Abstract
The development of effective immunoadjuvants for tumor immunotherapy is of fundamental importance. The use of Mycobacterium bovis bacillus Calmette‐Guérin cell wall skeleton (BCG‐CWS) in tumor immunotherapy has been examined in various clinical applications. Because BCG‐CWS is a macromolecule that cannot be chemically synthesized, the development of an alternative synthetic molecule is necessary to ensure a constant supply of adjuvant. In the present study, a new adjuvant was designed based on the structure of macrophage‐activating lipopeptide (MALP)‐2, which is a Toll‐like receptor (TLR)‐2 ligand similar to BCG‐CWS. Macrophage‐activating lipopeptide‐2, [S‐(2,3‐bispalmitoyloxypropyl)Cys (P2C) – GNNDESNISFKEK], originally identified in a Mycoplasma species, is a lipopeptide that can be chemically synthesized. A MALP‐2 peptide was substituted with a functional motif, RGDS, creating a novel molecule named P2C‐RGDS. RGDS was selected because its sequence constitutes an integrin‐binding motif and various integrins are expressed in immune cells including dendritic cells (DCs). Thus, this motif adds functionality to the ligand. P2C‐RGDS activated DCs and splenocytes more efficiently than MALP‐2 over short incubation times in vitro, and the RGDS motif contributed to their activation. Furthermore, P2C‐RGDS showed higher activity than MALP‐2 in inducing migration of DCs to draining lymph node, and in inhibiting tumor growth in vivo. This process of designing and developing synthetic adjuvants has been named “adjuvant engineering,” and the evaluation and improvement of P2C‐RGDS constitutes a first step in the development of stronger synthetic adjuvants in the future. (Cancer Sci 2010)
Bacterial adjuvants that were used as biological response modifiers (BRM) for cancer immunotherapy in the 1970s have recently been re‐evaluated.( 1 , 2 , 3 ) Cancer antigens that had been identified in many laboratories were tested as peptide vaccines for clinical applications, but the peptides alone were not sufficient to fully activate the immune system.( 4 ) These results suggested that the activation of the innate immune system, including dendritic cells (DCs), by a supporting adjuvant was important.( 5 , 6 , 7 , 8 ) In peptide vaccine therapy, the T cells of the acquired immune system play an important role in recognizing and attacking tumor cells.( 9 ) Dendritic cells play a key role in the regulation of the acquired immune system by presenting antigen and inducing a primary immune response. The identification of the Toll‐like receptor (TLR) family advanced the understanding of DC function and of the role of adjuvants, because almost all microbial adjuvants work as TLR ligands and activate DCs.( 10 , 11 , 12 ) These findings provide the basis for the understanding of the mechanism of adjuvant therapy.
Dr Azuma developed BCG‐CWS, a cell‐wall skeleton preparation of Mycobacterium bovis bacillus Calmette‐Guérin,( 13 , 14 ) as an antitumor immunotherapeutic adjuvant. Although many BRM studies have been discontinued, basic and clinical research on BCG‐CWS has continued at Osaka Medical Center for Cancer. We reported that BCG‐CWS is a ligand of TLR2/4( 15 , 16 , 17 ) and acts as an effective adjuvant to induce CTLs in irradiated tumors in a mouse experimental model. These activities are mediated by the myeloid differentiation protein 88 (MyD88),( 18 ) which is a TLR adaptor molecule. The effectiveness of BCG‐CWS in improving the prognosis for cancer patients after surgery was confirmed through clinical research.( 19 )
Interleukin (IL)‐23 and interferon (IFN)‐γ are the main cytokines induced by BCG‐CWS in vivo ( 14 , 19 , 20 ) and are important for antitumor immunity.( 21 , 22 ) Interleukin‐12 is well known as an antitumor cytokine,( 23 ) and IL‐23 shares the IL12p40 subunit with IL‐12.( 22 ) Unexpectedly, IL‐23 advanced tumor growth in experiments with IL‐23R−/− mice or neutralizing antibodies by interacting with Th17 cells.( 24 , 25 , 26 ) However, systemic administration of IL‐23 was also reported to have antitumor effects similar to those of IL‐12,( 27 ) and TLR2 ligands exhibit antitumor activity( 28 , 29 , 30 ) that may be mediated by the induction of IL‐23.
Although BCG‐CWS is effective as an adjuvant, its clinical use is limited in purity, stability, and a stable supply because it cannot be chemically synthesized and is therefore prepared from bacterial cells. These factors indicate that there is a need to develop new synthetic adjuvants as effective as BCG‐CWS. The present report describes the design of such adjuvants based on the structure of the TLR2 ligand and in consideration of the need for IL‐23 induction. Macrophage‐activating lipopeptide (MALP)‐2, a lipopeptide of mycoplasmic origin, is a TLR2 ligand that can be chemically synthesized. No functional consensus peptide sequences were identified in MALP‐2. The N‐terminal cysteine of the 13‐amino‐acid peptide of bacterial origin was modified with 2 palmitates [Pam2Cys or P2C, S‐(2,3‐bispalmitoyloxypropyl)‐cysteine],( 31 ) but P2C alone does not work as a TLR2 ligand.( 32 ) Bacterial and synthetic TLR2 ligands (MALP‐2, FSL‐1,( 32 ) P2C‐SKKKK( 33 )) contain mostly hydrophilic peptides, and the presence of solubilizers critically affects their TLR2 agonistic ability,( 34 ) suggesting that the activity of compounds as TLR2 agonists correlates with their solubility.
CD11c is a member of the integrin superfamily and is known to be a marker of DCs.( 35 ) Dendritic cells also express other integrin molecules such as αV/β3 and α5/β1, and the RGDS motif specifically binds to these integrins.( 36 , 37 ) Virus particles expressing proteins containing the RGD motif efficiently infect DCs.( 37 ) Therefore, a new TLR2 ligand was developed by replacing the peptide of bacterial origin with a hydrophilic functional motif (adjuvant engineering). P2C and the RGDS peptide were linked to increase the efficiency of ligand adherence to DCs or other immune cells, and the effect of the new adjuvant on antitumor activities in vitro and in vivo was examined.
