Abstract
The cyclooxygenase (COX)‐2 inhibitor has been reported to impede the progression of gastric cancer, but underlying mechanisms remain unclear. We therefore investigated the effect of a COX‐2 inhibitor, JTE‐522, on the ability of orthotopic fibroblasts to stimulate invasion of scirrhous gastric carcinoma cells. The human scirrhous gastric cancer cell lines OCUM‐2D or OCUM‐2M, and human gastric fibroblasts (NF‐21) were cultured in the absence or presence of JTE‐522 at various concentrations. Cancer cells were then assayed for invasiveness in vitro by invasion assay. The effect of prostaglandins (PG) on growth factor production in NF‐21 cells was examined by ELISA. Finally, the effects of orally administrated JTE‐522 on orthotopically transplanted tumors were examined in nude mice. NF‐21 cells stimulated invasion by OCUM‐2D cells, an effect suppressed by JTE‐522 at 5 × 10−6 M. Hepatocyte growth factor (HGF) and PGE2 production by NF‐21 cells were suppressed by JTE‐522 (P < 0.01). PGE2 stimulated HGF production by NF‐21 cells in a dose‐dependent manner. JTE‐522 significantly suppressed orthotopic tumor growth and lymph node metastasis, and also decreased HGF expression by fibroblasts within the gastric tumor. In conclusion, we found that gastric fibroblasts stimulated invasiveness in scirrhous gastric cancer cells, whereas a selective COX‐2 inhibitor inhibited this paracrine effect by decreasing fibroblast PGE2 production, resulting in downregulation of HGF production. (Cancer Sci 2005; 96: 451–455)
Abbreviations:
- COX‐2
cyclooxygenase‐2
- DMEM
Dulbecco's modified Eagle medium
- ELISA
enzyme linked immunosorbent assay
- FCS
fetal calf serum
- HGF
hepatocyte growth factor
- PBS
phosphate‐buffered saline
- PG
prostaglandin
- RT–PCR
reverse transcription–polymerase chain reaction
- TGF
transforming growth factor.
Human scirrhous gastric carcinoma (also termed diffusely infiltrating carcinoma or linitis plastica carcinoma) is characterized by extensive carcinoma cell infiltration and proliferation accompanied with abundant stromal fibrosis.( 1 ) In recent years the prognosis of patients with gastric carcinoma has generally improved, but that of patients with scirrhous gastric carcinoma remains poor.( 2 , 3 ) One reason for the continued poor prognosis of this type of cancer is rapid infiltration of cancer cells.( 4 ) When scirrhous gastric cancer cells invade deep to the mucosa of the stomach, they infiltrate diffusely through the gastric wall in association with fibroblast proliferation, frequently disseminating within the abdominal cavity.( 5 , 6 ) We previously reported that hepatocyte growth factor (HGF) and transforming growth factor (TGF)‐β1 produced by fibroblasts enhanced the invasiveness of scirrhous gastric cancer cells.( 7 , 8 )
Recent studies have indicated that non‐steroidal anti‐inflammatory drugs (NSAIDs) contributed to the decrease of incidence of colon cancer,( 9 , 10 , 11 , 12 , 13 ) whereas sulindac reduced the size and number of adenomas in patients with familial adenomatous polyposis (FAP).( 14 ) Cyclooxygenase (COX), a molecular target of NSAIDs, has two isoforms, COX‐1 and COX‐2. Whereas COX‐1 is expressed constitutively at a constant rate, COX‐2 expression is regulated by various factors. In particular, COX‐2 is expressed by stromal cells in various tumors, and is regulated by cytokines. Overexpression of COX‐2 has been linked to the development of various types of human cancers.( 15 , 16 , 17 , 18 ) Several reports have indicated that COX‐2 affects the invasiveness of cancer cells.( 19 , 20 , 21 , 22 , 23 , 24 ) However, the mechanisms responsible for the antitumor effect of COX‐2 inhibitors remain unclear. In colon polyps COX‐2 is overexpressed in stromal cells such as macrophages and fibroblasts, but not in adenomatous cells. COX‐2 produced by stromal cells brings about proliferation and invasion of human carcinoma by stimulating the stromal production of various cytokines.( 25 )
The oral chemotherapeutic agent S‐1 has been clinically available for patients with gastric cancer in Japan.( 26 ) S‐1 is a novel 5‐fluorouracil (FU) derivative that provides high clinical response rates without severe adverse effects. It has been reported that the serum concentration of 5‐FU when S‐1 is administered is high, and is equal to that achieved by continuous 5‐FU infusion. In the present study we investigated the inhibitory effect of a selective COX‐2 inhibitor, JTE‐522,( 27 ) on the interactions between scirrhous gastric cancer cells and gastric fibroblasts, which induce scirrhous gastric cancer cells to proliferate and invade. Furthermore, we investigated the synergistic effect of COX‐2 inhibitor and S‐1 on scirrhous gastric cancer.
