Abstract
Nobiletin is a citrus polymethoxyflavonoid that suppresses tumor growth and metastasis, both of which depend on angiogenesis. We recently identified nobiletin as a cell differentiation modulator. Because cell differentiation is a critical event in angiogenesis, it might be possible that nobiletin could exhibit antiangiogenic activity, resulting in suppression of these tumor malignant properties. To verify this possibility, we examined the antiangiogenic effects of nobiletin in vitro and in vivo. Nobiletin had concentration‐dependent inhibitory effects on multiple functions of angiogenesis‐related endothelial cells (EC); it suppressed the proliferation, migration and tube formation on matrigel of human umbilical vein EC (HUVEC) stimulated with endothelial cell growth supplement (ECGS), a mixture of acidic and basic fibroblast growth factors (FGFs). Gelatin zymography and northern blotting revealed that nobiletin suppressed pro‐matrix metalloproteinase‐2 (proMMP‐2) production and MMP‐2 mRNA expression in ECGS‐stimulated HUVEC. Nobiletin also downregulated cell‐associated plasminogen activator (PA) activity and urokinase‐type PA mRNA expression. Furthermore, nobiletin inhibited angiogenic differentiation induced by vascular endothelial growth factor and FGF, an in vitro angiogenesis model. This inhibition was accompanied by downregulation of angiogenesis‐related signaling molecules, such as extracellular signal‐regulated kinase 1/2 and c‐Jun N‐terminal kinase, and transcriptional factors (c‐Jun and signal transducer and activator of transcription 3), and activation of the caspase pathway. In a chick embryo chorioallantoic membrane assay, nobiletin showed an antiangiogenic activity, the ID50 value being 10 μg (24.9 nmol) per egg. These results indicate that nobiletin is a novel antiangiogenic compound that exhibits its activity through combined inhibition of multiple angiogenic EC functions. (Cancer Sci 2010; 101: 2462–2469)
Angiogenesis is a key factor for tumor development and metastasis that are major causes of difficulty in cancer treatment.( 1 , 2 ) The angiogenesis process includes the dedifferentiation of differentiated and quiescent endothelial cells (EC) into undifferentiated and angiogenic ones, which in turn degrade the extracellular matrix (ECM) via production of ECM‐degrading enzymes such as matrix metalloproteinases (MMP) and urokinase‐type plasminogen activator (uPA), migrate, proliferate and eventually redifferentiate to form new capillary vessels.( 3 , 4 , 5 ) Furthermore, endothelial progenitor cells, which are derived from bone marrow, have been found to differentiate and incorporate into sites of neovessels.( 6 ) These facts imply that a cell differentiation (or dedifferentiation) would represent an important target for inhibition of angiogenesis, and led us to speculate that a cell differentiation‐modulating activity could be a useful indicator for searching a new inhibitor of angiogenesis. A study along this line has yielded interesting findings. Namely, chemicals with cell differentiation‐modulating activity that we previously examined all exhibited an antiangiogenic activity. They include retinoids, vitamin D3 derivatives and radicicol.( 7 , 8 )
Nobiletin is a flavonoid found in citrus fruits such as Citrus depressa and Citrus reticulate.( 9 ) It suppresses carcinogenesis, tumor growth and metastasis in vivo.( 10 , 11 , 12 , 13 ) These tumor malignant properties are well known to be highly dependent on angiogenesis. Recently, we and another group revealed that nobiletin exhibited a cell differentiation‐modulating activity.( 14 , 15 ) Overall, these findings indicate the possibility that nobiletin could interfere with these angiogenesis‐dependent malignant properties of tumors through inhibition of angiogenesis. However, there is no report on the antiangiogenic property of nobiletin, to the best of our knowledge.
We here examined whether or not nobiletin interfered with in vitro and in vivo angiogenesis models.
Materials and Methods
Materials. Nobiletin was kindly donated by Dr Masamichi Yano (National Institute of Fruit Tree Science, Shizuoka, Japan).( 16 ) Ethylene‐vinyl acetate copolymer 40 (EV40) pellets were kindly donated by Mitsui‐DuPont Polychemicals (Tokyo, Japan). Fetal bovine serum (FBS) was purchased from Moregate (Melbourne, Australia), plasminogen for chromogenic substrate assay form Roche Applied Science (Hague Lord, IN, USA), matrigel from Becton, Dickinson and Company (Franklin Lakes, NJ, USA), Atelocollagen bovine dermis (type I collagen) from Koken (Tokyo, Japan), human recombinant vascular endothelial growth factor (VEGF) from Humanzyme (Chicago, IL, USA) and human recombinant basic fibroblast growth factor (bFGF) from WAKO (Osaka, Japan). Plasminogen for PA zymography and H‐D‐Val‐L‐Leu‐L‐Lys‐p‐nitroanilide (S‐2251), a chromogenic substrate for plasmin, were obtained from Chromogenix (Molndal, Sweden). Endothelial cell growth supplement (ECGS) and anti‐phospho‐c‐Jun (S73) antibody were purchased from Millipore (Billerica, MA, USA). Anti‐c‐Jun N‐terminal kinase (JNK) and anti‐glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other primary and secondary antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Unless otherwise stated, all other chemicals were purchased from Sigma (St Louis, MO, USA).
Cell culture. Human umbilical vein EC (HUVEC) and human dermal microvascular EC (HDMEC) were obtained from Cell Systems (Kirkland, WA, USA) and cultured in growth medium (MCDB‐131 medium supplemented with 10% FBS, 10 ng/mL epidermal growth factor, 10 μg/mL heparin, 10 μg/mL ECGS and 1% [v/v] penicillin/streptomycin mixture [Gibco, Grand Island, NY, USA]).