Materials and Methods
Mice, cells, and reagents. Toll/IL‐1 receptor homology‐containing adaptor molecule (TICAM)‐1−/− mice were generated in our laboratory.( 2 ) Toll‐like receptor (TLR)‐2−/− and MyD88−/− mice were provided by Shizuo Akira (Osaka University).( 38 ) The mice were maintained under specific pathogen‐free conditions in the animal facility of the Osaka Medical Center. They were backcrossed with C57BL/6 mice >8 times before use. Wild‐type (WT) C57BL/6 mice were purchased from Japan Clea (Tokyo, Japan). All animal experiments were approved by the committee at Osaka Medical Center for Cancer. EG7 cells are ovalbumin‐transfected EL4 and were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA).( 39 ) B16D8 was established in our laboratory as a subline of the B16 melanoma cell line.( 18 ) Cell lysates were prepared by the freeze‐thaw method.
Preparation of mouse bone marrow‐derived DCs (BMDCs), splenocytes, and lymph node cells. Bone marrow‐derived DCs were prepared as previously described( 35 , 40 ) with minor modifications, and were cultured in RPMI‐1640 (Invitrogen, Carlsbad, CA, USA) containing 10 ng/mL mouse granulocyte‐macrophage colony‐stimulating factor (PeproTech EC, London, UK), 50 μM 2‐mercaptoethanol (Invitrogen), 10 mM HEPES, and 10% FCS (Bio Whittaker, Walkersville, MD, USA). The inguinal lymph node cells and splenocytes were prepared by using Lympholyte‐M (Cedarlane, Burlington, ON, Canada). CD11c‐positive and ‐negative cells, and CD90‐positive and ‐negative cells were separated from splenocytes by using CD11c or CD90 microbeads (Miltenyi Biotec, Auburn, CA, USA).
In vitro assay. For the simple stimulation assay in vitro, BMDCs or splenocytes were cultured with 10 μg/mL of BCG‐CWS,( 18 ) 100 nM of MALP‐2 and the designed lipopeptide (purity >90%; Biologica, Aichi, Japan) for 24 h (BMDCs, FACS), 48 h (BMDCs, ELISA), or 72 h (splenocytes, ELISA). For the inhibition assay, BMDCs or splenocytes were pre‐incubated with the indicated concentrations of RGDS peptide or anti‐CD29 antibody (eBioscience, San Diego, CA, USA) at 4°C for 30 min before stimulating them with TLR2 ligands at 4°C for 60 min. The cells were then washed and re‐cultured for 48 h (BMDCs, ELISA) or 72 h (splenocytes, ELISA). The Mixed Lymphocyte Reaction (MLR) assay was performed as previously described,( 40 ) and the results were analyzed as uptake of [3H]thymidine (1 μCi/well; Amersham Biosciences, Piscataway, NJ, USA). Bone marrow‐derived DCs stimulated with TLR2 ligands for 24 h (C57BL/6, 5 × 104 cells) were co‐cultured with CD90‐positive T cells (BALB/c, 105 cells) for 72 h. To exclude the possible effect of contaminating lipopolysaccharide, lipopeptides were pretreated with polymyxin B (Sigma‐Aldrich, St. Louis, MO, USA) at 37°C for 60 min.
Fluorescence‐activated cell sorter (FACS) analysis, intracellular cytokine staining, and ELISA. For FACS analysis, cells were suspended in PBS containing 0.1% sodium azide and 1% FCS, and then incubated for 30 min at 4°C with FITC‐conjugated antimouse CD80, antimouse CD86, antimouse CD8, or isotype control antibody; or with phycoerythrin‐conjugated antimouse CD4 or isotype control antibody (eBioscience). The cells were washed, and their fluorescence intensities were measured by FACS analysis. For intracellular cytokine staining, splenocytes were stimulated with P2C‐RGDS for 72 h and Brefeldin A (GolgiPlug; BD Biosciences, San Diego, CA, USA) for the last 6 h. Cells were stained with phycoerythrin‐conjugated antimouse CD3e, antimouse CD4, antimouse CD8a antibodies, allophycocyanin‐conjugated antimouse CD11c, or antimouse NK1.1 antibodies (eBioscience), followed by fixation and permeabilization with the Cytofix/Cytoperm plus Kit (BD Biosciences). Cells were stained intracellularly with FITC‐labeled anti‐IFN‐γ antibody (XMG1.2; eBioscience). For ELISA, samples were stored at –80°C and analyzed with ELISA kits for IFN‐γ, TNF‐α, and IL12p40 (Biosource, Camarillo, CA, USA).
In vivo therapy model. C57BL/6 mice were shaved on the back and injected subcutaneously with 200 μL of 1–2 × 106 syngeneic EG7 cells in PBS on Day 0. Thereafter, the treatment was performed three times, on Days 16, 20, and 23, and tumor volumes were measured using a caliper every 2–3 days. A volume of 50 μL of a mixture consisting of 10 nmol of lipopeptide and the cell lysate of 2 × 105 EG7 cells with or without 10 nmol of RGDS peptide was injected intradermally around the transplanted tumor. Tumor volume was calculated using the formula: Tumor volume (cm3) = (long diameter) × (short diameter) × (short diameter) × 0.4. Statistical analysis was performed with the Student’s t‐test.
Ex vivo assay. C57BL/6 mice were treated intradermally with a mixture of 10 nmol of lipopeptide and the cell lysate of 2 × 105 EG7 cells every 3 days for >4 treatments. At 24 h after the last treatment, the mice were sacrificed by etherization, and then the splenocytes and lymph node cells were prepared and cultured for 4 days to be primed by DCs and macrophages. The cytolytic activities of lymph node cells were then analyzed with a 51Cr release assay.( 18 ) The percentage of specific lysis was calculated using the formula: %Specific lysis = [(experimental release – spontaneous release)/(total release – spontaneous release)] × 100. The proportions of CD8‐ and CD11c‐positive cells in the lymph nodes or spleen were analyzed by FACS.
