 69 |
| uPAR | 1–130 | 24 (23) | 8 | 15 | 37 |
| Ang‐2 | 0–6147 | 1381 (1337) | 415 | 938 | 2197 |
| Follistatin | 235–2903 | 924 (614) | 502 | 686 | 1197 |
| HGF | 201–12213 | 2700 (2516) | 1076 | 1628 | 3930 |
| IL‐8 | 5–234 | 52 (41) | 24 | 34 | 74 |
| G‐CSF | 0–4775 | 832 (1133) | 79 | 247 | 1161 |
| PDGF‐BB | 6–6219 | 737 (831) | 220 | 439 | 922 |
| VEGF | 0–724 | 186 (164) | 67 | 120 | 262 |
| Leptin | 0–32847 | 3155 (4433) | 1149 | 2134 | 3851 |
| PECAM‐1 | 1188–15837 | 5562 (3472) | 2901 | 4487 | 7348 |
| IL‐12 | 0–32 | 5 (6) | 2 | 3 | 6 |
| FGF‐basic | 0–235 | 21 (30) | 4 | 13 | 25 |
| TNF‐α | 0–72 | 4 (9) | 1 | 2 | 4 |
The concentrations are ng/mL for interleukin 6 receptor (IL‐6R), matrix metallopeptidase 9 (MMP‐9), TIMP metallopeptidase inhibitor 1 (TIMP‐1), TIMP‐2, endostatin, P‐selectin, intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), Tie‐2, plasminogen activator inhibitor‐1 (PAI‐1), macrophage migration inhibitory factor (MIF), and plasminogen activator urokinase receptor (uPAR), and the others are pg/mL. FGF‐basic, fibroblast growth factor 2; G‐CSF, colony stimulating factor 3; HGF, hepatocyte growth factor; PDGF‐BB, platelet‐derived growth factor beta polypeptide; PECAM‐1, platelet/endothelial cell adhesion molecule; TNF‐α, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Tumor‐node‐metastasis (TNM) stage and plasma concentrations of angiogenesis‐related molecules. The TNM stage can be accurately used to stratify patients at a high risk for cancer progression and is thought to reflect the malignant potential of each tumor. To estimate the contributions of the angiogenesis‐related molecules to the malignant potentials of the tumors, we examined the correlation between the plasma concentrations of each molecule and the TNM stage. A linear trend analysis showed that the plasma concentrations of 13 molecules increased significantly with an increasing TNM stage (P < 0.05): IL‐6R, TIMP‐1, TIMP‐2, P‐selectin, Tie‐2, PAI‐1, uPAR, Ang‐2, follistatin, HGF, IL‐8, PDGF‐BB, and VEGF (Table 3). Next, we performed an exploratory multivariate analysis using a proportional odds model with the TNM stage (I–IV) assigned as the objective variable and each of the angiogenesis‐related molecules assigned as explanatory variables. The multivariate analysis identified TIMP‐1, P‐selectin, Ang‐2, HGF, IL‐8, PDGF‐BB, and VEGF as being significantly correlated with the TNM stage. These results indicated that the plasma concentrations of several molecules increased significantly with an increasing TNM stage, strongly suggesting that these molecules might be candidate biomarkers for an unfavorable outcome in patients with CRC.
Table 3.
