Abstract
Papillomavirus binding factor (PBF) was first identified as a transcription factor regulating the promoter activity of human papillomavirus. We previously demonstrated that PBF is an osteosarcoma‐associated antigen and 92% of osteosarcoma tissues express PBF in the nucleus. Moreover, PBF‐positive osteosarcoma has a significantly poorer prognosis than that with negative expression of PBF. In the present study, we assessed the biological role of PBF in cell survival. Overexpression of PBF induced cell death‐mediated lactate dehydrase (LDH) release from 293EBNA cells. Cleaved poly(ADP‐ribose) polymerase and active caspase‐3 were also detected. However, PBF‐induced apoptosis did not affect caspase‐9 activity. Next, to identify the apoptosis regulator of PBF, we screened a cDNA library constructed from mRNA of the osteosarcoma cell line OS2000 using a yeast two‐hybrid system and isolated Scythe/BAT3. Scythe/BAT3 mRNA was detected in 56% of osteosarcoma tissues and ubiquitously in various normal tissues. Although Scythe/BAT3 was localized to the cytoplasm in normal tissue, it was localized to the nucleus in osteosarcoma tissue. PBF and Scythe/BAT3 also colocalized to the cytoplasm in 293T cells and the nucleus in OS2000. Furthermore, overexpression of Scythe/BAT3 suppressed cell death events that resulted from overexpression of PBF in OS2000, but not in 293EBNA cells. Thus, our results support the ideas that: (i) PBF could induce apoptotic cell death via a caspase‐9‐independent pathway; (ii) the apoptosis regulator Scythe/BAT3 is a PBF‐associated molecule acting as a nucleus–cytoplasm shuttling protein; and (iii) colocalization of PBF and Scythe/BAT3 in the nucleus might be an important factor for survival of osteosarcoma cells. (Cancer Sci 2009; 100: 47–53)
Papillomavirus binding factor (PBF) was first identified as a transcription factor regulating promoter activity of the human papillomavirus type 8 genome.( 1 ) We demonstrated that PBF is an osteosarcoma‐associated antigen recognized by an autologous cytotoxic T lymphocyte clone.( 2 ) Immunohistochemical analysis revealed that 92% of biopsy specimens of osteosarcoma express PBF. Moreover, PBF‐positive osteosarcoma has a significantly poorer prognosis than that with negative expression of PBF.( 3 ) Generally, conventional osteosarcoma is a malignant neoplasm of mesenchymal origin and there is no specific cause such as viral infection.( 4 ) Therefore, it is suggested that PBF has certain functions not only in transcription of the human papillomavirus genome, but also in the cell survival and apoptosis of osteosarcoma.
Apoptosis is tightly controlled programmed cell death. Generally, it is induced by two major pathways, an extrinsic pathway and an intrinsic pathway.( 5 ) The initiation of the extrinsic pathway of apoptosis is triggered by extracellular death signals. The binding of extracellular ligands to the death receptors of the tumor necrosis factor receptor superfamily leads to formation of the death‐inducing signaling complex, which is capable of activating the initiator caspase‐8.( 6 , 7 , 8 ) Meanwhile, initiation of the intrinsic pathway of apoptosis is triggered by cellular stress. Cellular death signals induce mitochondrial membrane permeabilization, mitochondrial cytochrome c release and activation of the initiator caspase‐9.( 5 , 8 , 9 ) Apart from these two major pathways, mitochondrial membrane permeabilization also leads to the release of apoptosis‐inducing factor (AIF), which induces caspase‐independent cell death.( 10 ) Recently, Sichtig et al. reported that PBF is an inducer of cell death and that 14‐3‐3 regulates the localization of PBF and PBF‐induced cell death in skin keratinocytes.( 11 ) 14‐3‐3 is associated with apoptotic cell death, the cell cycle, and regulation of various oncogenes and tumor‐suppressor genes.( 12 , 13 ) Moreover, 14‐3‐3 binds to the Bcl‐2 family member Bax and inhibits Bax‐induced cytochrome c release from the mitochondria upon apoptotic stimuli.( 14 ) Although these findings suggest a certain function of PBF as an apoptosis regulator, the function of PBF in osteosarcoma cells remains unknown.
In the present study, we demonstrated that PBF‐induced cell death results from apoptosis via a caspase‐9‐independent pathway. Next, we identified Scythe/BAT3( 15 , 16 ) as a PBF‐associated molecule using a yeast two‐hybrid system. Finally, we showed that PBF‐induced apoptotic cell death is inhibited by Scythe/BAT3 colocalized to the nuclei of osteosarcoma cells. Taken together, these findings suggest that both PBF and Scythe/BAT3 might play important roles in apoptosis of osteosarcoma cells.
Materials and Methods
The present study was approved under institutional guidelines for the use of human subjects in research. The patients and their families gave informed consent for the use of tissue specimens in our research.
Cell lines. The osteosarcoma cell lines OS2000, HOS, Saos‐2, and U2OS, as well as the human embryonic kidney cell lines 293EBNA and 293T, were used. 293EBNA and 293T cells did not express endogenous PBF protein (data not shown). OS2000 was established in our laboratory.( 17 ) The other cell lines were purchased from American Type Culture Collection (Manassas, VA, USA).