Results
To design a new TLR2 ligand with activity equivalent to that of BCG‐CWS, the minimum lipopeptide unit, P2C, was connected to the RGDS integrin‐binding motif to increase adherence to DCs, forming P2C‐RGDS (Fig. 1a). The hydrophobicity and pI of P2C‐RGDS were similar to those of MALP‐2, and the molecular weight of P2C‐RGDS was half that of MALP‐2 (Fig. 1b).
Figure 1.

The structure of the adjuvant developed in the present study, a synthetic Toll‐like receptor (TLR)‐2 ligand containing the RGDS motif. (a) The structure of macrophage‐activating lipopeptide (MALP)‐2. The N‐terminal cysteine of a peptide derived from a mycobacterium is modified with 2 palmitates [Pam2Cys, P2C, S‐(2,3‐bispalmitoyloxypropyl)cysteine]. RGDS was conjugated to P2C to form P2C‐RGDS because of its hydrophilicity and its additional function as an integrin‐binding motif for cell adhesion. (b) The structure, molecular weight, isoelectric point (pI), and hydrophobicity of synthetic (P2C‐RGDS, P2C‐SKKKK) and natural (MALP‐2, FSL‐1) lipopeptides used in this study. The pI and hydrophobicity of the peptide were calculated using ProtParam tools (http://br.expasy.org/tools/protparam.html). The pI and hydrophobicity of P2C‐RGDS were almost equivalent to those of MALP‐2. The molecular weight of P2C‐RGDS was about half that of MALP‐2. N, natural TLR2 ligand; S, synthetic TLR2 ligand. The asterisks (*) indicate acidic peptides. The gray boxes indicate hydrophobic amino acids.
First, the synthetic adjuvants were tested for their capacity to activate BMDCs in vitro when these compounds were added to the culture medium. P2C‐RGDS enhanced the expression of CD80 and CD86 in BMDCs at a level equal to that of MALP‐2, the positive control (Fig. 2a). P2C‐RGDS also enhanced the production of IL12p40 and TNF‐α (Fig. 2b) and the proliferation of allogeneic T cells co‐cultured with the BMDCs. Thus, P2C‐RGDS and MALP‐2 stimulate DCs equally in vitro, whereas P2C does not show the same activity.
Figure 2.
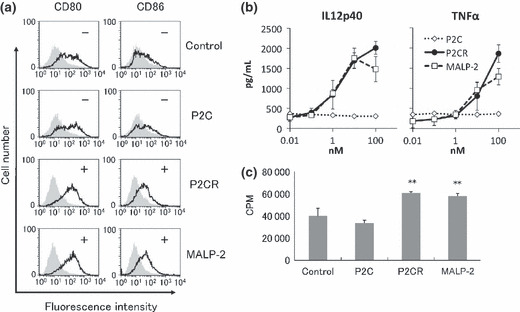
P2C‐RGDS activates bone marrow‐derived dendritic cells (BMDCs) as much as macrophage‐activating lipopeptide (MALP)‐2 in vitro. (a) The enhancement of CD80/CD86 expression of BMDCs stimulated with the indicated compounds (100 nM) for 24 h was observed by FACS analysis. (b) Interleukin (IL)12p40 and tumor necrosis factor (TNF)‐α production in BMDCs stimulated with each compound for 48 h was determined by ELISA. (c) The proliferation of allogeneic T cells co‐cultured with activated‐BMDCs for 72 h was measured by the [3H] thymidine uptake method. Bone marrow‐derived dendritic cells were treated with each compound for 24 h before co‐culture with T cells. CPM, count per minute. **P < 0.01 vs control (Student’s t‐test). P2CR, P2C‐RGDS.
Macrophage‐activating lipopeptide‐2 is a ligand of TLR2/6 that activates DCs through MyD88. To examine the TLR2 and TLR signaling pathway‐dependence of P2C‐RGDS, BMDCs were prepared from TLR2−/−, MyD88−/−, or TICAM‐1−/− mice and stimulated with these synthetic lipopeptides. Both MALP‐2 and P2C‐RGDS enhanced the expression of CD80 and CD86 in BMDCs derived from WT or TICAM‐1−/− mice, but not TLR2−/− or MyD88−/− mice (Fig. 3), suggesting that P2C‐RGDS is a TLR2 ligand with activity similar to that of MALP‐2 in vitro.
Figure 3.
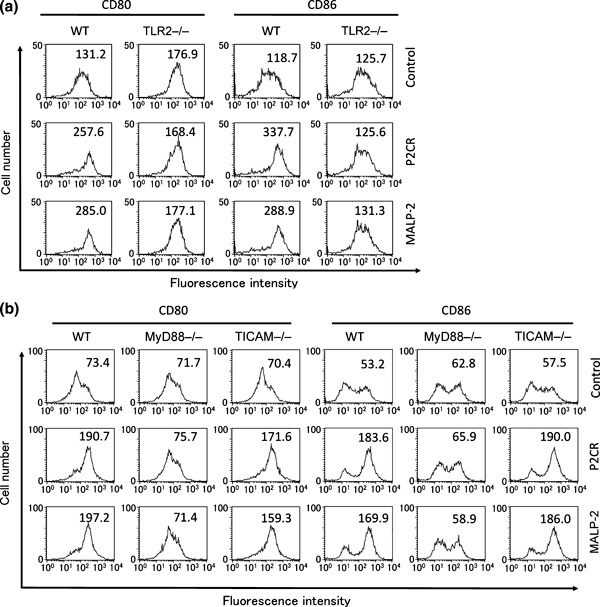
P2C‐RGDS activates bone marrow‐derived dendritic cells (BMDCs) in a Toll‐like receptor (TLR)‐2 and myeloid differentiation protein (MyD)‐88‐dependent manner in vitro. (a,b) Bone marrow‐derived dendritic cells were prepared from mice lacking TLR2 (TLR2−/−) and TLR adaptor molecules (MyD88−/− and TICAM‐1−/−). CD80 and CD86 expression was observed by FACS analysis at 24 h after BMDCs were stimulated with MALP‐2 or P2C‐RGDS. The numbers in the panels represent mean fluorescence intensities. P2C‐RGDS and MALP‐2 activated BMDCs via TLR2 and MyD88, but not via the Toll/IL‐1 receptor homology‐containing adaptor molecule (TICAM)‐1 pathway. P2CR, P2C‐RGDS.