Tumor‐node‐metastasis (TNM) stage and plasma concentrations in 100 colorectal cancers
| Molecules | TNM stage | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | P‐value | P‐value | |
| IL‐6R | 50 | 57 | 52 | 76 | 0.01 | n.s. |
| MMP‐9 | 34 | 50 | 28 | 33 | n.s. | n.s. |
| TIMP‐1 | 107 | 122 | 114 | 185 | <0.0001 | 0.02 |
| TIMP‐2 | 24 | 24 | 24 | 33 | 0.003 | n.s. |
| Endostatin | 184 | 198 | 182 | 201 | n.s. | n.s. |
| P‐selectin | 56 | 67 | 63 | 109 | 0.0003 | 0.04 |
| ICAM‐1 | 276 | 315 | 266 | 298 | n.s. | n.s. |
| VCAM‐1 | 135 | 136 | 141 | 144 | n.s. | n.s. |
| Tie‐2 | 116 | 162 | 129 | 375 | 0.005 | n.s. |
| PAI‐1 | 18 | 24 | 21 | 38 | 0.003 | n.s. |
| MIF | 53 | 55 | 51 | 78 | n.s. | n.s. |
| uPAR | 20 | 27 | 22 | 53 | 0.01 | n.s. |
| Ang‐2 | 978 | 1458 | 1447 | 2914 | 0.007 | 0.03 |
| Follistatin | 778 | 953 | 928 | 1704 | 0.006 | n.s. |
| HGF | 1933 | 2932 | 2803 | 5342 | 0.01 | 0.04 |
| IL‐8 | 38 | 56 | 53 | 108 | 0.002 | 0.02 |
| G‐CSF | 595 | 1114 | 797 | 1132 | n.s. | n.s. |
| PDGF‐BB | 442 | 822 | 802 | 1483 | 0.02 | 0.03 |
| VEGF | 129 | 209 | 192 | 375 | 0.006 | 0.03 |
| Leptin | 3236 | 2815 | 3235 | 3752 | n.s. | n.s. |
| PECAM‐1 | 5026 | 5914 | 5625 | 6356 | n.s. | n.s. |
| IL‐12 | 3 | 8 | 5 | 5 | n.s. | n.s. |
| FGF‐basic | 19 | 32 | 16 | 28 | n.s. | n.s. |
| TNF‐α | 4 | 6 | 3 | 2 | n.s. | n.s. |
Values indicate the average. Univariate: linear trend analysis, multivariate: proportional odds model. The concentrations are ng/mL for interleukin 6 receptor (IL‐6R), matrix metallopeptidase 9 (MMP‐9), TIMP metallopeptidase inhibitor 1 (TIMP‐1), TIMP‐2, endostatin, P‐selectin, intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), Tie‐2, plasminogen activator inhibitor‐1 (PAI‐1), macrophage migration inhibitory factor (MIF), and plasminogen activator urokinase receptor (uPAR), and the others are pg/mL. FGF‐basic, fibroblast growth factor 2; G‐CSF, colony stimulating factor 3; HGF, hepatocyte growth factor; n.s., not significant; PDGF‐BB, platelet‐derived growth factor beta polypeptide; PECAM‐1, platelet/endothelial cell adhesion molecule; TNF‐α, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Post‐operative recurrence and plasma concentrations of angiogenesis‐related molecules. To predict post‐operative recurrence using this system, we analyzed the 96 patients with CRC who underwent curative operations, excluding the four patients with distal metastasis. Among these 96 patients, 11 developed recurrences during the follow‐up period; the remaining 85 patients did not show any signs of recurrence. When the plasma levels of the angiogenesis‐related molecules were compared between the patients with recurrences and those without recurrences, t‐tests demonstrated that the plasma concentrations of IL‐6R (63.2 ± 13.8 and 51.3 ± 17.0 ng/mL, respectively), P‐selectin (76.1 ± 24.4 and 60.0 ± 24.8 ng/mL, respectively), VCAM‐1 (163.9 ± 61.0 and 134.6 ± 35.0 ng/mL, respectively), and PAI‐1 (28.2 ± 15.4 and 19.8 ± 10.2 ng/mL, respectively) were significantly higher among the patients with recurrences (Fig. 1a–d, Table 4). A multivariate logistic regression analysis revealed that the plasma concentrations of VCAM‐1 and PAI‐1 were significantly higher among the patients with recurrences (P = 0.039 and P = 0.028, respectively). A stepwise method selected VCAM‐1 and PAI‐1 as the most useful explanatory parameters, suggesting that the combination of these two molecules might synergistically improve the prediction of post‐operative recurrence. Finally, a prediction model incorporating VCAM‐1 and PAI‐1 successfully discriminated post‐operative recurrence, with an area under the curve (AUC) of 0.82, a sensitivity of 0.75, and a specificity of 0.73 (Fig. 2a). To assess the prediction performance, a leave‐one‐out