Antibodies. The mouse monoclonal antibodies used in the present study were anti‐cleaved poly(ADP‐ribose) polymerase (PARP) (D216, 1:1000 dilution; Cell Signaling, Danvers, MA, USA), anti‐caspase‐9 (clone 5B4, :1000 dilution; MBL International, Woburn, MA, USA), anti‐β‐actin (clone AC‐15, 1:1000 dilution; Sigma‐Aldrich, St Louis, MO, USA), anti‐myc (clone 9E10, 1:1000 dilution; American Type Culture Collection), and anti‐human influenza hemagglutinin (HA) (HA‐7, 1:10 000 dilution and 1:2000 in western blotting and immunofluorescence analysis, respectively; Sigma‐Aldrich, St Louis, MO, USA). Rabbit polyclonal antibodies used were anti‐Scythe/BAT3, kindly provided by Dr Peter J. McKinnon (St Jude Children's Research Hospital, Minneapolis, TN, USA; #23, used at 1:8000 dilution and 1:200 in western blotting and immunofluorescence analysis, respectively),( 18 ) and anti‐PBF (used at 1:800 dilution and 1:200 in western blotting and immunofluorescence analysis, respectively; SigmaGenosys, Sapporo, Japan).( 2 )
Screening of a cDNA library using the yeast two‐hybrid system. A cDNA library was constructed from OS2000 mRNA using a FastTrack 2.0 mRNA Isolation Kit (Invitrogen, Carlsbad, CA, USA) and the Superscript Choice System (Invitrogen). The cDNA was ligated to EcoRI–XhoI adapters and digested with XhoI. The resultant cDNA was cloned into the pACT2 vector (Clontech Laboratories, Mountain View, CA, USA). Recombinant plasmids were electroporated into Electromax DH10B cells (Invitrogen) and selected with ampicillin (100 µg/mL). The resultant 30 000 clones were amplified and plasmid DNA was extracted using a Qiagen Plasmid Maxi Kit (Qiagen, Hilden, Germany). Full‐length PBF subcloned into the pGBKT7 vector (Clontech) was used as bait to screen the cDNA library. Screening of the cDNA library was carried out according to the Mammalian Matchmaker Two‐hybrid Assay Kit protocol (Clontech).
Reverse transcription–polymerase chain reaction analysis. Expression of Scythe/BAT3 was determined by reverse transcription–polymerase chain reaction (PCR). Normal‐tissue cDNA was purchased (Multiple Tissue cDNA Panels; Clontech). Total RNA was extracted from nine osteosarcoma biopsy specimens and reverse transcribed. PCR was carried out with KOD dash DNA polymerase (Toyobo, Tokyo, Japan), using the forward primer (XhoI‐Scythe/BAT3‐FW) 5′‐GGGGGGCTCGAGAAATGGAGCCTAATGATAGTACCAGT‐3′ and the reverse primer (Scythe/BAT3‐NotI‐Rv) 5′‐AAAAAAGCGGCCGCCTAAGGATCATCAGCAAAGGCCCGC‐3′. The mixture was denatured at 98°C for 2 min, followed by 30 cycles at 98°C for 15 s, 56°C for 2 s, and 74°C for 3 min. Reaction products were analyzed by electrophoresis on 1.0% agarose gels with ethidium bromide. Full‐length Scythe/BAT3 cDNA, amplified from OS2000 cDNA by PCR, was digested with XhoI and NotI and subcloned into the pCMV‐HA tag vector (Clontech).
Transfection. 293EBNA or 293T cells were transfected with the indicated cDNA or small interfering RNA (siRNA) using Lipofectamine 2000 (Invitrogen) in Dulbecco's modified Eagle's medium containing 10% fetal calf serum in six‐well plates according to the manufacturer's protocol. OS2000 or U2OS cells were transfected with cDNA using Cell Line Nucleofector Kit V (Amaxa, Cologne, Germany) according to the manufacturer's protocol.
Western blotting. Cell lines and biopsy specimens were homogenized and suspended in ice‐cold Nonidet P‐40 (NP‐40) buffer for 20 min as described previously.( 19 ) The lysates were mixed with 2× sample buffer and boiled for 5 min. Then the lysates were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) on 7 or 13% gels and transferred to polyvinylidene fluoride membranes (Millipore, Billercia, MA, USA). The membranes were blocked and probed with the indicated antibodies for 40 min at room temperature. The membranes were then stained with peroxidase‐labeled secondary antibody and visualized using an enhanced chemiluminescence detection system (Amersham Life Science, Arlington Heights, IL, USA).
Silencing of Scythe/BAT3. Three Stealth siRNA targeting Scythe/BAT3 and a negative control siRNA were purchased from Invitrogen. Three micrograms of each siRNA was transfected into 293EBNA cells and OS2000 cells as described above. After screening of their silencing effect using western blotting (data not shown), one of the three siRNA (BAT3‐HSS111846) was selected for further analysis.
Immunoprecipitation. The cells were lysed on ice with NP‐40 lysis buffer. Lysates were precleared with protein A–sepharose beads (Phadia, Uppsala, Sweden) before immunoprecipitation. The anti‐HA‐7 monoclonal antibody and aliquots of lysates were mixed and incubated at 4°C for 24 h. Immunoprecipitates were recovered from the mixture using protein A–sepharose beads, washed three times with phosphate‐buffered saline (PBS) containing 0.1% NP‐40, mixed with SDS‐PAGE gel electrophoresis running buffer, and boiled for 5 min. Supernatants containing immunoprecipitates were fractionated on 13% SDS‐PAGE, dried, and exposed to X‐ray films with an intensifying screen.