Next, the functional dependence of P2C‐RGDS as a TLR2 ligand on not only its hydrophilicity, but also on the motif‐specificity of the peptide sequences, was tested. Since MALP2 and P2C‐RGDS activated DCs to the same extent at 37°C for 48 h, DCs were instead stimulated at 4°C for 1 h, then washed and re‐cultured at 37°C for 48 h. Under these conditions, P2C‐RGDS induced IL12p40 more efficiently than MALP‐2 (Fig. 4a). To analyze the specificity of the RGDS peptide, IL12p40 production was inhibited by the addition of an RGDS competitor peptide or anti‐integrin β1 antibody to the assay. The addition of the RGDS competitor peptide and the integrin blocking antibodies partially attenuated the production of IL12p40 induced by P2C‐RGDS, but not that induced by MALP‐2 (Fig. 4b,c). These results indicate that not only hydrophilicity but also the functional RGDS motif contributes to the activation of DCs in vitro at short incubation times.
Figure 4.
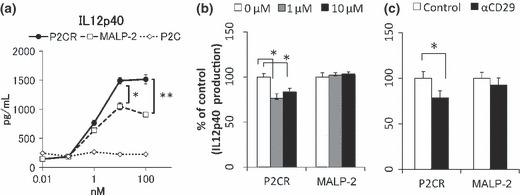
P2C‐RGDS efficiently activates bone marrow‐derived dendritic cells (BMDCs) in an RGDS motif–dependent manner in vitro over short incubation times. (a) Interleukin (IL)12p40 production of BMDCs stimulated with each Toll‐like receptor (TLR)‐2 ligand at 4°C for 1 h. Bone marrow‐derived dendritic cells were washed after the stimulation and re‐cultured for 48 h. Interleukin12p40 production was determined by ELISA. (b) Bone marrow‐derived dendritic cells were pretreated with the indicated concentrations of RGDS peptide (P2C‐modification free) as a competitor at 4°C for 30 min. Then, the BMDCs were stimulated with TLR2 ligands (10 nM) at 4°C for 1 h. Interleukin12p40 production by BMDCs was determined by ELISA. Data are shown as percentages of each control value. (c) The inhibition effects of α‐CD29 (integrin β1) antibody on IL12p40 production of BMDCs. Bone marrow‐derived dendritic cells were pre‐treated with 10 μg/mL of each antibody at 4°C for 30 min before stimulation. P2CR, P2C‐RGDS.
The P2C‐RGDS‐induced production of IFN‐γ was evaluated next, using in vitro whole splenocyte stimulation. The activities of P2C‐RGDS and MALP‐2, as measured by IFN‐γ production, were comparable and weaker than that of BCG‐CWS when splenocytes were simply stimulated with each compound for 72 h (Fig. 5a). However, when splenocytes were stimulated with each compound at 4°C for 1 h and re‐cultured at 37°C for 72 h, the P2C‐RGDS‐induced production of IFN‐γ was stronger than that induced by MALP‐2 and it was attenuated mostly by the RGDS peptide. Furthermore, the splenocytes stimulated with P2C‐RGDS produced as much IFN‐γ as those stimulated with BCG‐CWS at 4°C for 1 h (Fig. 5b). Interferon‐γ production was not detected in splenocytes depleted of CD11c‐positive dendritic cells, and IFN‐γ production could be restored under these conditions by adding back CD11c‐positive cells. These data indicate that IFN‐γ production by splenocytes following stimulation with P2C‐RGDS was mediated by DC activation (Fig. 5c). IFN‐γ production was also impaired by depletion of CD90 (Thy1)‐positive T cells (Fig. 5c). Further assessment of IFN‐γ producing cells by intracellular cytokine staining revealed that IFN‐γ was mainly detected in CD3‐ or CD8‐positive cells, and in CD4‐, NK1.1‐, or CD11c‐negative cells stimulated with P2C‐RGDS (Fig. 5d).
Figure 5.
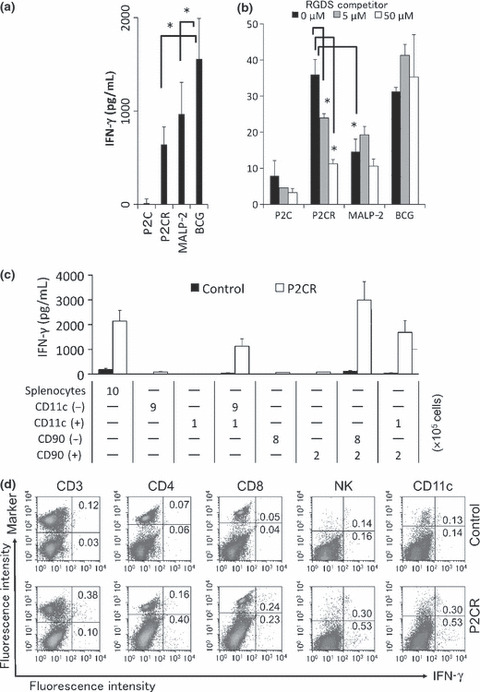
P2C‐RGDS efficiently activates splenocytes in an RGDS motif‐dependent manner. (a) Interferon (IFN)‐γ production by splenocytes stimulated with each compound for 72 h. (b) The effect of RGDS competitor peptide pretreatment on IFN‐γ production. The RGDS competitor attenuated IFN‐γ production from splenocytes stimulated with P2C‐RGDS at 4°C for 1 h. (c) The roles of CD11c‐positive cells (dendritic cells) and CD90‐positive cells (T cells) on IFN‐γ production by splenocytes. The splenocytes were separated into CD11c+ and CD11c− cells, or CD90+ and CD90− cells by using MACS beads. Cells were prepared based on the recovery ratio and stimulated with P2C‐RGDS for 72 h. *P < 0.05, **P < 0.01 (Student’s t‐test); n.d, not detected. (d) Intracellular IFN‐γ staining of splenocytes with various expression markers. Density plots show the expression of each surface marker and intracellular staining for IFN‐γ, and the numbers indicate the proportion of IFN‐γ‐positive cells (%). P2CR, P2C‐RGDS.