cross‐validation was applied to the model. The cross‐validated error rate was 12.5% (12/96). In stage III patients, the prediction model had a sensitivity of 0.625 (5/8) and a specificity of 0.865 (32/37) for predicting post‐operative recurrence (Fig. S2). On the other hand, when apparent distal metastases of CRCs were applied to the VCAM‐I/PAI‐I prediction model, three out of four metastatic cases were determined as “recurrence (+) cases”. These results suggest that apparent metastatic tumors could be discriminated using the model. Although a validation study is necessary, our results raise the possibility that the combined use of the pre‐operative plasma concentrations of VCAM‐1 and PAI‐1 might be useful for predicting post‐operative recurrence in patients with CRC. Finally, we retrospectively analyzed the plasma PAI‐1 concentrations of metastatic and non‐metastatic CRC in another study of an independent cohort using conventional ELISA. The plasma concentrations in the metastatic CRC patients were significantly higher than those in the non‐metastatic patients (P = 0.005), even in the independent cohort (Fig. S1). The CEA level was not significantly different between the recurrence (+) versus the recurrence (−) groups (P = 0.335) in our study.
Figure 1.
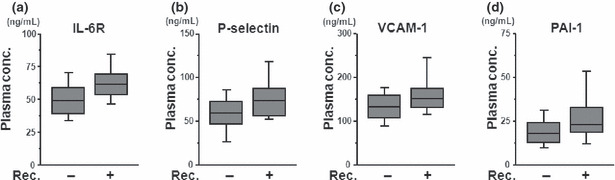
The concentrations of (a) interleukin 6 receptor (IL‐6R), (b) P‐selectin, (c) vascular cell adhesion molecule 1 (VCAM‐1), and (d) plasminogen activator inhibitor‐1 (PAI‐1) were significantly higher in the recurrence group in colorectal cancer. The upper bar, box, and lower bar represent the 90%, 75%, 50%, 25% and 10% percentiles. The plasma concentrations of each molecule were measured using a Bio‐Plex suspension array system. Rec, recurrence.
Table 4.
Results of multivariate analysis for recurrence after curative surgery in 96 colorectal cancers
| Molecules | Recurrence* | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| + | − | t‐test | Logistic | Stepwise | |
| P‐value | P‐value | P‐value | |||
| IL‐6R | 63 | 51 | 0.03 | n.s. | |
| MMP‐9 | 37 | 35 | n.s. | n.s. | |
| TIMP‐1 | 119 | 113 | n.s. | n.s. | |
| TIMP‐2 | 25 | 24 | n.s. | n.s. | |
| Endostatin | 193 | 186 | n.s. | n.s. | |
| P‐selectin | 76 | 60 | 0.04 | n.s. | |
| ICAM‐1 | 285 | 281 | n.s. | n.s. | |
| VCAM‐1 | 164 | 135 | 0.02 | 0.039 | 0.009 |
| Tie‐2 | 183 | 127 | n.s. | n.s. | |
| PAI‐1 | 28.3 | 19.8 | 0.02 | 0.005 | 0.005 |
| MIF | 64 | 51 | n.s. | n.s. | |
| uPAR | 30 | 21 | n.s. | n.s. | |
| Ang‐2 | 1514 | 1292 | n.s. | n.s. | |
| Follistatin | 962 | 883 | n.s. | n.s. | |
| HGF | 3155 | 2517 | n.s. | n.s. | |
| IL‐8 | 53 | 49 | n.s. | n.s. | |
| G‐CSF | 892 | 810 | n.s. | n.s. | |
| PDGF‐BB | 972 | 671 | n.s. | n.s. | |
| VEGF | 211 | 174 | n.s. | n.s. | |
| Leptin | 2623 | 3196 | n.s. | n.s. | |
| PECAM‐1 | 6159 | 5447 | n.s. | n.s. | |
| IL‐12 | 4 | 5 | n.s. | n.s. | |
| FGF‐basic | 16 | 22 | n.s. | n.s. | |
| TNF‐α | 4 | 4 | n.s. | n.s. | |
*Values indicate the average. Recurrence, post‐operative recurrence; logistic, logistic regression model. The concentrations are ng/mL for interleukin 6 receptor (IL‐6R), matrix metallopeptidase 9 (MMP‐9), TIMP metallopeptidase inhibitor 1 (TIMP‐1), TIMP‐2, endostatin, P‐selectin, intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), Tie‐2, plasminogen activator inhibitor‐1 (PAI‐1), macrophage migration inhibitory factor (MIF), and plasminogen activator urokinase receptor (uPAR), and the others are pg/mL. FGF‐basic, fibroblast growth factor 2; G‐CSF, colony stimulating factor 3; HGF, hepatocyte growth factor; n.s., not significant; PDGF‐BB, platelet‐derived growth factor beta polypeptide; PECAM‐1, platelet/endothelial cell adhesion molecule; TNF‐α, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Figure 2.