Materials and Methods
COX‐2 inhibitor. JTE‐522, a selective COX‐2 inhibitor, was provided by Japan Tobacco (Tokyo, Japan). For in vitro studies, JTE‐522 first was dissolved in ethanol, and then diluted with conditioned medium to various concentrations. For in vivo studies, JTE‐522 was suspended in 0.5% carboxymethyl cellulose (CMC; Wako, Osaka, Japan) sodium salt for oral administration.
Cell culture and cell lines. The culture medium was composed of DMEM with 2% heat‐inactivated FCS (Gibco, Grand Island, NY, USA), 100 IU/mL penicillin (ICN Biomedicals, Costa Mesa, CA, USA), 100 µg/mL streptomycin (ICN Biomedicals), 2 mM glutamine (Bioproducts, Waikersville, MD, USA) and 0.5 mM of sodium pyruvate (Bioproducts). The human gastric cancer cell lines OCUM‐2D and OCUM‐2M, as well as NF‐21 human gastric fibroblasts were obtained from a scirrhous gastric carcinoma. The fibroblast monocultures of NF‐21 cells were confirmed by staining with vimentin and cytokeratin. These were seeded in a 100‐mm dish (Falcon, Lincoln Park, NJ, USA) and cultured in 10 mL of medium at 37°C in a humidified atmosphere of 5% CO2 in air.
RT–PCR. Total cellular RNA was extracted from OCUM‐2D, OCUM‐2M, and NF‐21 cells using Trizol (Gibco) according to the manufacturer's protocol. The various cDNA were synthesized with a Maloney mouse leukemia virus (M‐MLV)‐RT kit (Gibco) using random hexamers. Relevant cDNA were amplified by PCR with Taq DNA polymerase (Nippon Gene, Tokyo, Japan) in a thermal cycler, with 30 cycles used for each of the three repeated steps. Primers used were: COX‐2, sense primer 5′‐CCGAGGTGTATGTATGAG‐3′, antisense primer 5′‐ATCAGGCACAGGAGGAAG‐3′, and as an internal control, glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), sense 5′‐ACCTGACCTGCCGTCTAGAA‐3′, antisense 5′‐TCCACCACCCTGTTGCTGTA‐3′. PCR conditions were as follows: predenaturation, 94°C for 5 min; denaturation, 94°C for 30 s; annealing, 60°C for 30 s; extension, 72°C for 1 min; and final incubation, 72°C for 1 min. PCR products were applied to 2% agarose gel and electrophoresed. COX‐2 PCR products were 336 bp in size.
Preparation of serum‐free conditioned medium. Serum‐free conditioned medium (SF‐CM) from fibroblasts was prepared as follows. Gastric fibroblasts (NF‐21; 5 × 104 cells/mL) were seeded into 100‐mm plastic dishes with 10 mL of DMEM containing 2% FCS and incubated for 3 days, after which time the number of fibroblasts in each dish was approximately 2.5 × 106. To obtain the SF‐CM, fibroblasts were washed twice with Dulbecco's phosphate‐buffered saline (PBS). They were then incubated for 3 days with 3 mL of DMEM containing various concentrations of JTE‐522 (0, 10−7, 10−6 or 10−5 M). JTE‐522 had no influence on the proliferation of NF‐21 cells. Each conditioned medium was collected and centrifuged at 1000g for 5 min. The supernatant was stored as SF‐CM at −20°C until use. As a negative control, DMEM was used in place of SF‐CM.