Cell proliferation assay. The HUVEC or HDMEC (5 × 103 cells/cm2) were seeded onto 24‐multiwell plates and allowed to adhere for 4 h. The cells were treated with growth medium containing vehicle (0.1% dimethyl sulfoxide [DMSO]) or the indicated concentrations of nobiletin for 72 h. Cell numbers were measured with a Coulter Counter Z1 (Coulter Japan, Tokyo, Japan).
Gelatin zymography. The HUVEC were treated with vehicle or nobiletin in serum‐free growth medium for 18 h. The protein content of the supernatants was determined by Protein assay (Bio‐Rad, Hercules, CA, USA). Aliquots of the supernatants corresponding to the same protein content were electrophoresed under a nonreducing condition. After rinsing the gel, it was incubated in a reaction buffer (100 mM Tris‐HCl [pH 7.5], 200 mM NaCl, 5 mM CaCl2 and 1 μM ZnCl2) at 37°C for 24 h. The visualization of proteolysis by MMP was performed by staining with 0.25% (w/v) Coomassie Brilliant Blue R‐250 in 10% acetic acid and 45% methanol. The intensity of bands was determined with a Leica Q500MC image analyzer equipped with QWin image analysis software (Cambridge, UK).
Northern blotting. Northern blotting was performed as described previously.( 17 ) Briefly, HUVEC were treated with vehicle or nobiletin in serum‐free growth medium containing 0.1% bovine serum albumin (BSA) for 18 h. Total RNA was extracted using Isogen reagent (Nippon Gene, Toyama, Japan). Ten microgram of total RNA was separated on a 1% formaldehyde agarose gel and then transferred to a Biodyne B membrane (Pall Corporation, NY, USA). cDNA probes for uPA (gene accession number: NM_002658, 615–1088 bp) and MMP‐2 (gene accession number: NM_004530, 1828–2105 bp) were prepared from genomic DNA. The radioactive band was detected and analyzed by an image analyzer (Fuji Photo Film, Tokyo, Japan).
Chromogenic substrate assay for PA. Cell‐associated PA activity was determined as described previously.( 18 ) Briefly, HUVEC or HDMEC were treated with vehicle, nobiletin or medroxyprogesterone acetate (MPA) in serum‐free growth medium containing 0.1% BSA for 18 h. The PA activity in the cell lysates was determined using plasminogen and S‐2251, and expressed as milliunits (mU)/μg protein. The protein content was determined by DC protein assay (Bio‐Rad).
Plasminogen activator zymography. The PA zymography was performed as described previously.( 19 ) Briefly, the cell lysates used in a chromogenic substrate assay were adjusted to correspond to the same protein content. Aliquots of the adjusted cell lysates were electrophoresed under nonreducing conditions. After rinsing the gel, it was overlaid on substrate gels containing 0.1 CU/mL plasminogen and 1% skim milk powder as a source of casein. After a 16‐h incubation at 37°C to allow proteolysis, the substrate gels were stained with 0.1% (w/v) Amide Black 10B in 10% acetic acid and 10% methanol. The intensity of the bands was determined as described in the gelatin zymography.
Cell migration assay. The EC migration was determined with a wound healing migration assay as described previously.( 19 ) Briefly, confluent HUVEC or HDMEC were pretreated with vehicle or nobiletin for 2 h and then scratched with a razor blade to produce acellular areas. The cells were allowed to migrate toward the acellular areas for 18 h in serum‐free growth medium containing 0.1% BSA with vehicle or nobiletin. After Giemsa‐staining, the migrated cells across the wound edge were determined with an NIH image program (National Institutes of Health, Bethesda, MD, USA).
In vitro angiogenesis model. Angiogenic differentiation, an in vitro angiogenesis model, was performed as described previously with slight modifications.( 20 ) Briefly, HUVEC (9.0 × 104 cells/cm2) were seeded between two layers of collagen gel (0.21% type I collagen) and incubated in the medium containing 1% FBS, 10 ng/mL VEGF, 10 ng/mL bFGF and 25 μg/mL ascorbic acid with vehicle or nobiletin for 24 h. For quantification, the cells were stained with 0.1% toluidine blue in 30% methanol. Tube area (area ratio of the formed tubes per pictured field) was quantified using the NIH Image program.
For the preparation of western blotting samples, HUVEC were suspended at 4.2 × 105 cells per 48‐well plate in 140 μL of collagen gel and allowed to induce a 3‐D tube formation. The cells were incubated with the medium containing 1% FBS, 30 ng/mL VEGF, 30 ng/mL bFGF and 25 μg/mL ascorbic acid with vehicle or nobiletin for up to 12 h.
Western blotting. Western blotting was conducted as previously described.( 21 ) Signal intensities were quantified using the NIH Image program.
Tube formation on matrigel. Tube formation on matrigel was conducted as described previously.( 19 ) Briefly, HUVEC or HDMEC were pretreated with nobiletin for 24 h and then plated onto matrigel at 5.0 × 104 cells/cm2 in growth medium containing 1% FBS with vehicle or nobiletin. After a 16‐h incubation, the total length of the formed tubular structures in nine randomly chosen microscopic fields per well was determined with a Leica Q500MC image analyzer.
Chorioallantoic membrane (CAM) assay. A CAM assay was performed as described previously.( 22 ) Briefly, the 5‐day‐old CAM were treated with EV40 pellets alone or in combination with various doses of nobiletin and incubated at 37°C for 2 days. An appropriate volume of 20% fat emulsion was injected into the chorioallantois to further visualize the vascular network. The antiangiogenic response was assessed as positive when the avascular zone in a treated CAM was 3 mm or more in diameter.
Statistical analysis. The values in in vitro experiments were expressed as means ± SE. Differences were ascertained by an analysis of variance (anova). Multiple comparisons among treatments were checked with Dunnett’s test (*P < 0.05, **P < 0.01). Data on the CAM assay were analyzed by Fisher’s exact probability test (*P < 0.05, **P < 0.01).