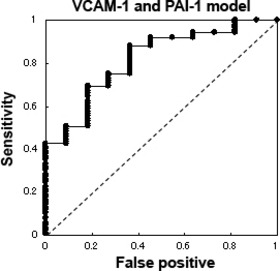
The receiver–operator curve (ROC) for post‐operative recurrence. A stepwise method selected vascular cell adhesion molecule 1 (VCAM‐1) and plasminogen activator inhibitor‐1 (PAI‐1) as the most useful explanatory parameters; these two molecules were subsequently used to construct a prediction model. The ROC indicates the results of this model, which was capable of discriminating post‐operative recurrence with an area under the curve (AUC) of 0.82, a sensitivity of 0.75, and a specificity of 0.73.
Discussion
Vascular cell adhesion molecule 1 (VCAM‐1)/CD106 is a member of the Ig superfamily and encodes a cell surface sialoglycoprotein expressed by cytokine‐activated endothelium. This type I membrane protein mediates leukocyte‐endothelial cell adhesion and signal transduction, and may play a role in the development of atherosclerosis( 12 ) and rheumatoid arthritis.( 13 ) In the field of oncology, accumulating evidence suggests that VCAM‐1 is associated with a poor outcome.( 14 , 15 , 16 , 17 ) Recently, Shariat et al. ( 18 ) reported that standard clinical variables alone exhibited an accuracy of 71.6% for predicting the risk of biochemical recurrence following a radical prostatectomy in patients with prostate cancer, whereas the addition of preoperative blood levels of TGF‐β1, sIL‐6R, IL‐6, VCAM‐1, VEGF, endoglin, and uPA increased the predictive accuracy by 15–86.6%. Vascular endothelial growth factor (VEGF) and uPA were not significant predictors of post‐operative recurrence in our data set for CRC, but VCAM‐1 and sIL‐6R were significant, consistent with Shariat’s study. The mechanism by which VCAM‐1 mediates an unfavorable phenotype remains unclear, but the most probable explanation is that the tumor cells escape T‐cell immunity by overexpressing the endothelial cell adhesion molecule VCAM‐1, which normally mediates leukocyte extravasation to sites of tissue inflammation.( 19 )
Plasminogen activator inhibitor‐1 (PAI‐1)/SERPINE1 belongs to the plasmin/plasminogen system and is secreted into the blood, where it prevents the generation of plasmin, promoting the persistence and expansion of thrombi.( 20 ) Plasminogen activator inhibitor‐1 (PAI‐1) is known as tumor biological prognostic factor and has been thoroughly validated with regard to its clinical utility in breast cancer.( 21 , 22 ) The 2007 Breast Tumor Markers Guidelines recommend that uPA/PAI‐1 be measured using an ELISA with a minimum of 300 mg of fresh or frozen breast cancer tissue for determining the prognosis of patients with newly diagnosed, node‐negative breast cancer. Furthermore, CMF‐based adjuvant chemotherapy provides a substantial benefit, compared with observation alone, in patients with a high risk of recurrence as determined by the presence of high levels of uPA and PAI‐1.( 23 ) Previous reports have demonstrated that higher levels of PAI‐1, but not PAI‐2, are associated with large tumors, metastatic stage, and a worse prognosis in patients with CRC.( 24 , 25 , 26 ) Our study differed in that it evaluated the clinical parameter of post‐operative recurrence in a prospective study. The biological mechanism by which PAI‐1 promotes tumor progression is thought to involve a reduction in cell adhesion to the extracellular matrix as a consequence of excess PAI‐1 interfering with uPAR binding to vitronectin, thereby facilitating cell invasion and migration.( 27 ) Interestingly, accumulating data indicate that both VCAM‐1 and PAI‐1 promote tumor metastasis and cellular adhesion. These activities are likely involved in post‐operative recurrence. We plan to perform a validation study to predict post‐operative recurrence using the plasma concentrations of VCAM‐1 and PAI‐1 in the near future.