Finally, the antitumor activity of P2C‐RGDS in vivo was investigated using a tumor‐implantation model. The mice were transplanted with EG7 on day 0, and treated with synthetic lipopeptide (10 nmol) and the cell lysate of EG7 cells (2 × 105) on Days 16, 20, and 23. The minimum lipopeptide unit P2C showed no in vivo antitumor activity similar to the activation of DCs in vitro. Although MALP‐2‐treated mice showed a slightly smaller tumor volume than control mice, this difference was not significant. However, P2C‐RGDS showed a significant antitumor effect (Student’s t‐test, P < 0.05 vs control; Fig. 6a). Moreover, the mixture of P2C and the RGDS peptide showed no antitumor activity (Fig. 6b). Next, lymph node cells from mice immunized with EG7 lysate and P2C‐RGDS or MALP‐2 were isolated, and the cytotoxicity against EG7 and B16D8 cells was measured using a 51Cr release assay. P2C‐RGDS specifically induced a stronger cytotoxic activity against EG7 than MALP‐2, but the lymph node cells were not sufficiently cytotoxic against the negative target B16D8 cells (Fig. 6c). In addition, lymph node cells were cultured continuously with live EG7 cells for 96 h and the proportion of CD8‐positive cells was analyzed by FACS. CD8‐positive cells in lymph nodes derived from P2C‐RGDS‐treated mice remained at a level of approximately 16% of total cells, but most CD8‐positive cells from MALP‐2‐treated mice were lost after culture with EG7 (Fig. 6d). The proportion of CD8‐positive cells in a splenocyte population derived from immunized mice was also evaluated using FACS analysis. CD8‐positive cells were proportionally higher among splenocytes derived from mice treated with P2C‐RGDS than among splenocytes derived from mice treated with MALP‐2 (Fig. 6e). Necrosis was observed on the surface of tumors after P2C‐RGDS but not after MALP‐2 treatment, and CD8‐positive cells were detected around the necrosis tissue by immunostaining (data not shown). These data suggest that P2C‐RGDS induces and activates CTLs more efficiently than MALP‐2. Moreover, to analyze the mechanism underlying the strong antitumor effect of P2C‐RGDS in vivo, the proportions of CD11c‐positive cells in draining lymph nodes were analyzed in mice treated with MALP‐2 or P2C‐RGDS for 24 h. P2C‐RGDS induced the migration of CD11c‐positive cells to the draining lymph nodes more effectively than MALP2 (P = 0.055, Fig. 6f).
Figure 6.
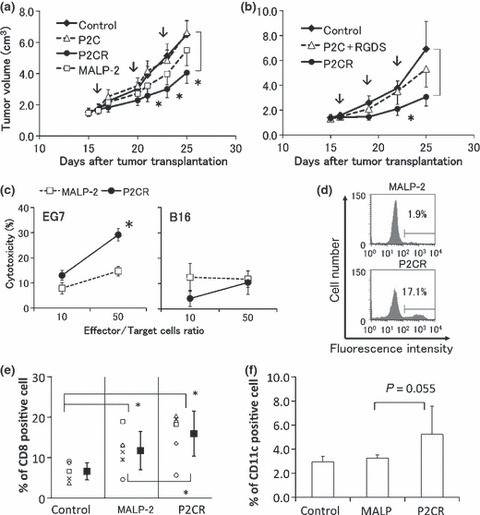
P2C‐RGDS is more effective at retarding tumor growth and inducing CD8‐positive cells than macrophage‐activating lipopeptide (MALP)‐2 in vivo and ex vivo. Antitumor effect of P2C‐RGDS and MALP‐2 (a), and of a mixture of P2C and RGDS peptide (b), in the EG7‐implanted mouse model (C57BL6‐EG7 model). Mice were treated with Toll‐like receptor (TLR)‐2 ligand and EG7 lysate on Days 16, 20, and 23 (arrows). The data are shown as means ± SE (n = 9). *P < 0.05 vs control (Student’s t‐test). (c) Lymph node cells derived from P2C‐RGDS and EG7 lysate‐immunized mice showed stronger cytotoxicity than cells from MALP‐2‐immunized mice against EG7 but not B16 (51Cr release assay). *P < 0.05 vs MALP‐2 (Student’s t‐test). (d) CD8‐positive T cells were more effectively induced in lymph nodes derived from mice immunized with P2C‐RGDS and EG7 lysate than in those immunized with MALP‐2. The lymph node cells in Figure 6(c) were cultured with live EG7 for 96 h, and then the proportion of CD8‐positive T cells was analyzed. (e) Immunization with P2C‐RGDS and EG7 lysate induced CD8‐positive T cells in splenocytes more efficiently than immunization with MALP‐2. The proportions of CD8‐positive cells in splenocytes were determined by FACS analysis at 96 h after the initiation of splenocyte cultivation. Each symbol indicates an individual experiment. The closed squares represent average ± SD. *P < 0.05 (paired t‐test). (f) The proportion of CD11c‐positive cells in the draining lymph nodes at 24 h after immunization. CD11c‐positive cells were examined by FACS analysis. P = 0.055 vs MALP‐2 (Student’s t‐test). P2CR, P2C‐RGDS.
Discussion
Natural TLR2 ligands of bacterial origin, such as MALP‐2( 31 , 41 , 42 ) and FSL‐1,( 32 ) have been reported to effectively activate CD8‐positive T cells and induce antitumor activity. Because these lipopeptides are hydrophilic, it was predicted that the hydrophilicity of the peptide might be important for its activity.( 31 , 32 , 33 , 34 ) Several researchers have relied on a chemobiologic strategy of producing amino acid replacements to explore which portion of the lipopeptide sequence possesses effective adjuvant activity.( 43 ) Using this strategy, Takeda developed Tan‐1511 analogues that show high levels of activity in the induction of granulopoiesis.( 43 ) Another strategy has been to randomly search for sequences or peptides effective at enhancing TLR2 ligand activity.( 44 ) The purpose of the current project was to design a TLR2 ligand with an additional function through the addition of a hydrophilic, functional peptide that could be developed as a new synthetic adjuvant (Fig. 1). In comparison to a prior developmental strategies, the present design has the advantage of allowing the selection of various functions, and because the designed lipopeptides do not exist in nature, they could show new or enhanced properties. In the present study, the TLR2 ligand was designed to possess stronger adhesive capacity through the linking of the RGDS peptide to P2C.