Invasion assay. Invasiveness was measured in vitro by the methods of Albini et al.( 28 ) with some modifications. We used Chemotaxicell chambers (Millipore, Bedford, MA, USA) with a 12‐µm filter; the upper surface of each filter was coated with 5 µg of Matrigel (reconstituted basement membrane; Collaborative Research, Lexington, MA, USA) in cold DMEM to form a matrix barrier. The chamber (upper compartment) was placed in a 24‐well culture plate (lower compartment). OCUM‐2D cells with or without SF‐CM from NF‐21 were resuspended at a final concentration of 105 cells/mL in DMEM with 7.5% FCS. Then 100 µL of gastric cancer cell suspension and 900 µL of DMEM with 10% FCS were added to the upper and lower compartments, respectively, and the plate was incubated for 72 h at 37°C. After incubation, cancer cells on the upper surface of the membrane were removed by wiping with cotton swabs. The filter was fixed with methanol and stained with hematoxylin. Cancer cells that invaded through the Matrigel‐coated filter to reach the lower surface of the membrane were counted manually under a microscope at ×200 magnification.
ELISA. Hepatocyte growth factor, TGF‐β1, and matrix metalloprotease (MMP)‐2 in SF‐CM from NF‐21 cells treated with JTE‐522 at a concentration of 0, 10−5, 10−6 or 10−7 M each were quantified using an ELISA kit (Genzyme, Cambridge, MA, USA). PGE‐2 in SF‐CM from NF‐21 cells was quantified using another ELISA kit (Amersham, Aylesbury, UK).
Tumor growth studies in vivo. Female BALB/c nude mice (Nihon CLEA, Tokyo, Japan) were used for the in vivo studies. The mice were housed under conditions free of specific pathogens and were provided with standard chow pellets and water ad libitum. Experiments were performed according to the standard guidelines for animal experiments of the Osaka City University Medical School. We examined the effect of JTE‐522 on orthotopically transplanted tumors as follows. On day 0, OCUM‐2M cells (107 cells in 100 µL of DMEM per mouse) were injected at the right flank into the serosa of the stomach. Medication was administered orally five times weekly for 4 weeks beginning the week after inoculation. Medication‐defined groups were CMC (control group), JTE‐522 (30 mg/kg per day), S‐1 (10 mg/kg per day; Taiho Pharmaceutical Co., Tokyo, Japan), and JTE‐522 (30 mg/kg per day) plus S‐1 (10 mg/kg per day). S‐1 consists of tegafur (a prodrug of 5‐FU) and two modulators, 5‐chloro‐2,4‐dihydroxypyridine and potassium oxonate, at a molar ratio of 1 : 0.4 : 1.( 26 ) All mice were killed on day 35, when tumor size and total weight of lymph nodes with metastases were determined.
Histopathological and immunohistochemical examinations. To clarify the histopathological findings and quantify HGF expression in nude mice, we examined sections after hematoxylin–eosin (HE) staining, and after anti‐HGF immunohistochemical processing. After mice were killed, gastric tumors and lymph nodes containing metastases were removed. These specimens were washed in PBS and fixed in 10% formalin for paraffin sectioning. Sections were stained with HE or stained immunohistochemically using an antibody against mouse HGFα (American Research Products, Belmont, MA, USA) and then examined under a light microscope. Two investigators independently determined the degree of HGFα antibody reactivity.
Statistical analysis. Significant differences between control results and results with JTE‐522 treatment were assessed using the t‐test. A value of P less than 0.05 was considered to indicate statistical significance.
Results
Expression of COX‐2. We recognized a 336‐bp band representing COX‐2 mRNA in both scirrhous gastric cancer cell lines (OCUM‐2D and OCUM‐2M), and in gastric fibroblasts (NF‐21; Fig. 1).
Figure 1.
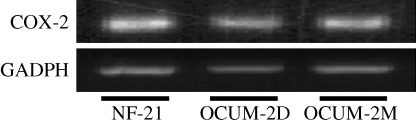
COX‐2 mRNA expression. NF‐21 cells, OCUM‐2D cells and OCUM‐2M cells showed a COX‐2 mRNA positive band. The product was 336 bp in length.