Results
Inhibitory effect of nobiletin on proliferation. The effects of nobiletin on proliferation of HUVEC and HDMEC in response to ECGS that is composed mainly of acidic and basic FGFs were determined by counting the cell number after vehicle or nobiletin treatment for 72 h. Nobiletin treatment resulted in a concentration‐dependent decrease in the proliferation of both EC (Fig. 1). The IC50 values were 26 and 24 μM for HUVEC and HDMEC, respectively.
Figure 1.
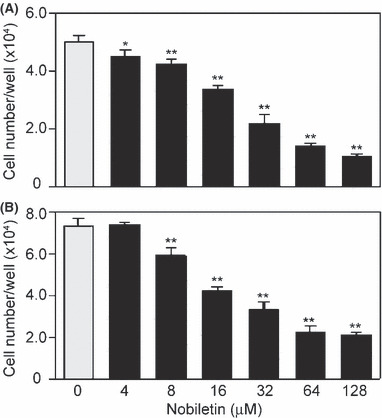
Nobiletin inhibited endothelial cell (EC) proliferation. After a 72‐h culture in the presence of various concentrations of nobiletin, cell numbers of (A) human umbilical vein EC and (B) human dermal microvascular EC were determined with a Coulter counter. Values are expressed as means ± SE (n = 3). *P < 0.05, **P < 0.01, as compared with the control.
Suppression of proMMP‐2 production and MMP‐2 expression in EC by nobiletin. Production of MMP‐2 and MMP‐9 by HUVEC in the presence or absence of nobiletin was determined with gelatin zymography. It caused a concentration‐dependent decrease in proMMP‐2 production, the IC50 being 52 μM (Fig. 2A). The bands corresponding to proMMP‐9 and activeMMP‐2 were undetectable in culture medium of HUVEC in our experimental condition. Northern blotting showed that nobiletin suppressed MMP‐2 expression in a concentration‐dependent manner and its expression was decreased to 56% (% of control) at 128 μM (Fig. 2B).
Figure 2.
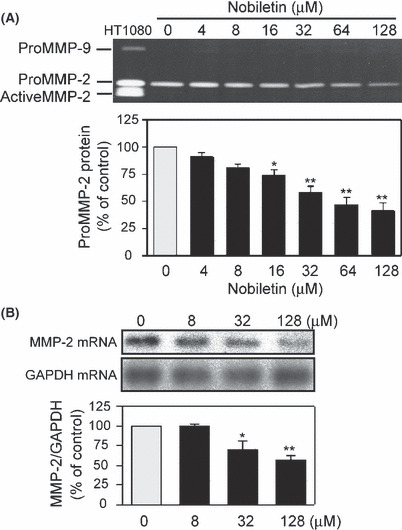
Nobiletin reduced proMMP‐2 levels via downregulation of MMP‐2 mRNA expression in human umbilical vein endothelial cells. (A) The cells were treated with indicated concentrations of nobiletin for 18 h. Gelatinase activity in the conditioned medium was analyzed using a gelatin zymography. The conditioned medium of HT‐1080 cells was used as a marker for MMP‐2 and MMP‐9. (B) MMP‐2 and GAPDH mRNA expressions were analyzed by northern blotting. The ratio of MMP‐2 to GAPDH was calculated after each radioactive band was quantified. All values are expressed as means ± SE (n = 3). *P < 0.05, **P < 0.01, as compared with the control.
Suppression of PA activity and uPA expression in EC by nobiletin. Secreted PA associates with a cell‐surface PA receptor. The cell‐associated PA contributes to perivascular proteolysis by cleaving plasminogen into plasmin. The effect of nobiletin on cell‐associated PA activity was assessed by a chromogenic substrate assay, where MPA, an antiangiogenic compound, was used as a positive control.( 18 ) Nobiletin inhibited cell‐associated PA activity in HUVEC in a concentration‐dependent manner (Fig. 3A). Such an inhibitory effect was also observed in HDMEC (data not shown). The IC50 values were 46 and 27 μM for HUVEC and HDMEC, respectively. Consistent with the inhibitory effects on PA activity, nobiletin reduced the uPA protein level in HUVEC (Fig. 3B) in a PA zymography. The IC50 values were 60 and 44 μM for HUVEC and HDMEC, respectively. On the other hand, the band corresponding to tissue‐type PA (tPA) was undetectable, indicating that the PA activity in our experimental condition was mainly due to uPA, but not tPA. Furthermore, northern blotting revealed that nobiletin suppressed uPA expression and its expression decreased by 49% at 128 μM (Fig. 3C).
Figure 3.
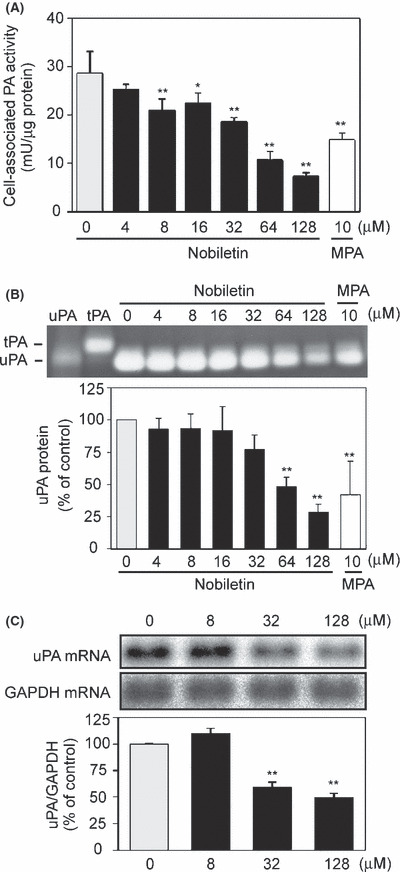
Nobiletin suppressed cell‐associated plasminogen activator (PA) activity via downregulation of uPA mRNA expression in human umbilical vein endothelial cells. (A) The cells were treated with vehicle, nobiletin or medroxyprogesterone acetate (MPA) for 18 h. Cell‐associated PA activity was determined using plasminogen and S‐2251, a chromogenic substrate for plasmin. (B) The PA levels in cell lysates were analyzed using a PA zymography. Human urokinase (hUK) and tPA proteins were loaded as molecular markers. (C) uPA and GAPDH expressions were analyzed by northern blotting. Ratio of uPA to GAPDH was calculated after each radioactive band was quantified. All values are expressed as means ± SE (n = 3). *P < 0.05, **P < 0.01, as compared with the control.