Immunofluorescence analysis. OS2000, U2OS and 293T cells cultured on glass coverslips (Asahi Techno Glass, Tokyo, Japan) were fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton X‐100. After washing with PBS, cells were incubated with the polyclonal anti‐PBF antibody and monoclonal anti‐HA antibody to detect the subcellular localization of PBF protein and HA‐tagged Scythe/BAT3 protein. The monoclonal anti‐myc antibody and polyclonal anti‐Scythe/BAT3 antibody were used to detect exogenous myc‐tagged PBF protein and endogenous Scythe/BAT3 protein. 4′,6‐Diamidine‐2′‐phenylindole dihydrochloride (DAPI) was used for counterstaining nuclei. After washing with PBS, cells were immunostained with secondary antibodies (Alexa Fluor 488‐ or Alexa Fluor 594‐conjugated goat anti‐mouse or anti‐rabbit antibodies; Invitrogen). After staining, cells were visualized by confocal laser microscopy (R2100AG2; Bio‐Rad Laboratories, Hercules, CA, USA). Formalin‐fixed paraffin‐embedded sections of one osteosarcoma biopsy specimen and autopsy specimens of liver, pancreas, and kidney (obtained from a brain infarction patient) were deparaffinized and incubated with the polyclonal anti‐Scythe/BAT3 antibody overnight at 4°C. After washing with PBS, cells were immunostained with Alexa Fluor 488‐conjugated goat anti‐rabbit IgG, followed by visualization using confocal laser microscopy. DAPI was used for counterstaining of nuclei.
Lactate dehydrase (LDH) release assay. 293EBNA, 293T or OS2000 cells were transfected with cDNA as described above. After a 24‐h incubation period at 37°C, the supernatant was replaced with AIM‐V. The amount of LDH in the supernatant was measured by colorimetric assay using an LDH Cytotoxicity Detection Kit (Takara, Ohtsu, Japan) after an additional 24 h incubation period. LDH release was calculated using the following equation: LDH release =([sample release] – [spontaneous release]).
Caspase‐3 colorimetric assay. 293EBNA cells were transfected with cDNA as described above. After a 24‐h incubation period at 37°C, the amount of active caspase‐3 in the supernatant was measured by colorimetric assay using an APOPCYTO Caspase‐3 Colorimetric Assay Kit (Medical and Biological Laboratories, Nagoya, Japan) according to the manufacturer's protocol.
Statistical analysis. Significant differences among samples in the LDH release assay and caspase‐3 colorimetric assay were determined by Welch's t‐test using StatMate III for Macintosh v3.10 (ATMS, Tokyo, Japan). A probability of less than 0.05 was considered statistically significant.
Results
Overexpression of PBF induces apoptotic cell death via a caspase‐independent pathway. To investigate the function of PBF as an apoptosis regulator, we analyzed the cell death and apoptotic events that occurred in 293EBNA cells transfected transiently with PBF. As shown in Figure 1a, overexpression of PBF induced cell death‐mediated LDH release from 293EBNA and OS2000 cells. As depicted in Figure 1b, the proportions of mock‐induced and PBF‐induced cell death in 293EBNA cells were 10.6 ± 1.02 and 27.3 ± 8.57%, respectively. Next, we examined whether PBF overexpression could induce major intrinsic apoptosis via a caspase‐9‐dependent pathway.( 5 ) Overexpressed PBF and cleaved PARP, which is a classical substrate for caspase‐3 during the end stage of the caspase cascade, were detected from 12 h after transfection, followed by an increase in cleaved PARP products (Fig. 1d). Moreover, overexpressed PBF also activated caspase‐3 (Fig. 1c). However, overexpression of PBF in 293EBNA cells did not affect caspase‐9 activity (Fig. 1e). These results suggest that overexpression of PBF could induce apoptotic cell death via a capsase‐9‐independent pathway.
Figure 1.
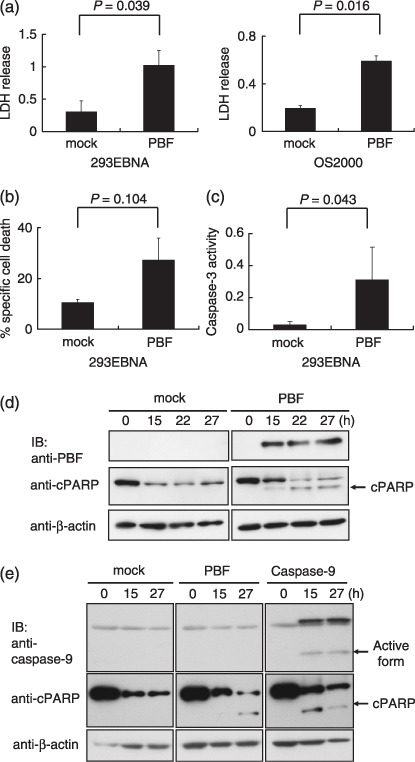
Apoptotic cell death induced by overexpressed papillomavirus binding factor (PBF). (a) LDH release from 293EBNA cells and OS2000 cells transiently transfected with PBF‐myc/pcDNA3.1+ or pcDNA3.1+ as mock transfection. (b) The proportion of cell death in 293EBNA cells transiently transfected with PBF‐myc/pcDNA3.1+ or mock transfection. (c) Expression of PBF and cleaved poly(ADP‐ribose) polymerase (cPARP) in 293EBNA cells was assessed from 0 to 27 h after transient transfection with PBF‐myc/pcDNA3.1+ or mock transfection. (d) Caspase‐3 activity in 293EBNA cells transiently transfected with PBF‐myc/pCMV or pCMV as mock transfection. (e) Expression of PBF, caspase‐9, and cPARP in 293EBNA cells was assessed after transient transfection with the indicated plasmids. IB, immunoblotting.