Invasion‐stimulating effect of fibroblast‐conditioned medium on cancer cells and inhibition by JTE‐522. The number of invading OCUM‐2D cells without SF‐CM was 130 ± 21 cells per field, whereas in cells with SF‐CM from NF‐21, this was 235 ± 32 cells per field, representing a significant increase of invasiveness (P = 0.0016). The invasion‐stimulating ability of SF‐CM from fibroblasts was significantly decreased by JTE‐522 concentrations of 5 × 10−7 M or above (Fig. 2). In contrast, JTE‐522 at 5 × 10−7 M did not affect the invasion ability of OCUM‐2D cells or the proliferation of OCUM‐2D cells and NF‐21 cells
Figure 2.
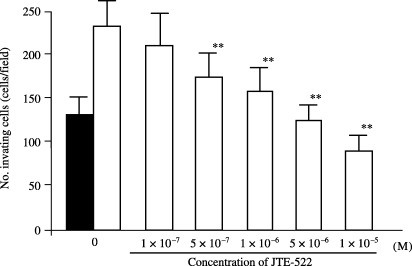
Effect of JTE‐522 on the invasion‐stimulating activity of serum‐free conditioned medium (SF‐CM) from fibroblasts. The number of invading cancer cells was significantly increased by the SF‐CM from NF‐21 cells (□) compared with OCUM‐2D alone (▪). The number of the invading OCUM‐2D cells with SF‐CM from NF‐21 was significantly suppressed by JTE‐522 at a concentration of 1 × 10−6 M. Data are presented as the mean and standard deviation (error bars) of four independent experiments. **P < 0.01.
Inhibitory effect of JTE‐522 on production of cytokines by NF‐21. The HGF concentration was 2.71 ng/mL in SF‐CM from NF‐21 without JTE‐522. At JTE‐522 concentrations of 10−7, 10−6 and 10−5 M, production of HGF was 2.3, 1.8 and 1.5 ng/mL. Thus, HGF production by fibroblasts was suppressed significantly by JTE‐522 at or above 10−6 M (Fig. 3a). Production of MMP‐2 and TGF‐β1 was not affected by the drug (data not shown). However, production of PGE2 detected in SF‐CM from NF‐21 cells was suppressed by JTE‐522e, re at 10−6 M (Fig. 3b). Finally, HGF productionetected in SF‐CM from NF‐21 cells was increased by exogenous PGE2 in a dose‐dependent manner (Fig. 4).
Figure 3.
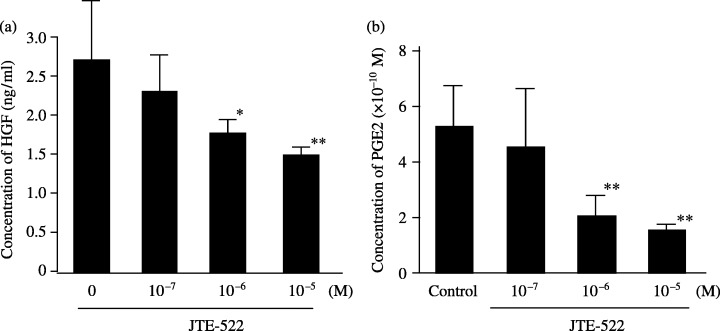
Effect of JTE‐522 on the production of HGF and PGE2 from gastric fibroblasts. (a) JTE‐522 significantly decreased HGF production from fibroblasts at a concentration of 10−6 M, compared with the control. (b) PGE2 production from fibroblasts was inhibited at a concentration of 10−6 M. Data are presented as the mean and standard deviation (error bars) of four independent experiments. *P < 0.05, **P < 0.01.
Figure 4.
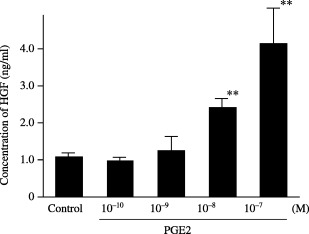
Effect of PGE2 on the production of HGF from gastric fibroblasts. HGF production from gastric fibroblasts was increased by PGE2 in a dose‐dependent manner. Data are presented as the mean and standard deviation (error bars) of four independent experiments. **P < 0.01.