The compound P2C‐RGDS was developed and shown to be as effective as MALP‐2 in generating BMDC responses in vitro, such as the enhancement of a maturation marker (CD80 and CD86) and cytokine induction when DCs were cultured with each compound for 24–48 h (Fig. 2). P2C‐RGDS and MALP‐2 also activated DCs through the TLR2–MyD88 pathway (Fig. 3),( 38 ) but P2C‐RGDS activated DCs more efficiently than MALP2, and the RGDS integrin binding motif was found to be important for DCs activation over short incubation times (Fig. 4). Because DCs were treated with compounds for only 1 h, then washed and re‐cultured at 37°C for 48 h in these experiments, it was predicted that P2C‐RGDS might efficiently adhere to the DCs in short incubation times, and then stimulate DCs continuously at the surface or in the phagosome of DCs. Whole splenocytes stimulated with P2C‐RGDS also produced more IFN‐γ than MALP‐2 over short incubation times (Fig. 5b), and the production of IFN‐γ by splenocytes depended on CD11c‐positive DCs (Fig. 5c). These data suggest that the adhesion properties of P2C‐RGDS caused the efficient activation of DCs, and reflected splenocyte activation. The stronger IFN‐γ induction by P2C‐RGDS might also be due to its retention in the culture system by adherence to various cells among the splenocytes. Moreover, P2C‐RGDS may be retained in local regions for a long time via integrin binding in vivo, leading to the efficient activation of immune cells such as dermal DCs. P2C‐RGDS induced the migration of CD11c‐positive cells into the draining lymph nodes more effectively than MALP‐2 in in vivo experiments (Fig. 6f). These DCs might activate CD8‐positive cells, enhance cytotoxicity (Fig. 6c), and lead to retardation of tumor growth (Fig. 6a). These data suggest that the greater activation of DCs by P2C‐RGDS compared to MALP2 influences IFN‐γ production by splenocytes, thereby resulting in increased cytotoxicity and antitumor effects in vivo.
The induction of IFN‐γ production by BCG‐CWS treatment is one of the indexes for continuing treatment in clinical applications,( 14 , 19 ) and the response can be confirmed in mouse experiments. Interferon‐γ stimulation up‐regulates the expression of MHC class I in tumor cells,( 45 ) presumably improving tumor recognition by immune cells, and leading to increased suppression of tumor growth. With short stimulation periods, P2C‐RGDS induced as much IFN‐γ as BCG‐CWS. Although the present compound was designed without considering IFN‐γ induction, results show that CD8‐positive cells produced IFN‐γ in splenocytes stimulated with P2C‐RGDS alone in the absence of antigen peptide (Fig. 5). The mechanism of IFN‐γ induction by P2C‐RGDS should be analyzed in the future. The tumor volume of the BCG‐CWS treatment group was about 60% of that of the control on Day 22 (data not shown), and the therapeutic effects of P2C‐RGDS were almost equivalent to those of BCG‐CWS. Because BCG‐CWS must be emulsified with drakeol, the use of P2C‐RGDS has significant advantages.
The integrin binding sequence has served as the basis for the design of drugs that depend on adhesive activity. In the present work, this adhesive function was applied to TLR ligands to enhance immunoadjuvant activity. Cilengitide, a cyclic RGD peptide, was developed as an integrin αV antagonist, which impairs angiogenesis, tumor growth, and metastasis( 46 ) because integrins are expressed on various tumor cells.( 47 ) Although EG7 expresses integrin αV, β1, and β3, P2C‐RGDS and RGDS peptide did not show direct cytotoxic activity against EG7 cells at concentrations up to 100 nM (data not shown). Furthermore, the adjuvant activities of P2C‐RGDS were compared to those of a mixture of P2C and RGDS peptide. P2C had no adjuvant activity, such as the activation of DCs and splenocytes in vitro or antitumor effect in vivo by lipopeptides (2, 5, 6). The mixture did not show any effects in vivo such as appreciable antitumor activity (Fig. 6b). Based on these results, the stronger antitumor activity of P2C‐RGDS compared to that of MALP‐2 is thought to occur through an increase in cell adhesive ability, but not through the inhibition of angiogenesis.
Concerning the relationship between TLR and its ligand, it is suggested that a co‐receptor plays a key role for TLR binding and signaling( 1 , 7 , 48 ) as observed previously for CD14 in the Lipopolysaccharide (LPS)–TLR4 signaling pathway. Although integrin binding is predicted to support the capture and phagocytosis of ligands by DCs, integrin signaling in addition to TLR signaling might influence adjuvant activity. Stronger adjuvants will be developed by selecting for other properties, in addition to TLR signaling, that are essential for adjuvant activity, and the integrin signal could be one of the candidates.
In the present work, the inclusion of an integrin‐binding motif in a TLR2 ligand was examined for its effect in increasing the activity of the compound as an antitumor adjuvant by enhancing adhesion of the ligand to DCs and other cells. Dendritic cells efficiently recognized P2C‐RGDS, which they adhered to and maintained around cells, and P2C‐RGDS showed stronger antitumor activity than MALP‐2 in vivo. The present adjuvant‐engineering project is a new strategy to incorporate biological findings into drug design. Targeting peptides are used to elicit a strictly selective response among immune cells. A targeted strategy can effectively activate immune cells at a low concentration while not affecting other cells whose activation might lead to side effects. More than 20 TLR2 ligands with 10 alternative functions have already been synthesized by adjuvant engineering and our group is working to develop the strongest adjuvant through continued evaluation and improvements.