Inhibitory effect of nobiletin on EC migration. The effect of nobiletin on EC migration was tested using a wound healing migration assay. Nobiletin markedly inhibited the migration of HUVEC (Fig. 4A). The inhibition was in a concentration‐dependent manner and the IC50 values were 33 and 31 μM for HUVEC and HDMEC, respectively (Fig. 4B,C).
Figure 4.
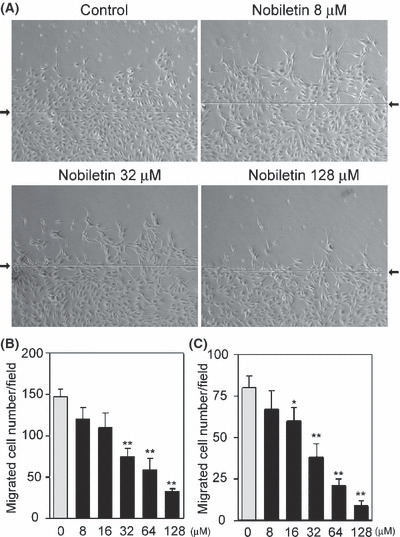
Nobiletin suppressed endothelial cell (EC) migration. (A) Human umbilical vein EC (HUVEC) were pretreated with nobiletin for 2 h and then allowed to migrate for 18 h. Representative images are shown. Arrows indicate a wound edge. (B,C) The migrated cell numbers across the wound edge in (B) HUVEC and (C) human dermal microvascular EC were counted. Values are expressed as means ± SE (n = 3). *P < 0.05, **P < 0.01, as compared with the control.
Effect of nobiletin on both VEGF‐ and bFGF‐induced angiogenic differentiation and the signaling pathway. The effect of nobiletin on angiogenic differentiation, an in vitro angiogenesis model, was tested. The angiogenic morphological change of HUVEC induced by VEGF and bFGF was significantly suppressed by nobiletin (Fig. 5). Western blotting showed that treatment with both VEGF and bFGF resulted in enhanced phosphorylation of extracellular signal‐regulated kinase 1/2 (ERK1/2) and JNK (p46) in tube‐forming HUVEC (Fig. 6A,B). This phosphorylation was significantly attenuated by nobiletin. These two angiogenic stimuli also elicited phosphorylation of c‐Jun, a component of AP‐1, and signal transducer and activator of transcription 3 (STAT3), both of which are angiogenesis‐related key transcriptional factors (Fig. 6C,D). Nobiletin completely suppressed both VEGF‐ and bFGF‐induced phosphorylation of these transcriptional factors. Furthermore, nobiletin induced activation of caspase‐3 and cleavage of poly ADP‐ribose polymerase (PARP), a substrate of caspase, both of which are molecular markers of apoptosis (Fig. 6E).
Figure 5.
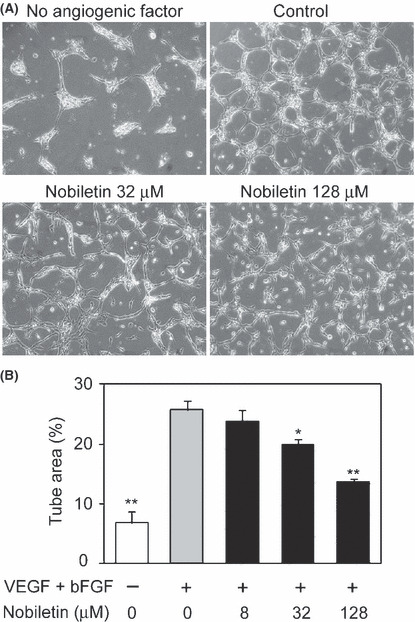
Nobiletin inhibited both vascular endothelial growth factor (VEGF)‐ and basic fibroblast growth factor (bFGF)‐induced angiogenic differentiation. (A) Human umbilical vein endothelial cells were cultured between the two layers of collagen gel with vehicle or nobiletin in the presence or absence of VEGF and bFGF for 24 h. Representative images are shown. (B) The formed tube area per field was determined. Values are expressed as means ± SE (n = 3). *P < 0.05, **P < 0.01, as compared with the control.
Figure 6.
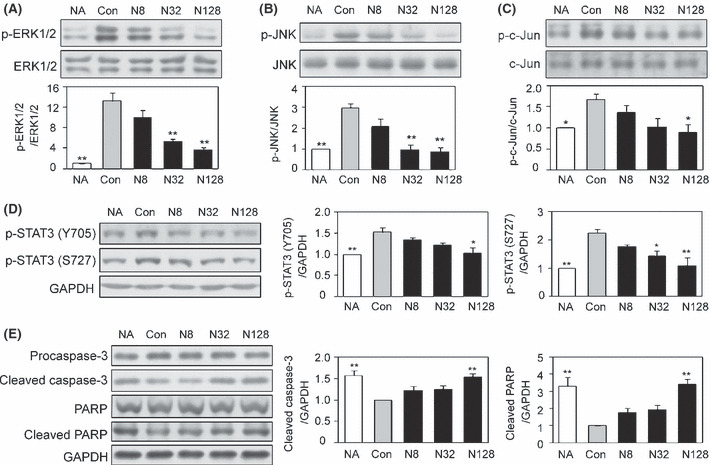
Nobiletin attenuated the phosphorylation of both vascular endothelial growth factor (VEGF)‐ and basic fibroblast growth factor (bFGF)‐induced signaling and transcriptional molecules, and evoked activation of the caspase pathway in tube‐forming human umbilical vein endothelial cells (HUVEC). The tube‐forming HUVEC were treated with vehicle or nobiletin in the presence or absence of VEGF and bFGF for 2 h (A‐D) or 12 h (E). Representative images are shown. Values are expressed as means ± SE (n = 3). NA, no angiogenic factor; Con, control; N, nobiletin. *P < 0.05, **P < 0.01, as compared with the control.