Effect of JTE‐522 on tumor development. The mean areas of orthotopically transplanted tumors in control, JTE‐522, S‐1 and JTE‐522 plus S‐1 micspectively, were 56, 15, 17 and 11 mm2. Thus tumors in mice receiving JTE‐522 and/or S‐1 were significantly smaller than those in controls (Table 1). The mean weights of metastatic lymph nodes were 106, 51, 35 and 13 mg in control, JTE‐522, S‐1, and JTE‐522 plus S‐1 mice. Although JTE‐522 or S‐1 decreased the weight of metastatic lymph nodes (Table 1), a synergistic effect of JTE‐522 and S‐1 together against lymph node metastasis was evident. Toxicity or body weight loss were not observed in any group.
Table 1.
Antitumor effect of JTE‐522 and S‐1 on gastric primary tumor and lymph node metastasis
| Tumor size (mm2) | Total weight of metastatic lymph nodes (mg) | |
|---|---|---|
| Control (n = 6) | 56.0 ± 19.9a,b,c | 105.8 ± 37.7d,e,f |
| JTE‐522 (n = 5) | 15.0 ± 12.5a,h | 51.0 ± 27.0d,i |
| S‐1 (n = 6) | 17.2 ± 8.3b,g | 34.8 ± 16.5e,j |
| JTE‐522 and S‐1 (n = 6) | 10.7 ± 9.4c,g,h | 12.7 ± 15.0f,i,j |
Data are represented as mean ± SD. Superscript letters indicate significance between values with the same letter: a–f, P < 0.01; g–j, not significant.
Inhibitory effect of dJTE‐522 on expression of HGF protein by fibroblasts in nude mice. JTE‐522 treatment decreased HGF expression by fibroblasts in the stroma of orthotopic tumors (Fig. 5). In contrast, no marked difference between JTE‐522 treatment and non‐treatment was noted in the histological examinations of the HE‐stained orthotopic tumors.
Figure 5.
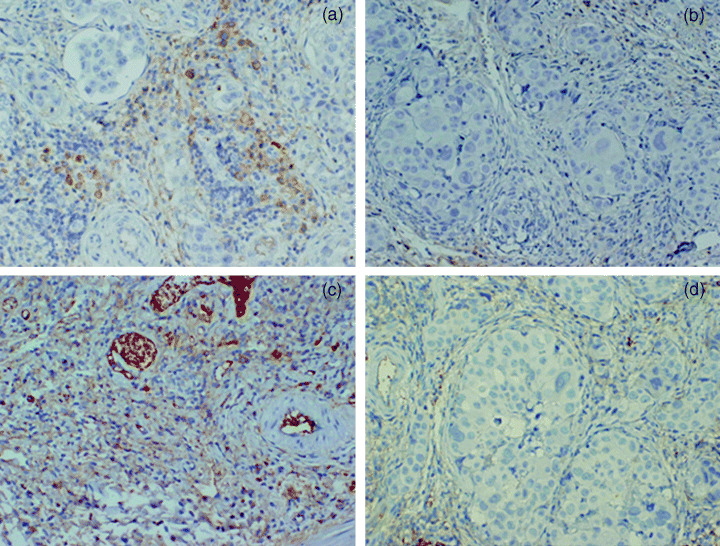
HGF expression of tumors in nude mice. (a) Carboxymethyl cellulose sodium salt (CMC) control group; (b) JTE‐522; (c) S‐1; (d) JTE‐522 and S‐1. Compared with the CMC control groups, HGF expression in stromal cells was suppressed by JTE‐522. Administration of JTE‐522 and S‐1 also suppressed the expression of HGF in stromal cells, but administration of S‐1 did not.