Identification of PBF‐associated molecules in osteosarcoma cells. For identification of PBF‐associated molecules as apoptotic regulators in osteosarcoma cells, we screened a cDNA library constructed from OS2000 mRNA using a yeast two‐hybrid system. After screening of 30 000 cDNA clones, 106 positive clones were identified. Among them, cDNA clone 93 (Fig. 2a) was identical to the longest open reading frame of Scythe/BAT3. Clone 93 contained 852 bp of the 3′ sequence of Scythe/BAT3 with deletion of the 5′ sequence. There were no frameshifts or point mutations. Subsequently, the association of PBF with Scythe/BAT3 was confirmed by coimmunoprecipitation experiments in mammalian cells. As depicted in Figure 2b, exogenous myc‐tagged PBF was precipitated specifically with the anti‐HA antibody from the cell lysate of 293T cells cotransfected with myc‐tagged PBF and HA‐tagged Scythe/BAT3.
Figure 2.
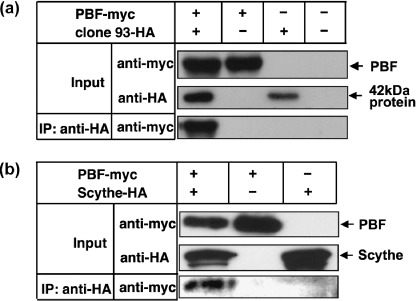
Association of papillomavirus binding factor (PBF) and Scythe/BAT3 in mammalian cells. Lysates of 293T cells transiently transfected with the indicated plasmids (PBF‐myc/pCMV, Scythe/BAT3‐HA/pCMV, and mock plasmid) were immunprecipitated with an anti‐HA monoclonal antibody and immunoblotted with an anti‐myc monoclonal antibody. Expression of each transfected cDNA in the input lysate was assessed by western blotting with anti‐HA or anti‐myc monoclonal antibodies. IP, immunoprecipitation.
Expression and subcellular localization of Scythe/BAT3 in osteosarcoma and normal tissues. Expression of Scythe/BAT3 mRNA was analyzed by reverse transcription‐PCR. Scythe/BAT3 mRNA was detected in five of nine primary osteosarcoma tissues, both osteosarcoma cell lines examined, and ubiquitously in various normal tissues (Fig. 3a,b). Comparing the expression levels in osteosarcoma cell lines, mRNA expression in primary osteosarcoma tissue seemed to be lower. Next, expression of Scythe/BAT3 protein was assessed by western blotting and immunofluorescence analysis. Although Scythe/BAT3 protein was similarly detected in all four osteosarcoma cell lines and 293EBNA cells (Fig. 4a), the subcellular localization of Scythe/BAT3 was clearly different between osteosarcoma and normal tissues (Fig. 4b). In normal liver, pancreas, and kidney, Scythe/BAT3 was localized to the cytoplasm, not the nucleus. In contrast, Scythe/BAT3 was localized to both the cytoplasm and nucleus in one osteosarcoma tissue.
Figure 3.
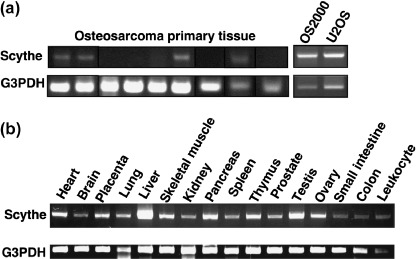
Expression of Scythe/BAT3 mRNA by reverse transcription–polymerase chain reaction analysis. The expression of Scythe/BAT3 was determined in (a) nine osteosarcoma primary tissues and two osteosarcoma cell lines and (b) 16 adult normal tissues. The glyceraldehyde‐3‐phosphate dehydrogenase (G3PDH) housekeeping gene was used as a positive control.
Figure 4.
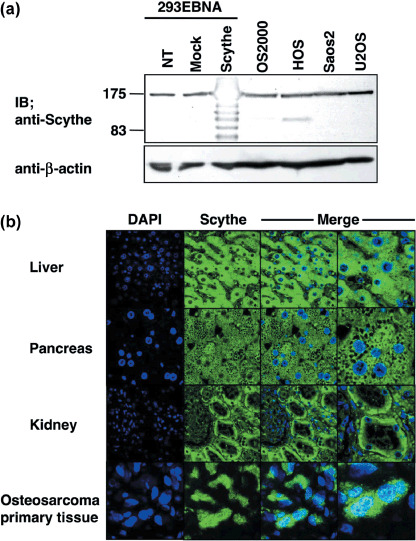
Expression of Scythe/BAT3 protein. (a) Lysates of 293EBNA cells transiently transfected with the indicated plasmids and four osteosarcoma cell lines were immunoblotted with an anti‐Scythe/BAT3 antibody. (b) Immunofluorescence analysis. Formalin‐fixed paraffin‐embedded sections of liver, pancreas, kidney, and an osteosaroma primary tissue were stained with an anti‐Scythe/BAT3 antibody and assessed by confocal laser microscopy. DAPI, 4′,6‐diamidine‐2′‐phenylindole dihydrochloride; IB, immunoblotting; NT, no treatment.
Colocalization of PBF and Scythe/BAT3 in the nucleus suppresses PBF‐induced cell death in osteosarcoma cells. PBF has been reported as a nuclear‐shuttling transcription factor, and the subcellular localization of PBF might be important to regulate apoptotic function.( 11 ) Therefore, we analyzed the colocalization of PBF and Scythe/BAT3 in 293T, OS2000, and U2OS cells. As shown in Figure 5a, immunofluorescence analysis revealed that exogenous PBF and exogenous Scythe/BAT3 were colocalized to the cytoplasm in 293T cells. However, the majority of both of these proteins was colocalized to the nucleus in OS2000 and U2OS cells. We also observed that exogenous PBF and Scythe/BAT3 were similarly colocalized to the nucleus in OS2000 and U2OS cells (Fig. 5b).