Nobiletin also caused a concentration‐dependent inhibition of tube formation on matrigel by HUVEC and HDMEC. The IC50 values were 70 and 58 μM for HUVEC and HDMEC, respectively (Fig. 7).
Figure 7.
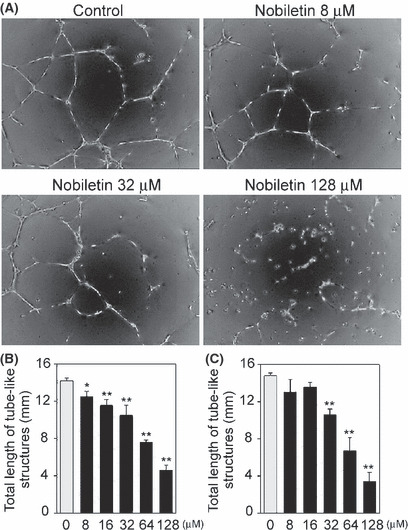
Nobiletin prevented tube formation of endothelial cells (EC) on matrigel. (A) Human umbilical vein EC (HUVEC) were pretreated with nobiletin for 24 h. The cells seeded onto matrigel and were induced to form tube‐like structures in the presence of nobiletin for 16 h. Representative images are shown. (B,C) The total length of tube‐like structures per field in (B) HUVEC and (C) human dermal microvascular EC was determined. Values are expressed as means ± SE (n = 3). *P < 0.05, **P < 0.01, as compared with the control.
Inhibitory effect of nobiletin on angiogenesis in vivo on a chick embryo CAM. The amount of nobiletin available was limited. Therefore, to determine whether nobiletin exhibits antiangiogenic activity in vivo, a chick embryo CAM assay was performed, because it required a considerably smaller amount of test sample than other in vivo angiogenesis assays, such as a dorsal air sac test. In a control egg, the formation of vascular networks on a CAM was observed, whereas such vascular formation was potently inhibited by nobiletin treatment (100 μg per egg) (Fig. 8A). This inhibition was dose‐dependent and the ID50 value was 10 μg (24.9 nmol) per egg (Fig. 8B).
Figure 8.
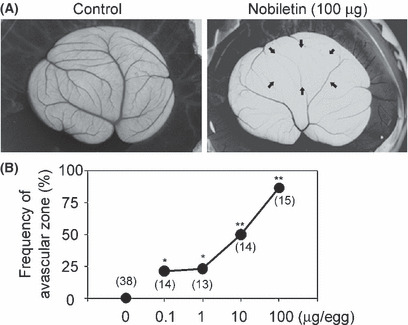
Nobiletin inhibited angiogenesis in vivo on a chick embryo chorioallantoic membrane (CAM). (A) The CAM were treated with an empty EV40 pellet alone (i.e. control) or a nobiletin‐containing EV40 pellet (100 μg/egg) for 2 days. Representative images are shown. Arrows indicate an avascular zone. (B) The points indicate the frequencies (%) of avascular zones showing an antiangiogenic response. The values in parentheses are the number of CAM examined. *P < 0.05; **P < 0.01 compared with the value for control CAM (n = 33), which did not show any avascular zone.
Discussion
In the present study, we demonstrated that nobiletin suppressed multiple angiogenic EC functions, probably resulting in the inhibition of in vitro and in vivo angiogenesis models. This suppression involved downregulation of signaling pathway(s) and transcriptional factors induced by VEGF and bFGF.
Chemotherapeutic agents easily access cancer cells in culture dishes in vitro, but it appears to be harder to reach cancer cells in solid tumors in vivo because there are some barriers to drug delivery, including abnormally high pressure seen in the tumor interstitium.( 23 ) By contrast, vascular EC are likely to more easily receive drugs for cancer treatment, compared with cancer cells in vivo. Thus, it was important to determine whether nobiletin modulated EC proliferation and apoptosis, although it was reported to exhibit anti‐proliferative and proapoptotic actions against various tumor cells.( 24 , 25 , 26 , 27 ) The present study revealed that nobiletin inhibited ECGS‐stimulated EC proliferation and activated caspase pathway in both VEGF‐ and bFGF‐induced tube‐forming EC. We and others have previously shown that ERK1/2 inactivation in EC caused suppression of EC proliferation and induction of caspase‐dependent apoptosis.( 20 , 21 , 28 , 29 ) Overall, it might be reasonable to assume that nobiletin exerted such effects on angiogenic EC, at least in part, via suppression of angiogenic stimuli‐induced ERK1/2 activation.
Perivascular ECM degradation by MMP like MMP‐2 is an essential process for the following angiogenesis responses such as proliferation, migration and tube formation. Deletion of MMP‐2 in mice and treatment of CAM with PEX, a natural inhibitor of MMP‐2 activity, caused a dramatic impairment of pathological and physiological angiogenesis in vivo.( 30 , 31 ) The present study showed that nobiletin downregulated MMP‐2 expression and inhibited CAM angiogenesis. Therefore, it might be possible that its downregulation was involved in its downregulation of CAM angiogenesis.