In conclusion, we have demonstrated that a combination prediction model based on the plasma concentrations of VCAM‐1 and PAI‐1 was a useful biomarker for predicting post‐operative recurrence in patients with colorectal cancer. Our strategy, which utilizes a multiplex immunoassay system, may be a powerful tool for identifying biomarkers in clinical settings.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Metastasis (+) vs (−) in independent samples of colorectal cancer (CRC) (n = 28).
Fig. S2. Post‐operative recurrence (+) vs (−) in stage III colorectal cancer (CRC) (n = 45).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Acknowledgments
This study was supported by the Third‐Term Comprehensive 10‐Year Strategy for Cancer Control, a Grant‐in‐Aid for Scientific Research; the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NiBio); a Grant‐in‐Aid for Cancer Research (H20‐20‐9) from the Ministry of Health, Labour and Welfare; and a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (19209018). The following people have played very important roles in the conduct of this project: Hiromi Orita, Hideko Morita, and Mari Araake.
References
- 1. Greenlee RT, Hill‐Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin 2001; 51: 15–36. [DOI] [PubMed] [Google Scholar]
- 2. Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F. Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum 1998; 41: 1033–49. [DOI] [PubMed] [Google Scholar]
- 3. Mutch MG. Molecular profiling and risk stratification of adenocarcinoma of the colon. J Surg Oncol 2007; 96: 693–703. [DOI] [PubMed] [Google Scholar]
- 4. Locker GY, Hamilton S, Harris J et al. ; ASCO . ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006;24:5313–27. [DOI] [PubMed] [Google Scholar]
- 5. Park YJ, Park KJ, Park JG, Lee KU, Choe KJ, Kim JP. Prognostic factors in 2230 Korean colorectal cancer patients: analysis of consecutively operated cases. World J Surg 1999; 23: 721–6. [DOI] [PubMed] [Google Scholar]
- 6. Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst 1998; 90: 675–84. [DOI] [PubMed] [Google Scholar]
- 7. Andreyev HJ, Norman AR, Cunningham D et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer 2001; 85: 692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005; 23: 609–18. [DOI] [PubMed] [Google Scholar]
- 9. Aschele C, Debernardis D, Lonardi S et al. Deleted in colon cancer protein expression in colorectal cancer metastases: a major predictor of survival in patients with unresectable metastatic disease receiving palliative fluorouracil‐based chemotherapy. J Clin Oncol 2004; 22: 3758–65. [DOI] [PubMed] [Google Scholar]
- 10. Duffy MJ, Van Dalen A, Haglund C et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer 2007; 43: 1348–60. [DOI] [PubMed] [Google Scholar]
- 11. Kimura H, Kasahara K, Sekijima M, Tamura T, Nishio K. Plasma MIP‐1beta levels and skin toxicity in Japanese non‐small cell lung cancer patients treated with the EGFR‐targeted tyrosine kinase inhibitor, gefitinib. Lung Cancer 2005; 50: 393–9. [DOI] [PubMed] [Google Scholar]
- 12. Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 2007; 27: 2292–301. [DOI] [PubMed] [Google Scholar]
- 13. Carter RA, Wicks IP. Vascular cell adhesion molecule 1 (CD106): a multifaceted regulator of joint inflammation. Arthritis Rheum 2001; 44: 985–94. [DOI] [PubMed] [Google Scholar]