Discussion
The invasiveness of gastric cancer cells was promoted significantly by conditioned medium from gastric fibroblasts with COX‐2 expression. This invasion‐stimulating activity by gastric fibroblasts was decreased significantly by a COX‐2 inhibitor, JTE‐522, whereas JTE‐522 did not affect the invasion ability of OCUM‐2D cells. These findings suggest that the COX‐2 inhibitor suppressed the invasion‐enhancing effect of gastric fibroblasts on scirrhous gastric cancer cells. The invasiveness of scirrhous gastric cancer has been reported to be affected by HGF, TGF‐β1, and MMP‐2 originating from gastric fibroblasts.( 7 ) In the present study, JTE‐522 inhibited HGF production by fibroblasts, but not the production of TGF‐β1 or MMP‐2. Fibroblast HGF production was reported to be reduced by COX‐2 inhibitor actions at the EP2 receptor,( 29 ) which contributes to the invasiveness of cancer cells.( 21 , 22 ) In the present study, HGF production by fibroblasts was increased by PGE2, and PGE2 production was inhibited by a COX‐2 inhibitor. Gastric tumor size was suppressed significantly by oral administration of a COX‐2 inhibitor. We previously reported that keratinocyte growth factor (KGF) production from fibroblasts affects the growth of scirrhous gastric carcinoma.( 6 ) COX‐2 inhibitor may suppress the paracrine effect of fibroblasts. Lymph node metastasis following orthotopic inoculation of tumor cells was decreased significantly by oral administration of a COX‐2 inhibitor. Immunohistochemical findings from the orthotopic tumors indicated a reduction of HGF expression by gastric fibroblasts within the tumor in response to the COX‐2 inhibitor. We previously reported that OCUM‐2D cells express the c‐Met receptor. These findings suggest that the COX‐2 inhibitor decreased HGF production from gastric fibroblasts by suppressing PGE2 productions, resulting in decreased invasive ability of gastric cancer cells.
Administration of JTE‐522 and S‐1 suppressed orthotopic tumor growth, and their combined administration decreased lymph node metastasis more effectively than either alone. These findings suggest that administering S‐1 and a COX‐2 inhibitor in combination can obtain a synergistic effect. This technique might prove useful in treating scirrhous gastric cancer.
Acknowledgments
This study was supported in part by Grants‐in‐Aid for Scientific Research (C) 13671329 and (B) 13470260 from the Ministry of Education, Science, Sports, Culture and Technology of Japan, and by a Grant‐in‐Aid from the Osaka City University Medical Research Foundation.
References
- 1. Tahara E. Growth factors and oncogenes in human gastrointestinal carcinomas. J Cancer Res Clin Oncol 1990; 116: 121–31. [DOI] [PubMed] [Google Scholar]
- 2. Akama Y, Yasui W, Kuniyasu H et al. Genetic status and expression of the cyclin‐dependent kinase inhibitors in human gastric carcinoma cell lines. Jpn J Cancer Res 1996; 87: 824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yasui W, Akama Y, Yokozaki H et al. Expression of p21WAF1/CIP1 in colorectal adenomas and adenocarcinomas and its correlation with p53 protein expression. Pathol Int 1997; 47: 470–7. [DOI] [PubMed] [Google Scholar]
- 4. Sowa M, Kato Y, Nishimura M, Yoshino H, Kubo T, Umeyama K. Clinico‐histochemical studies on type 4 carcinoma of the stomach: with special reference to mucopolysaccharides and sialic acid in tumor tissue. Jpn J Surg 1989; 19: 153–62. [DOI] [PubMed] [Google Scholar]
- 5. Yashiro M, Chung YS, Kubo T, Hato F, Sowa M. Differential responses of scirrhous and well‐differentiated gastric cancer cells to orthotopic fibroblasts. Br J Cancer 1996; 74: 1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakazawa K, Yashiro M, Hirakawa K. Keratinocyte growth factor produced by gastric fibroblasts specifically stimulates proliferation of cancer cells from scirrhous gastric carcinoma. Cancer Res 2003; 63: 8848–52. [PubMed] [Google Scholar]