Acknowledgments
This work was supported in part by KAKENHI (15790069; 19790301; 20200075; 21790400) from the Ministry of Education, Culture, Science, and Technology of Japan; The Uehara Memorial Foundation; Osaka Community Foundation; and The Charitable Trust Osaka Cancer Researcher Fund. We are grateful to Drs K. Toyoshima, H. Koyama, S. Imaoka, S. Hori, K. Kato, and M. Tatsuta (Osaka Medical Center for Cancer, Osaka) for their support of this work. We are grateful to Dr K. Kikuchi (Sapporo Medical University, Sapporo), who gave the name “adjuvant engineering” to our project. Thanks are also due to N. Kanto, and T. Yasuda, E. Takahara, Y. Mimura, and M. Yabu (Osaka Medical Center for Cancer, Osaka) for their assistance.
References
- 1. Seya T, Akazawa T, Tsujita T, Matsumoto M. Role of Toll‐like receptors in adjuvant‐augmented immune therapies. Evid Based Complement Alternat Med 2006; 3(1): 31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akazawa T, Ebihara T, Okuno M et al. Antitumor NK activation induced by the Toll‐like receptor 3‐TICAM‐1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci U S A 2007; 104(1): 252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okamoto M, Oshikawa T, Tano T et al. Involvement of Toll‐like receptor 4 signaling in interferon‐g production and antitumor effect by streptococcal agent OK‐432. J Natl Cancer Inst 2003; 95(4): 316–26. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10(9): 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celis E. Toll‐like receptor ligands energize peptide vaccines through multiple paths. Cancer Res 2007; 67(17): 7945–7. [DOI] [PubMed] [Google Scholar]
- 6. Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll‐like receptor agonists and antagonists. Nat Med 2007; 13(5): 552–9. [DOI] [PubMed] [Google Scholar]
- 7. Seya T, Akazawa T, Uehori J, Matsumoto M, Azuma I, Toyoshima K. Role of toll‐like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer Res 2003; 23(6a): 4369–76. [PubMed] [Google Scholar]
- 8. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 2006; 124: 783–801. [DOI] [PubMed] [Google Scholar]
- 9. Miconnet I, Coste I, Beermann F et al. Cancer vaccine design: a novel bacterial adjuvant for peptide‐specific CTL induction. J Immunol 2001; 166(7): 4612–19. [DOI] [PubMed] [Google Scholar]
- 10. Iwasaki A, Medzhitov R. Toll‐like receptor control of the adaptive immune responses. Nat Immunol 2004; 5: 987–95. [DOI] [PubMed] [Google Scholar]
- 11. Kaisho T, Akira S. Toll‐like receptors as adjuvant receptors. Biochim Biophys Acta 2002; 1589(1): 1–13. [DOI] [PubMed] [Google Scholar]
- 12. Hemmi H, Takeuchi O, Kawai T et al. A Toll‐like receptor recognizes bacterial DNA. Nature 2000; 408(6813): 740–5. [DOI] [PubMed] [Google Scholar]
- 13. Azuma I, Ribi EE, Meyer TJ, Zbar B. Biologically active components from mycobacterial cell walls: I Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst 1974; 52: 95–101. [DOI] [PubMed] [Google Scholar]
- 14. Hayashi A, Doi O, Azuma I, Toyoshima K. Immuno‐friendly use of BCG‐cell wall skeleton remarkably improves the survival rate of various cancer patients. Proc Jpn Acad 1998; 74 Ser. B: 50–5. [Google Scholar]
- 15. Uehori J, Fukase K, Akazawa T et al. Dendritic cell maturation induced by muramyl dipeptide (MDP) derivatives: monoacylated MDP confers TLR2/TLR4 activation. J Immunol 2005; 174(11): 7096–103. [DOI] [PubMed] [Google Scholar]
- 16. Tsuji S, Matsumoto M, Takeuchi O et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette‐Guérin: involvement of toll‐like receptors. Infect Immun 2000; 68: 6883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uehori J, Matsumoto M, Tsuji S et al. Simultaneous blocking of human Toll‐like receptor 2 and 4 suppresses myeloid dendritic cell activation induced by Mycobacterium bovis bacillus Calmette‐Guérin (BCG)‐peptidoglycan (PGN). Infect Immun 2003; 71: 4238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akazawa T, Masuda H, Saeki Y et al. Adjuvant‐mediated tumor regression and tumor‐specific cytotoxic response are impaired in MyD88‐deficient mice. Cancer Res 2004; 64(2): 757–64. [DOI] [PubMed] [Google Scholar]
- 19. Kodama K, Higashiyama M, Takami K et al. Innate immune therapy with a Bacillus Calmette‐Guérin cell wall skeleton after radical surgery for non‐small cell lung cancer: a case‐control study. Surg Today 2009; 39(3): 194–200. [DOI] [PubMed] [Google Scholar]
- 20. Begum NA, Ishii K, Kurita‐Taniguchi M et al. Mycobacterium bovis BCG cell wall‐specific differentially expressed genes identified by differential display and cDNA subtraction in human macrophages. Infect Immun 2004; 72(2): 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsumoto M, Seya T, Kikkawa S et al. Interferon gamma‐producing ability in blood lymphocytes of patients with lung cancer through activation of the innate immune system by BCG cell wall skeleton. Int Immunopharmacol 2001; 1(8): 1559–69. [DOI] [PubMed] [Google Scholar]
- 22. Oppmann B, Lesley R, Blom B et al. Novel p19 protein engages IL‐12p40 to form a cytokine, IL‐23, with biological activities similar as well as distinct from IL‐12. Immunity 2000; 13(5): 715–25. [DOI] [PubMed] [Google Scholar]
- 23. Zou JP, Yamamoto N, Fujii T et al. Systemic administration of rIL‐12 induces complete tumor regression and protective immunity: response is correlated with a striking reversal of suppressed IFN‐g production by anti‐tumor T cells. Int Immunol 1995; 7(7): 1135–45. [DOI] [PubMed] [Google Scholar]
- 24. Langowski JL, Zhang X, Wu L et al. IL‐23 promotes tumour incidence and growth. Nature 2006; 442(7101): 461–5. [DOI] [PubMed] [Google Scholar]