We also showed in the present study that nobiletin suppressed cell‐associated PA activity, at least in part, via inhibition of uPA expression. This result is consistent with our and other previous findings that antiangiogenic properties of cell differentiation modulators, including radicicol and peroxisome proliferator‐activated receptor γ ligands, might be partly due to a decrease in PA activity and/or the uPA level.( 32 , 33 ) However, it remains unclear whether nobiletin affected production of other angiogenesis‐related MMP, such as MMP‐9 and membrane‐type 1 MMP, and levels of inhibitors of MMP and PA in EC. Thus, further studies on these points are needed.
Both VEGF and bFGF trigger angiogenic responses via activation of a diverse signaling pathway, including mitogen‐activated protein kinases such as ERK1/2 and JNK, and their downstream transcriptional factors such as AP‐1 and STAT.( 34 , 35 , 36 , 37 ) ERK1/2 is known to upregulate the transcriptional activity of AP‐1 and Sp1,( 38 , 39 ) both of which are critical players of MMP‐2 and uPA expression.( 40 , 41 , 42 ) JNK also elevates the transcriptional activity of AP‐1 via phosphorylation of its component c‐Jun.( 43 ) Thus, the inactivation of ERK1/2, JNK and c‐Jun by nobiletin observed in our present in vitro angiogenesis model might be responsible for its downregulation of MMP‐2 and uPA expression. In this regard, nobiletin was found to act as an inhibitor of MEK, an upstream kinase of ERK1/2, in HT‐1080 cells, and to suppress AP‐1 and Sp1 activation in 12‐O‐tetradecanoylphorbol 13‐acetate‐stimulated HT‐1080 cells and lipopolysaccharide‐stimulated THP‐1 monocytic cells.( 44 , 45 , 46 )
Both VEGF and bFGF are known to phosphorylate STAT3.( 38 , 47 , 48 ) The phosporylation by VEGF was involved in EC migration and tube formation on collagen gel induced by the angiogenic factor.( 48 ) The present study revealed that nobiletin suppressed phosphorylation of STAT3 in VEGF‐ and bFGF‐stimulated tube‐forming HUVEC. So it was plausible to consider that this suppression by nobiletin might contribute to its blockage of EC migration and tube formation. Alternatively, inhibitory effects on EC migration and tube formation by nobiletin seemed to be due to a decreased uPA level by the agent, because there are some reports showing the contribution of uPA to EC migration and tube formation via its proteolytic activity‐dependent and ‐independent pathways.( 49 , 50 , 51 )
We previously found that a variety of seemingly unrelated substances have antiangiogenic effects in vivo using a CAM assay, which involves all of EC functions related to angiogenesis in vivo.( 22 , 32 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ) In the present study, topical application of nobiletin resulted in the suppression of embryonic angiogenesis, indicating that nobiletin has the ability to inhibit angiogenesis in vivo. One might ask the involvement of anti‐inflammatory activity of nobiletin in its suppression of CAM angiogenesis, because inflammation is one of several major inducing factors of angiogenesis in vivo and nobiletin is known to possess anti‐inflammatory activity.( 10 , 59 , 60 ) However, the possibility is highly unlikely, since a CAM assay provides a macrophage‐free environment until day 8.( 61 ) Thus, it is reasonable to assume that nobiletin suppress CAM angiogenesis via its direct antiangiogenic properties. The present study did not examine the effect of nobiletin on tumor‐triggered angiogenesis, due to the limited amount of agent available. Thus, further study on this point is needed.
In conclusion, we here demonstrated that nobiletin suppressed a series of angiogenic EC functions and embryonic angiogenesis in vivo. Our findings provide new insight that nobiletin might be a useful antiangiogenic compound for the prevention and treatment of cancer and other angiogenesis‐related disorders.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
The authors wish to thank Professor Yasufumi Sato (Institute of Development, Aging and Cancer, Tohoku University, Sendai, Japan) for advice regarding the cell migration assay. This work was supported by grants‐in‐aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan and a fund from the School of Nutrition and Dietetics, Kanagawa University of Human Services.
References
- 1. Folkman J. Clinical applications of research on angiogenesis. N Engl J Med 1995; 333: 1757–63. [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–57. [DOI] [PubMed] [Google Scholar]
- 3. Sato Y. Molecular diagnosis of tumor angiogenesis and anti‐angiogenic cancer therapy. Int J Clin Oncol 2003; 8: 200–6. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. [DOI] [PubMed] [Google Scholar]
- 5. Munoz‐Chapuli R, Quesada AR, Angel Medina M. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci 2004; 61: 2224–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asahara T, Murohara T, Sullivan A et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–7. [DOI] [PubMed] [Google Scholar]
- 7. Oikawa T. Strategies to find novel angiogenesis inhibitors as potential therapeutic agents for cancer. Curr Med Chem 1995; 1: 406–17. [Google Scholar]
- 8. Oikawa T. Control of angiogenesis by microbial products. In: Fan T‐PD, Kohn EC, eds. The New Angiotherapy. Totowa: Humana Press, 2001; 329–55. [Google Scholar]
- 9. Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem 2006; 70: 178–92. [DOI] [PubMed] [Google Scholar]