Figure 5.
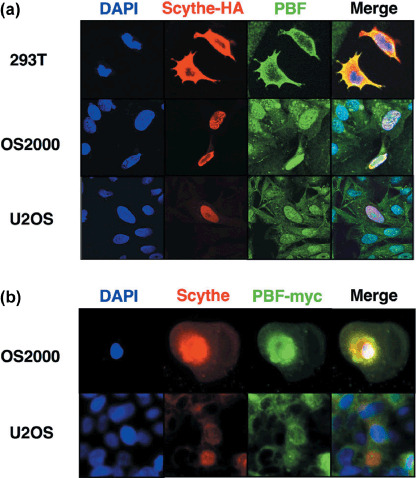
Cellular localization of papillomavirus binding factor (PBF) and Scythe/BAT3. (a) Immunofluorescence analysis using anti‐PBF antibody and anti‐HA antibody. Upper column: 293T cells were transiently transfected with PBF‐myc/pCMV and Scythe/BAT3‐HA/pCMV. 4′,6‐Diamidine‐2′‐phenylindole dihydrochloride (DAPI) was used for counterstaining of nuclei. Middle and lower columns: OS2000 (middle) or U2OS (lower) cells were transiently transfected with Scythe/BAT3‐HA. (b) Immunofluorescence analysis using anti‐myc antibody and anti‐Scythe/BAT3 antibody. OS2000 (upper) or U2OS (lower) cells were transiently transfected with PBF‐myc. DAPI was used for counterstaining of nuclei.
In Figure 1 we already showed that overexpressed PBF induced apoptotic cell death. Therefore, we assessed the apoptosis regulatory function of Scythe/BAT3 in non‐cancerous and osteosarcoma cells. As shown in Figure 6a, overexpression of Scythe/BAT3 did not affect cell death events that resulted from overexpression of PBF in 293EBNA cells. However, Scythe/BAT3 significantly suppressed PBF‐induced cell death events in OS2000 cells (Fig. 6b). Next, we assessed the effects of the downregulation of Scythe/BAT3 on PBF‐induced cell death using siRNA. In 293EBNA cells, the downregulation of Scythe/BAT3 did not change cell death events, the same as with overexpression of Scythe/BAT3 (Fig. 6c). In contrast, downregulation of Scythe/BAT3 significantly increased LDH release in OS2000 with the mock control but did not change it in OS2000 with PBF (Fig. 6d). The expression status of transfected PBF‐myc, Scythe/BAT3‐HA, and endogenous Scythe/BAT3 was confirmed by western blotting, as shown in Figure 6e,f. Considering these observations, we can briefly summarize the relationship between PBF and Scythe/BAT3 in 293EBNA and OS2000 cells as follows: (i) PBF expression in the nuclei of OS2000 cells could induce apoptosis, like PBF in the cytoplasm of 293EBNA cells; (ii) nuclear PBF‐induced apoptosis in OS2000 cells could be suppressed by colocalization of Scythe/BAT3; and (iii) in contrast, cytoplasmic PBF‐induced apoptosis in 293EBNA cells could not be suppressed by colocalization of Scythe/BAT3. Taken together, these results suggest that colocalization of PBF and Scythe/BAT3 in the nucleus, which was observed in OS2000 cells, could regulate PBF‐induced cell death and might be an important factor in the survival of osteosarcoma cells.
Figure 6.
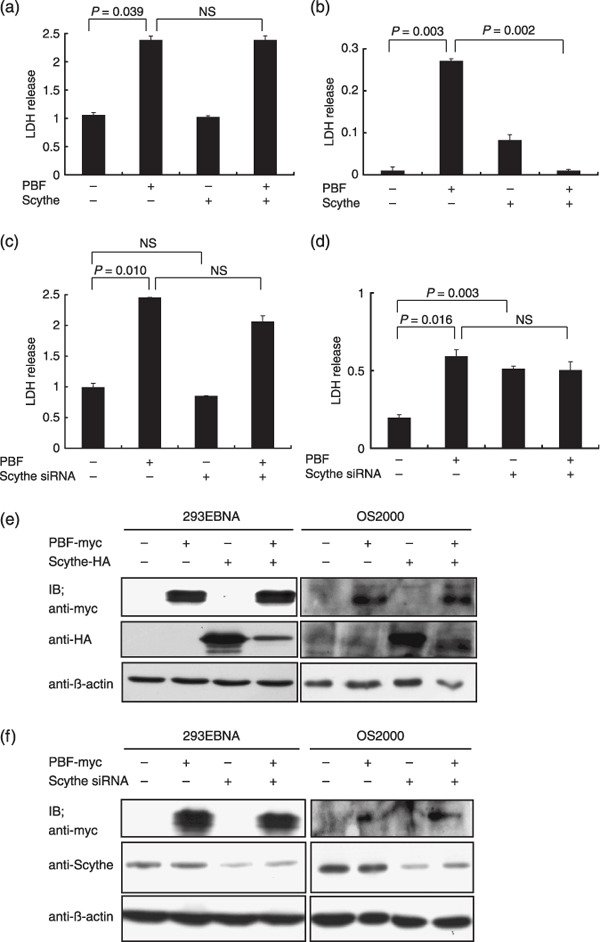
Effects of papillomavirus binding factor (PBF) and Scythe/BAT3 on apoptotic cell death. (a) 293EBNA or (b) OS2000 cells were transfected with PBF‐myc/pCMV, Scythe/BAT3‐HA/pCMV, or both plasmids. pCMV was used for mock transfection. LDH release into the supernatant from transfected cells (resulting from cell death) was assessed. (c) 293EBNA or (d) OS2000 cells were transfected with PBF‐myc/pCMV, small interfering RNA (siRNA) silencing Scythe/BAT3 or PBF‐myc/pCMV and Scythe/BAT3‐HA/pCMV PCMV and control siRNA were used for mock transfection. (e) Transfected PBF‐myc and Scythe/BAT3‐HA and (f) endogenous Scythe/BAT3 that was downregulated by siRNA in 293EBNA and OS2000 cells were detected. NS, not significant
Discussion
In the present study, we demonstrated that: (i) overexpressed PBF could induce apoptotic cell death via a caspase‐9‐independent pathway; (ii) Scythe/BAT3 was identified as a molecule associated with PBF after screening of a cDNA library of OS2000 using a yeast two‐hybrid system; (iii) Scythe/BAT3 mRNA was detected in 56% of osteosarcoma primary tissues and ubiquitously in various normal organs; (iv) Scythe/BAT3 protein was localized to the nuclei of osteosarcoma tissue but the cytoplasm of normal tissues; and (v) PBF‐induced apoptotic cell death was suppressed by colocalization of Scythe/BAT3 with PBF in the nuclei of OS2000 but not in the cytoplasm of 293 cells. These findings suggest that Scythe/BAT3 might play a role in regulating PBF‐mediated apoptosis and that the proliferation of osteosarcoma cells is also regulated by PBF and Scythe/BAT3 that colocalize to the nucleus.