- 14. Yao M, Huang Y, Shioi K et al. A three‐gene expression signature model to predict clinical outcome of clear cell renal carcinoma. Int J Cancer 2008; 123: 1126–32. [DOI] [PubMed] [Google Scholar]
- 15. De Cicco C, Ravasi L, Zorzino L et al. Circulating levels of VCAM and MMP‐2 may help identify patients with more aggressive prostate cancer. Curr Cancer Drug Targets 2008; 8: 199–206. [DOI] [PubMed] [Google Scholar]
- 16. Silva HC, Garcao F, Coutinho EC, De Oliveira CF, Regateiro FJ. Soluble VCAM‐1 and E‐selectin in breast cancer: relationship with staging and with the detection of circulating cancer cells. Neoplasma 2006; 53: 538–43. [PubMed] [Google Scholar]
- 17. Shioi K, Komiya A, Hattori K et al. Vascular cell adhesion molecule 1 predicts cancer‐free survival in clear cell renal carcinoma patients. Clin Cancer Res 2006; 12: 7339–46. [DOI] [PubMed] [Google Scholar]
- 18. Shariat SF, Karam JA, Walz J et al. Improved prediction of disease relapse after radical prostatectomy through a panel of preoperative blood‐based biomarkers. Clin Cancer Res 2008; 14: 3785–91. 18559597 [Google Scholar]
- 19. Wu TC. The role of vascular cell adhesion molecule‐1 in tumor immune evasion. Cancer Res 2007; 67: 6003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Durand MK, Bødker JS, Christensen A et al. Plasminogen activator inhibitor‐I and tumour growth, invasion, and metastasis. Thromb Haemost 2004; 91: 438–49. [DOI] [PubMed] [Google Scholar]
- 21. Harbeck N, Schmitt M, Kates RE et al. Clinical utility of urokinase‐type plasminogen activator and plasminogen activator inhibitor‐1 determination in primary breast cancer tissue for individualized therapy concepts. Clin Breast Cancer 2002; 3: 196–200. [DOI] [PubMed] [Google Scholar]
- 22. Harbeck N, Kates RE, Schmitt M. Clinical relevance of invasion factors urokinase‐type plasminogen activator and plasminogen activator inhibitor type 1 for individualized therapy decisions in primary breast cancer is greatest when used in combination. J Clin Oncol 2002; 20: 1000–7. [DOI] [PubMed] [Google Scholar]
- 23. Harris L, Fritsche H, Mennel R et al. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007; 25: 5287–312. [DOI] [PubMed] [Google Scholar]
- 24. Herszènyi L, Plebani M, Carraro P et al. The role of cysteine and serine proteases in colorectal carcinoma. Cancer 1999; 86: 1135–42. [DOI] [PubMed] [Google Scholar]
- 25. Sier CF, Vloedgraven HJ, Ganesh S et al. Inactive urokinase and increased levels of its inhibitor type 1 in colorectal cancer liver metastasis. Gastroenterology 1994; 107: 1449–56. [DOI] [PubMed] [Google Scholar]
- 26. Langenskiöld M, Holmdahl L, Angenete E, Falk P, Nordgren S, Ivarsson ML. Differential prognostic impact of uPA and PAI‐1 in colon and rectal cancer. Tumour Biol 2009; 30(4): 210–20. [DOI] [PubMed] [Google Scholar]
- 27. Berger DH. Plasmin/plasminogen system in colorectal cancer. World J Surg 2002; 26: 767–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Metastasis (+) vs (−) in independent samples of colorectal cancer (CRC) (n = 28).
Fig. S2. Post‐operative recurrence (+) vs (−) in stage III colorectal cancer (CRC) (n = 45).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item