- 10. Murakami A, Nakamura Y, Torikai K et al. Inhibitory effect of citrus nobiletin on phorbol ester‐induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res 2000; 60: 5059–66. [PubMed] [Google Scholar]
- 11. Kohno H, Yoshitani S, Tsukio Y et al. Dietary administration of citrus nobiletin inhibits azoxymethane‐induced colonic aberrant crypt foci in rats. Life Sci 2001; 69: 901–13. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki R, Kohno H, Murakami A et al. Citrus nobiletin inhibits azoxymethane‐induced large bowel carcinogenesis in rats. Biofactors 2004; 22: 111–4. [DOI] [PubMed] [Google Scholar]
- 13. Minagawa A, Otani Y, Kubota T et al. The citrus flavonoid, nobiletin, inhibits peritoneal dissemination of human gastric carcinoma in SCID mice. Jpn J Cancer Res 2001; 92: 1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kunimasa K, Kuranuki S, Matsuura N et al. Identification of nobiletin, a polymethoxyflavonoid, as an enhancer of adiponectin secretion. Bioorg Med Chem Lett 2009; 19: 2062–4. [DOI] [PubMed] [Google Scholar]
- 15. Saito T, Abe D, Sekiya K. Nobiletin enhances differentiation and lipolysis of 3T3‐L1 adipocytes. Biochem Biophys Res Commun 2007; 357: 371–6. [DOI] [PubMed] [Google Scholar]
- 16. Ishiwa J, Sato T, Mimaki Y, Sashida Y, Yano M, Ito A. A citrus flavonoid, nobiletin, suppresses production and gene expression of matrix metalloproteinase 9/gelatinase B in rabbit synovial fibroblasts. J Rheumatol 2000; 27: 20–5. [PubMed] [Google Scholar]
- 17. Yajima Y, Sato M, Sumida M, Kawashima S. Mechanism of adult primitive mesenchymal ST‐13 preadipocyte differentiation. Endocrinology 2003; 144: 2559–65. [DOI] [PubMed] [Google Scholar]
- 18. Ashino‐Fuse H, Takano Y, Oikawa T, Shimamura M, Iwaguchi T. Medroxyprogesterone acetate, an anti‐cancer and anti‐angiogenic steroid, inhibits the plasminogen activator in bovine endothelial cells. Int J Cancer 1989; 44: 859–64. [DOI] [PubMed] [Google Scholar]
- 19. Aoki K, Watanabe K, Sato M, Ikekita M, Hakamatsuka T, Oikawa T. Effects of rhizoxin, a microbial angiogenesis inhibitor, on angiogenic endothelial cell functions. Eur J Pharmacol 2003; 459: 131–8. [DOI] [PubMed] [Google Scholar]
- 20. Kunimasa K, Kobayashi T, Kaji K, Ohta T. Antiangiogenic effects of indole‐3‐carbinol and 3,3′‐diindolylmethane are associated with their differential regulation of ERK1/2 and Akt in tube‐forming HUVEC. J Nutr 2010; 140: 1–6. [DOI] [PubMed] [Google Scholar]
- 21. Kunimasa K, Ahn MR, Kobayashi T et al. Brazilian propolis suppresses angiogenesis by inducing apoptosis in tube‐forming endothelial cells through inactivation of survival signal ERK1/2. Evid Based Complement Alternat Med 2009; doi: 10.1093/ecam/nep024 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oikawa T, Hirotani K, Nakamura O, Shudo K, Hiragun A, Iwaguchi T. A highly potent antiangiogenic activity of retinoids. Cancer Lett 1989; 48: 157–62. [DOI] [PubMed] [Google Scholar]
- 23. Jain RK. Barriers to drug delivery in solid tumors. Sci Am 1994; 271: 58–65. [DOI] [PubMed] [Google Scholar]
- 24. Morley KL, Ferguson PJ, Koropatnick J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett 2007; 251: 168–78. [DOI] [PubMed] [Google Scholar]
- 25. Luo G, Guan X, Zhou L. Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line A549 in vitro and in vivo. Cancer Biol Ther 2008; 7: 966–73. [DOI] [PubMed] [Google Scholar]
- 26. Tang MX, Ogawa K, Asamoto M et al. Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer Sci 2007; 98: 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshimizu N, Otani Y, Saikawa Y et al. Anti‐tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment Pharmacol Ther 2004; 20(Suppl 1): 95–101. [DOI] [PubMed] [Google Scholar]
- 28. Wilhelm SM, Carter C, Tang L et al. BAY 43‐9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64: 7099–109. [DOI] [PubMed] [Google Scholar]
- 29. Mavria G, Vercoulen Y, Yeo M et al. ERK‐MAPK signaling opposes Rho‐kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 2006; 9: 33–44. [DOI] [PubMed] [Google Scholar]
- 30. Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A‐deficient mice. Cancer Res 1998; 58: 1048–51. [PubMed] [Google Scholar]
- 31. Brooks PC, Silletti S, Von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 1998; 92: 391–400. [DOI] [PubMed] [Google Scholar]
- 32. Oikawa T, Ito H, Ashino H et al. Radicicol, a microbial cell differentiation modulator, inhibits in vivo angiogenesis. Eur J Pharmacol 1993; 241: 221–7. [DOI] [PubMed] [Google Scholar]
- 33. Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator‐activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 1999; 274: 9116–21. [DOI] [PubMed] [Google Scholar]
- 34. Cross MJ, Dixelius J, Matsumoto T, Claesson‐Welsh L. VEGF‐receptor signal transduction. Trends Biochem Sci 2003; 28: 488–94. [DOI] [PubMed] [Google Scholar]
- 35. Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005; 16: 139–49. [DOI] [PubMed] [Google Scholar]
- 36. Yang X, Qiao D, Meyer K, Friedl A. Signal transducers and activators of transcription mediate fibroblast growth factor‐induced vascular endothelial morphogenesis. Cancer Res 2009; 69: 1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bartoli M, Gu X, Tsai NT et al. Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J Biol Chem 2000; 275: 33189–92. [DOI] [PubMed] [Google Scholar]