Papillomavirus binding factor has been identified as a transcription factor of a promotor in the human papillomavirus type 8 genome,( 1 ) a Huntington's disease‐associated protein,( 20 ) and a tumor‐associated antigen overexpressed in various sarcomas and carcinomas.( 2 , 21 ) Thereafter, Sichtig et al. reported that overexpression of PBF in the cytoplasm induces cell death and the subcellular localization of PBF is regulated by 14‐3‐3, which is a cytoplasmic protein, and could promote cell survival in a skin keratinocyte cell line.( 11 , 22 ) We also confirmed that overexpression of PDF was localized in cytoplasm of 293 cells and induces cell death, as shown in our current study.
Generally, endogenous cell stress, which is evoked by subcellular expression of apoptotic molecules, induces the intrinsic mitochondrial pathway for apoptosis induction, including the mitochondrial release of cytochrome c, which binds to apoptosis protease‐activating factor‐1. This complex, which is called the apoptosome, activates caspase‐9, triggering downstream caspase‐3.( 5 , 8 ) However, as shown in our current study, the overexpression of PBF induces apoptosis, indicated by caspase‐3 activity and cleavage of PARP, and resulted in cell death, but did not activate caspase‐9. This suggests that overexpression of PBF induces apoptosis via a caspase‐9‐independent pathway. Therefore, we screened PBF‐binding molecules using a yeast two‐hybrid system to identify apoptotic regulators of PBF, and isolated Scythe/BAT3.
Scythe has been identified as a nuclear protein regulating apoptosis, associated with Reaper in Drosophila melanogaster,( 15 ) and BAT3 has been identified as a human homolog of Scythe.( 16 ) Scythe/BAT3 is required for acetylation of p53 in response to DNA damage( 23 ) and drug‐induced apoptosis.( 18 ) However, Scythe/BAT3 was reported to be an anti‐apoptotic protein.( 24 , 25 ) Scythe/BAT3 is essential for cell proliferation,( 18 ) can suppress apoptotic activity of the Xenopus laevis elongation factor 1α oocyte form (XEF1AO), and might control development in Xenopus embryos.( 26 ) In addition, Scythe/BAT3 contains a Bcl‐2‐associated athanogene (BAG) domain at the C‐terminus and inhibits heat shock protein 70‐mediated protein refolding.( 27 ) In fact, Scythe/BAT3 and heat shock protein 70 are cochaperones of human small glutamine‐rich tetratricopeptide repeat (TRP)‐containing protein, which is an essential molecule for cell division.( 28 ) However, the functions of Scythe/BAT3 in tumor cells remain unknown.
In the present study, PBF‐induced cell death was suppressed by overexpressed Scythe/BAT3 in nuclei of osteosarcoma cells. However, overexpressed Scythe/BAT3 localized to the cytoplasm did not suppress PBF‐induced cell death in spite of colocalization of PBF and Scythe/BAT3. These findings suggest that overexpression of Scythe/BAT3 functions as an anti‐apoptotic factor in tumor cells. Expression of Scythe/BAT3 at the endogenous normal level is required for signaling apoptosis but its overexpression induces an anti‐apoptotic effect via upregulation of Bcl‐2.( 24 ) Recently, Scythe/BAT3 was shown to stabilize and interact with AIF,( 29 ) which can lead to chromatin condensation and DNA fragmentation by its relocation from the mitochondria to the nucleus.( 10 ) In severe DNA damage, PARP‐1 activation triggers AIF translocation from mitochondria to the nucleus, followed by mitochondrial depolarization, release of cytochrome c, and activation of caspase‐3, which cleaves PARP.( 30 , 31 ) Moreover, overexpression of Bcl‐2, which is upregulated by overexpression of Scythe/BAT3,( 24 ) also suppresses AIF release from mitochondria.( 30 ) Thus, it appears that overexpression of PBF might induce apoptotic cell death via PARP‐1 and an AIF‐dependent pathway, followed by mitochondrial release of caspase‐3 cleaving PARP‐1. Then Scythe/BAT3‐mediated suppression of PBF‐induced cell death might result from inhibition of AIF translocation to the nucleus by Bcl‐2 upregulated via Scythe/BAT3 overexpression.