- 7. Inoue T, Chung YS, Yashiro M et al. Transforming growth factor‐beta and hepatocyte growth factor produced by gastric fibroblasts stimulate the invasiveness of scirrhous gastric cancer cells. Jpn J Cancer Res 1997; 88: 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yashiro M, Chung YS, Inoue T et al. Hepatocyte growth factor (HGF) produced by peritoneal fibroblasts may affect mesothelial cell morphology and promote peritoneal dissemination. Int J Cancer 1996; 67: 289–93. [DOI] [PubMed] [Google Scholar]
- 9. Thun MJ, Namboodiri MM, Heath CW Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med 1991; 325: 1593–6. [DOI] [PubMed] [Google Scholar]
- 10. Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW Jr. Aspirin use and risk of fatal cancer. Cancer Res 1993; 53: 1322–7. [PubMed] [Google Scholar]
- 11. Giovannucci E, Egan KM, Hunter DJ et al. Aspirin and the risk of colorectal cancer in women. N Engl J Med 1995; 333: 609–14. [DOI] [PubMed] [Google Scholar]
- 12. Giardiello FM, Hamilton SR, Krush AJ et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328: 1313–6. [DOI] [PubMed] [Google Scholar]
- 13. Williams CS, Smalley W, DuBois RN. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest 1997; 100: 1325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz‐Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long‐term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology 2002; 122: 641–5. [DOI] [PubMed] [Google Scholar]
- 15. Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase‐2 modulates carcinoma growth. J Clin Invest 2000; 105: 1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kundu N, Fulton AM. Selective cyclooxygenase (COX)‐1 or COX‐2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res 2002; 62: 2343–6. [PubMed] [Google Scholar]
- 17. Kamate C, Baloul S, Grootenboer S et al. Inflammation and cancer, the mastocytoma P815 tumor model revisited: triggering of macrophage activation in vivo with pro‐tumorigenic consequences. Int J Cancer 2002; 100: 571–9. [DOI] [PubMed] [Google Scholar]
- 18. Sawaoka H, Kawano S, Tsuji S et al. Cyclooxygenase‐2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol 1998; 274: G1061–7. [DOI] [PubMed] [Google Scholar]
- 19. Chen WS, Wei SJ, Liu JM, Hsiao M, Kou‐Lin J, Yang WK. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase‐2 (COX‐2) expression and inhibited by a COX‐2‐selective inhibitor, etodolac. Int J Cancer 2001; 91: 894–9. [DOI] [PubMed] [Google Scholar]
- 20. Tsujii M, Kawano S, DuBois RN. Cyclooxygenase‐2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA 1997; 94: 3336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dohadwala M, Luo J, Zhu L et al. Non‐small cell lung cancer cyclooxygenase‐2‐dependent invasion is mediated by CD44. J Biol Chem 2001; 276: 20 809–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dohadwala M, Batra RK, Luo J et al. Autocrine/paracrine prostaglandin E2 production by non‐small cell lung cancer cells regulates matrix metalloproteinase‐2 and CD44 in cyclooxygenase‐2‐dependent invasion. J Biol Chem 2002; 277: 50 828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998; 93: 705–16. [DOI] [PubMed] [Google Scholar]
- 24. Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase‐1 in tumorigenesis. J Biol Chem 2003; 278: 19 396–405. [DOI] [PubMed] [Google Scholar]
- 25. Oshima M, Dinchuk JE, Kargman SL et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX‐2). Cell 1996; 87: 803–9. [DOI] [PubMed] [Google Scholar]
- 26. Nakata B, Mitachi Y, Tsuji A et al. Combination phase I trial of a novel oral fluorouracil derivative S‐1 with low‐dose cisplatin for unresectable and recurrent gastric cancer (JFMC27‐9902). Clin Cancer Res 2004; 10: 1664–9. [DOI] [PubMed] [Google Scholar]
- 27. Wakitani K, Nanayama T, Masaki M, Matsushita M. Profile of JTE‐522 as a human cyclooxygenase‐2 inhibitor. Jpn J Pharmacol 1998; 78: 365–71. [DOI] [PubMed] [Google Scholar]
- 28. Albini A, Iwamoto Y, Kleinman HK et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987; 47: 3239–45. [PubMed] [Google Scholar]
- 29. Takahashi M, Ota S, Hata Y et al. Hepatocyte growth factor as a key to modulate anti‐ulcer action of prostaglandins in stomach. J Clin Invest 1996; 98: 2604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