- 25. Kolls JK, Lindén A. Interleukin‐17 family members and inflammation. Immunity 2004; 21(4): 467–76. [DOI] [PubMed] [Google Scholar]
- 26. Shime H, Yabu M, Akazawa T et al. Tumor‐secreted lactic acid promotes IL‐23/IL‐17 proinflammatory pathway. J Immunol 2008; 180(11): 7175–83. [DOI] [PubMed] [Google Scholar]
- 27. Kaiga T, Sato M, Kaneda H, Iwakura Y, Takayama T, Tahara H. Systemic administration of IL‐23 induces potent antitumor immunity primarily mediated through Th1‐type response in association with the endogenously expressed IL‐12. J Immunol 2007; 178(12): 7571–80. [DOI] [PubMed] [Google Scholar]
- 28. Jackson DC, Lau YF, Le T et al. A totally synthetic vaccine of generic structure that targets Toll‐like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc Natl Acad Sci U S A 2004; 101(43): 15440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider C, Schmidt T, Ziske C et al. Tumour suppression induced by the macrophage activating lipopeptide MALP‐2 in an ultrasound guided pancreatic carcinoma mouse model. Gut 2004; 53(3): 355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murata M. Activation of Toll‐like receptor 2 by a novel preparation of cell wall skeleton from Mycobacterium bovis BCG Tokyo (SMP‐105) sufficiently enhances immune responses against tumors. Cancer Sci 2008; 99(7): 1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mühlradt PF, Kiess M, Meyer H, Süssmuth R, Jung G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med 1997; 185(11): 1951–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okusawa T, Fujita M, Nakamura J et al. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll‐like receptors 2 and 6. Infect Immun 2004; 72(3): 1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Omueti KO, Beyer JM, Johnson CM, Lyle EA, Tapping RI. Domain exchange between human toll‐like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J Biol Chem 2005; 280(44): 36616–25. [DOI] [PubMed] [Google Scholar]
- 34. Voss S, Ulmer AJ, Jung G, Wiesmüller KH, Brock R. The activity of lipopeptide TLR2 agonists critically depends on the presence of solubilizers. Eur J Immunol 2007; 37(12): 3489–98. [DOI] [PubMed] [Google Scholar]
- 35. Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II‐self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med 1997; 186: 665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harui A, Roth MD, Vira D, Sanghvi M, Mizuguchi H, Basak SK. Adenoviral‐encoded antigens are presented efficiently by a subset of dendritic cells expressing high levels of aV‐b3 integrins. J Leukoc Biol 2006; 79(6): 1271–8. [DOI] [PubMed] [Google Scholar]
- 37. Okada N, Masunaga Y, Okada Y et al. Gene transduction efficiency and maturation status in mouse bone marrow‐derived dendritic cells infected with conventional or RGD fiber‐mutant adenovirus vectors. Cancer Gene Ther 2003; 10(5): 421–31. [DOI] [PubMed] [Google Scholar]
- 38. Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin‐induced maturation of MyD88‐deficient dendritic cells. J Immunol 2001; 166(9): 5688–94. [DOI] [PubMed] [Google Scholar]
- 39. Helmich BK, Dutton RW. The role of adoptively transferred CD8 T cells and host cells in the control of the growth of the EG7 thymoma: factors that determine the relative effectiveness and Homing properties of Tc1 and Tc2 effectors. J Immunol 2001; 166: 6500–8. [DOI] [PubMed] [Google Scholar]
- 40. Akazawa T, Shingai M, Sasai M et al. Tumor immunotherapy using bone marrow‐derived dendritic cells overexpressing Toll‐like receptor adaptors. FEBS Lett 2007; 581(18): 3334–40. [DOI] [PubMed] [Google Scholar]
- 41. Matsumoto M, Nishiguchi M, Kikkawa S, Nishimura H, Nagasawa S, Seya T. Structural and functional properties of complement‐activating protein M161Ag, a Mycoplasma fermentans gene product that induces cytokine production by human monocytes. J Biol Chem 1998; 273: 12407–14. [DOI] [PubMed] [Google Scholar]
- 42. Nishiguchi M, Matsumoto M, Takao T et al. Mycoplasma fermentans lipoprotein M161Ag‐induced cell activation is mediated by Toll‐like receptor 2: role of N‐terminal hydrophobic portion in its multiple functions. J Immunol 2001; 166: 2610–16. [DOI] [PubMed] [Google Scholar]
- 43. Hida T, Hayashi K, Yukishige K, Tanida S, Kawamura N, Harada S. Synthesis and biological activities of TAN‐1511 Analogues. J Antibiot 1995; 48(7): 589–603. [DOI] [PubMed] [Google Scholar]
- 44. Reutter F, Jung G, Baier W, Treyer B, Bessler WG, Wiesmüller KH. Immunostimulants and Toll‐like receptor ligands obtained by screening combinatorial lipopeptide collections. J Pept Res 2005; 65(3): 375–83. [DOI] [PubMed] [Google Scholar]
- 45. Gobin SJ, Peijnenburg A, Keijsers V, Van Den Elsen PJ. Site a is crucial for two routes of IFN g‐induced MHC class I transactivation: the ISRE‐mediated route and a novel pathway involving CIITA. Immunity 1997; 6(5): 601–11. [DOI] [PubMed] [Google Scholar]
- 46. Buerkle MA, Pahernik SA, Sutter A, Jonczyk A, Messmer K, Dellian M. Inhibition of the alpha‐nu integrins with a cyclic RGD peptide impairs angiogenesis, growth and metastasis of solid tumours in vivo . Br J Cancer 2002; 86(5): 788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Janssen ML, Oyen WJ, Dijkgraaf I et al. Tumor targeting with radiolabeled alpha(v)beta(3) integrin binding peptides in a nude mouse model. Cancer Res 2002; 62(21): 6146–51. [PubMed] [Google Scholar]
- 48. Seya T, Matsumoto M, Tsuji S, Begum NA, Azuma I, Toyoshima K. Structural‐functional relationship of pathogen‐associated molecular patterns: lessons from BCG cell wall skeleton and mycoplasma lipoprotein M161Ag. Microbes Infect 2002; 4(9): 955–61. [DOI] [PubMed] [Google Scholar]