Regarding whether the normal level of Scythe/BAT3 could act as an anti‐apoptotic factor, we observed that silencing of Scythe/BAT3 significantly increased LDH release in OS2000 with the mock control but did not change it in OS2000 with PBF. Although we predicted that silencing of Scythe/BAT3 would increase cell death events induced by PBF, we thought that natural PBF expression might be the reason why there was no difference between OS2000 with the mock control and OS2000 with PBF in the condition of silencing of Scythe/BAT3. However, we also observed that silencing of Scythe/BAT3 did not change LDH release in the case of 293EBNA cells. These results suggest that natural cytoplasmic Scythe/BAT3 could not suppress PBF‐induced apoptosis in 293EBNA cells, as was also the case with the overexpressed cytoplasmic Scythe/BAT3.
Our results support the idea that Scythe/BAT3, as well as PBF, could act as a nucleus–cytoplasm shuttling protein regulating apoptosis. Tumor suppressors, such as p53, Forkhead BOX, and phosphatase and tensin homolog, have been reported to shuttle molecules between the nucleus and cytoplasm regulated by mono‐ubiquitylation.( 32 ) The localization of these proteins plays a key role in tumor suppression. Nucleus–cytoplasm shuttling of proteins is generally considered an important mechanism for apoptotic regulation.( 33 , 34 )
We previously reported that PBF is expressed in the nucleus of primary osteosarcoma tissues and that PBF‐positive osteosarcoma and Ewing's sarcoma have significantly poor prognoses.( 3 , 35 ) Although these findings suggest strongly that PBF regulates the proliferation of osteosaroma cells in humans, it is still unknown how PBF regulates cell death and proliferation in vivo. Scythe/BAT3 is a possible factor modulating the function of PBF in vitro. Thus, further studies including PBF‐depletion in vitro and in vivo should be considered.
In conclusion, we identified Scythe/BAT3 as a molecule associated with PBF. We have also demonstrated that overexpression of Scythe/BAT3 regulates PBF‐induced apoptotic cell death in osteosarcoma cells. Moreover, colocalization of PBF and Scythe/BAT3 in the nucleus might be an important factor for survival of osteosarcoma cells.
Acknowledgments
The authors thank Dr Peter J. MacKinnon, Department of Genetics and Tumor Cell Biology, St Jude Children's Research Hospital, for the kind donation of the anti‐Scythe/BAT3 antibody and Dr Ryosuke Minami, Department of Biochemistry, Graduate School of Pharmaceutical Sciences, Hokkaido University, for his helpful discussions and kind support. This work was supported by Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 16209013 to N. Sato), Practical Application Research from the Japan Science and Technology Agency (grant no. H14‐2 to N. Sato), the Ministry of Health, Labor and Welfare (grant no. H17‐Gann‐Rinsyo‐006 to T. Wada), Postdoctoral Fellowship of the Japan Society for the Promotion of Science (grant no. 02568 to T. Tsukahara), Northern Advancement Center for Science and Technology (grant no. H18‐Waka‐075 to T. Tsukahara), and The Uehara Memorial Foundation (grant no. H19‐Kenkyu‐Syorei to T. Tsukahara).
References
- 1. Boeckle S, Pfister H, Steger G. A new cellular factor recognizes E2 binding sites of papillomaviruses which mediate transcriptional repression by E2. Virology 2002; 293: 103–17. [DOI] [PubMed] [Google Scholar]
- 2. Tsukahara T, Nabeta Y, Kawaguchi S et al . Identification of human autologous cytotoxic T‐lymphocyte‐defined osteosarcoma gene that encodes a transcriptional regulator, papillomavirus binding factor. Cancer Res 2004; 64: 5442–8. [DOI] [PubMed] [Google Scholar]
- 3. Tsukahara T, Kawaguchi S, Torigoe T et al . Prognostic impact and immunogenicity of a novel osteosarcoma antigen, papillomavirus binding factor, in patients with osteosarcoma. Cancer Sci 2008; 99: 368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fletcher CDM, Van Den Berg E, Molenaar WM. Pathology and genetics of tumors of soft tissue and bone. In: Fletcher CDM, Unni KK, Mertens F, eds. World Health Organization Classification of Tumors. Lyon: IARC Press, 2002; 120–2. [Google Scholar]
- 5. Takayama S, Reed JC, Homma S. Heat‐shock proteins as regulators of apoptosis. Oncogene 2003; 22: 9041–7. [DOI] [PubMed] [Google Scholar]
- 6. Yin XM, Ding WX. Death receptor activation‐induced hepatocyte apoptosis and liver injury. Curr Mol Med 2003; 3: 491–508. [DOI] [PubMed] [Google Scholar]
- 7. MacFarlane M, Williams AC. Apoptosis and disease: a life or death decision. EMBO Rep 2004; 5: 674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol 2007; 8: 405–13. [DOI] [PubMed] [Google Scholar]
- 9. Beere HM. Death versus survival: functional interaction between the apoptotic and stress‐inducible heat shock protein pathways. J Clin Invest 2005; 115: 2633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Susin SA, Lorenzo HK, Zamzami N et al . Molecular characterization of mitochondrial apoptosis‐inducing factor. Nature 1999; 397: 441–6. [DOI] [PubMed] [Google Scholar]