- 38. Merchant JL, Du M, Todisco A. Sp1 phosphorylation by Erk 2 stimulates DNA binding. Biochem Biophys Res Commun 1999; 254: 454–61. [DOI] [PubMed] [Google Scholar]
- 39. Eyries M, Agrapart M, Alonso A, Soubrier F. Phorbol ester induction of angiotensin‐converting enzyme transcription is mediated by Egr‐1 and AP‐1 in human endothelial cells via ERK1/2 pathway. Circ Res 2002; 91: 899–906. [DOI] [PubMed] [Google Scholar]
- 40. D’Orazio D, Besser D, Marksitzer R et al. Cooperation of two PEA3/AP1 sites in uPA gene induction by TPA and FGF‐2. Gene 1997; 201: 179–87. [DOI] [PubMed] [Google Scholar]
- 41. Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP‐2 are required for constitutive matrix metalloproteinase‐2 gene expression in astroglioma cells. J Biol Chem 1999; 274: 29130–7. [DOI] [PubMed] [Google Scholar]
- 42. Pan M‐R, Hung W‐C. Nonsteroidal anti‐inflammatory drugs inhibit matrix metalloproteinase‐2 via suppression of the ERK/Sp1‐mediated transcription. J Biol Chem 2002; 277: 32775–80. [DOI] [PubMed] [Google Scholar]
- 43. Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103: 239–52. [DOI] [PubMed] [Google Scholar]
- 44. Sato T, Koike L, Miyata Y et al. Inhibition of activator protein‐1 binding activity and phosphatidylinositol 3‐kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases‐1 production and suppression of production of matrix metalloproteinases‐1 and ‐9 in human fibrosarcoma HT‐1080 cells. Cancer Res 2002; 62: 1025–9. [PubMed] [Google Scholar]
- 45. Miyata Y, Sato T, Imada K, Dobashi A, Yano M, Ito A. A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT‐1080 cells. Biochem Biophys Res Commun 2008; 366: 168–73. [DOI] [PubMed] [Google Scholar]
- 46. Hirata Y, Masuda Y, Kakutani H et al. Sp1 is an essential transcription factor for LPS‐induced tissue factor expression in THP‐1 monocytic cells, and nobiletin represses the expression through inhibition of NF‐kappaB, AP‐1, and Sp1 activation. Biochem Pharmacol 2008; 75: 1504–14. [DOI] [PubMed] [Google Scholar]
- 47. Deo DD, Axelrad TW, Robert EG, Marcheselli V, Bazan NG, Hunt JD. Phosphorylation of STAT‐3 in response to basic fibroblast growth factor occurs through a mechanism involving platelet‐activating factor, JAK‐2, and Src in human umbilical vein endothelial cells. Evidence for a dual kinase mechanism. J Biol Chem 2002; 277: 21237–45. [DOI] [PubMed] [Google Scholar]
- 48. Yahata Y, Shirakata Y, Tokumaru S et al. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor‐induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem 2003; 278: 40026–31. [DOI] [PubMed] [Google Scholar]
- 49. Pepper MS, Vassalli JD, Montesano R, Orci L. Urokinase‐type plasminogen activator is induced in migrating capillary endothelial cells. J Cell Biol 1987; 105: 2535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schnaper HW, Barnathan ES, Mazar A et al. Plasminogen activators augment endothelial cell organization in vitro by two distinct pathways. J Cell Physiol 1995; 165: 107–18. [DOI] [PubMed] [Google Scholar]
- 51. Odekon LE, Sato Y, Rifkin DB. Urokinase‐type plasminogen activator mediates basic fibroblast growth factor‐induced bovine endothelial cell migration independent of its proteolytic activity. J Cell Physiol 1992; 150: 258–63. [DOI] [PubMed] [Google Scholar]
- 52. Oikawa T, Hirotani K, Ogasawara H et al. Inhibition of angiogenesis by vitamin D3 analogues. Eur J Pharmacol 1990; 178: 247–50. [DOI] [PubMed] [Google Scholar]
- 53. Oikawa T, Yoshida Y, Shimamura M, Ashino‐Fuse H, Iwaguchi T, Tominaga T. Antitumor effect of 22‐oxa‐1 alpha,25‐dihydroxyvitamin D3, a potent angiogenesis inhibitor, on rat mammary tumors induced by 7,12‐dimethylbenz[a]anthracene. Anticancer Drugs 1991; 2: 475–80. [DOI] [PubMed] [Google Scholar]
- 54. Oikawa T, Okayasu I, Ashino H, Morita I, Murota S, Shudo K. Three novel synthetic retinoids, Re 80, Am 580 and Am 80, all exhibit anti‐angiogenic activity in vivo. Eur J Pharmacol 1993; 249: 113–6. [DOI] [PubMed] [Google Scholar]
- 55. Oikawa T, Murakami K, Sano M, Shibata J, Wierzba K, Yamada Y. A potential use of a synthetic retinoid TAC‐101 as an orally active agent that blocks angiogenesis in liver metastases of human stomach cancer cells. Jpn J Cancer Res 2001; 92: 1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oikawa T, Onozawa C, Kuranuki S et al. Dipalmitoylation of radicicol results in improved efficacy against tumor growth and angiogenesis in vivo. Cancer Sci 2007; 98: 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oikawa T, Sasaki T, Nakamura M et al. The proteasome is involved in angiogenesis. Biochem Biophys Res Commun 1998; 246: 243–8. [DOI] [PubMed] [Google Scholar]
- 58. Oikawa T, Sasaki M, Inose M et al. Effects of cytogenin, a novel microbial product, on embryonic and tumor cell‐induced angiogenic responses in vivo. Anticancer Res 1997; 17: 1881–6. [PubMed] [Google Scholar]
- 59. Lin N, Sato T, Takayama Y et al. Novel anti‐inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol 2003; 65: 2065–71. [DOI] [PubMed] [Google Scholar]
- 60. Murakami A, Nakamura Y, Ohto Y et al. Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti‐inflammatory polymethoxyflavonoid. Biofactors 2000; 12: 187–92. [DOI] [PubMed] [Google Scholar]
- 61. Ribatti D, Vacca A, Roncali L, Dammacco F. The chick embryo chorioallantoic membrane as a model for in vivo research on anti‐angiogenesis. Curr Pharm Biotechnol 2000; 1: 73–82. [DOI] [PubMed] [Google Scholar]