- 11. Sichtig N, Silling S, Steger G. Papillomavirus binding factor (PBF)‐mediated inhibition of cell growth is regulated by 14‐3‐3β. Arch Biochem Biophys 2007; 464: 90–9. [DOI] [PubMed] [Google Scholar]
- 12. Hermeking H. 14‐3‐3 proteins and cancer biology. Semin Cancer Biol 2006; 16: 161. [DOI] [PubMed] [Google Scholar]
- 13. Tzivion G, Gupta VS, Kaplun L, Balan V. 14‐3‐3 proteins as potential oncogenes. Semin Cancer Biol 2006; 16: 203–13. [DOI] [PubMed] [Google Scholar]
- 14. Nomura M, Shimizu S, Sugiyama T et al . 14‐3‐3 interacts directly with and negatively regulates pro‐apoptotic Bax. J Biol Chem 2003; 278: 2058–65. [DOI] [PubMed] [Google Scholar]
- 15. Thress K, Henzel W, Shillinglaw W, Kornbluth S. Scythe: a novel reaper‐binding apoptotic regulator. EMBO J 1998; 17: 6135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banerji J, Sands J, Strominger JL, Spies T. A gene pair from the human major histocompatibility complex encodes large proline‐rich proteins with multiple repeated motifs and a single ubiquitin‐like domain. Proc Natl Acad Sci USA 1990; 87: 2374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nabeta Y, Kawaguchi S, Sahara H et al . Recognition by cellular and humoral autologous immunity in a human osteosarcoma cell line. J Orthop Sci 2003; 8: 554–9. [DOI] [PubMed] [Google Scholar]
- 18. Desmots F, Russell HR, Lee Y, Boyd K, McKinnon PJ. The reaper‐binding protein scythe modulates apoptosis and proliferation during mammalian development. Mol Cell Biol 2005; 25: 10 329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamoto M, Torigoe T, Kamiguchi K et al . A novel isoform of TUCAN is overexpressed in human cancer tissues and suppresses both caspase‐8‐ and caspase‐9‐mediated apoptosis. Cancer Res 2005; 65: 8706–14. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka K, Shouguchi‐Miyata J, Miyamoto N, Ikeda JE. Novel nuclear shuttle proteins, HDBP1 and HDBP2, bind to a neuronal cell‐specific cis‐regulatory element in the promoter for the human Huntington's disease gene. J Biol Chem 2004; 279: 7275–86. [DOI] [PubMed] [Google Scholar]
- 21. Tsukahara T, Kawaguchi S, Nagoya S et al . Novel approach to immunotherapy for epithelial cancers, bone and soft‐tissue sarcomas. Ann Cancer Res Ther 2004; 12: 53–70. [Google Scholar]
- 22. Masters SC, Subramanian RR, Truong A et al . Survival‐promoting functions of 14‐3‐3 proteins. Biochem Soc Trans 2002; 30: 360–5. [DOI] [PubMed] [Google Scholar]
- 23. Sasaki T, Gan EC, Wakeham A, Kornbluth S, Mak TW, Okada H. HLA‐B‐associated transcript 3 (Bat3)/Scythe is essential for p300‐mediated acetylation of p53. Genes Dev 2007; 21: 848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu YH, Shih SF, Lin JY. Ricin triggers apoptotic morphological changes through caspase‐3 cleavage of BAT3. J Biol Chem 2004; 279: 19 264–75. [DOI] [PubMed] [Google Scholar]
- 25. Kikukawa Y, Minami R, Shimada M et al . Unique proteasome subunit Xrpn10c is a specific receptor for the antiapoptotic ubiquitin‐like protein Scythe. FEBS J 2005; 272: 6373–86. [DOI] [PubMed] [Google Scholar]
- 26. Minami R, Shimada M, Yokosawa H, Kawahara H. Scythe regulates apoptosis through modulating ubiquitin‐mediated proteolysis of the Xenopus elongation factor XEF1AO. Biochem J 2007; 405: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thress K, Song J, Morimoto RI, Kornbluth S. Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper. EMBO J 2001; 20: 1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winnefeld M, Grewenig A, Schnolzer M et al . Human SGT interacts with Bag‐6/Bat‐3/Scythe and cells with reduced levels of either protein display persistence of few misaligned chromosomes and mitotic arrest. Exp Cell Res 2006; 312: 2500–14. [DOI] [PubMed] [Google Scholar]
- 29. Desmots F, Russell HR, Michel D, McKinnon PJ. Scythe regulates apoptosis inducing factor stability during endoplasmic reticulum stress induced apoptosis. J Biol Chem 2008; 283: 3264–171. [DOI] [PubMed] [Google Scholar]
- 30. Yu SW, Wang H, Poitras MF et al . Mediation of poly(ADP‐ribose) polymerase‐1‐dependent cell death by apoptosis‐inducing factor. Science 2002; 297: 259–63. [DOI] [PubMed] [Google Scholar]
- 31. Aguilar‐Quesada R, Munoz‐Gamez JA, Martin‐Oliva D et al . Modulation of transcription by PARP‐1: consequences in carcinogenesis and inflammation. Curr Med Chem 2007; 14: 1179–87. [DOI] [PubMed] [Google Scholar]
- 32. Salmena L, Pandolfi PP. Changing venues for tumour suppression: balancing destruction and localization by monoubiquitylation. Nat Rev Cancer 2007; 7: 409–13. [DOI] [PubMed] [Google Scholar]
- 33. Wang SC, Hung MC. Cytoplasmic/nuclear shuttling and tumor progression. Ann NY Acad Sci 2005; 1059: 11–15. [DOI] [PubMed] [Google Scholar]
- 34. Chu CT, Plowey ED, Wang Y, Patel V, Jordan‐Sciutto KL. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol 2007; 66: 873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yabe H, Tsukahara T, Kawaguchi S et al . Overexpression of papillomavirus binding factor in Ewing's sarcoma family of tumors conferring poor prognosis. Oncol Rep 2008; 19: 129–34. [PubMed] [Google Scholar]


