Abstract
The aim of the current study was to investigate the role of promoter methylation of adenomatous polyposis coli (APC) and epithelial cadherin (E‐cadherin) genes in endometrial tumorigenesis. The methylation status of both genes was investigated in 43 cases of normal endometrium, 21 simple hyperplasia, 17 atypical hyperplasia, and 86 endometrial carcinoma (EC). Additionally, the methylation pattern of both genes was analyzed in 24 primary ECs and their corresponding metastases. DNA methylation of the APC gene increased from atypical hyperplasia (23.5%) to endometrial carcinoma, reaching its highest level of 77.4% in early stage cancer (FIGO I and II) and decreasing stepwise to 24.2% in advanced stage carcinomas (FIGO III and IV). No methylation of APC was found in normal endometrium or simple hyperplasia. Methylation of E‐cadherin was found only in EC (22.1%). The mean age of the patients with aberrant APC methylation was 68.8 years and was significantly higher compared to the mean age (60.9 years) of the patients without methylation of APC promoter (P = 0.02). APC promoter methylation significantly correlated with decreased protein expression of APC (P = 0.039), with increased expression of the Ki‐67 proliferative marker (P = 0.006) and decreased metastatic potential (P = 0.002). There was no correlation between APC and E‐cadherin methylation patterns and the other clinicopathologic features, nor with patient outcome. Our results suggest that hypermethylation of APC promoter region is an early event in endometrial tumorigenesis. (Cancer Sci 2009)
Abbreviations
- AH
atypical hyperplasia
- APC
adenomatous polyposis coli
- EC
endometrial carcinoma
- E‐cadherin
epithelial cadherin
- FIGO
International Federation of Gynecology and Obstetrics
- MSP
methylation‐specific PCR
- NE
normal endometrium
- PT
primary tumor
- SH
simple hyperplasia
Endometrial carcinoma represents the most frequently diagnosed malignancy of the female genital tract worldwide, but incidence varies among different countries.( 1 ) EC often precedes or coexists with endometrial hyperplasia, proposed to be a possible precursor lesion of EC.( 2 , 3 ) According to the World Health Organization, endometrial hyperplasia is classified into simple and complex hyperplasia, with and without atypia.( 4 , 5 ) Atypical endometrial hyperplasia is considered to be a precursor lesion of EC. Women with AH without concurrent invasive carcinoma have a 30% risk of developing EC.( 2 ) Although two pathways of endometrial carcinogenesis have been proposed,( 6 ) the exact genetic mechanisms remain to be established. Epigenetic changes, such as DNA methylation, regulate gene expression in normal mammalian development. The silencing of tumor suppressor genes associated with promoter hypermethylation is a common feature in human cancers, and serves as an alternative mechanism for loss of tumor suppressor gene function.( 7 )
The APC gene encodes a large multidomain protein that plays an integral role in Wnt signaling and in intracellular adhesion.( 8 ) APC regulates free ß‐catenin levels through glycogen synthetase kinase‐3ß and the ubiquitin‐proteasome pathway.( 8 ) The inactivation of APC results in an accumulation of high levels of ß‐catenin, which induces the Wnt signaling pathway and enhances the transcriptional activity of different target genes and oncogenes.( 9 ) Additionally, binding the cytoplasmic protein ß‐catenin to the intracellular part of E‐cadherin is necessary for its function.( 9 )
E‐cadherin is an adhesion molecule that keeps neighboring cells attached.( 10 ) Impaired function of this molecule can lead to dedifferentiation of the tumor cells and an increase in metastatic potential. The role of aberrations in the Wnt signaling pathway and E‐cadherin in human tumorigenesis has been reported for colorectal, ovarian, and only occasionally for EC.( 11 , 12 , 13 , 14 , 15 , 16 ) Gene mutation is the most important mechanism of APC alterations in colorectal cancer.( 14 , 15 ) Point mutations and gene dys‐regulation of APC have been detected in more than 80% of colorectal cancers.( 15 ) Another way of inactivating the APC gene is the methylation of its promoter region.( 17 , 18 ) The APC gene has two promoter regions, 1A and 1B, that initiate transcription from different sites.( 19 ) Hypermethylation of promoter 1A has been reported in a variety of cancers, whereas promoter 1B remains unmethylated.( 17 , 18 , 20 ) Reports on methylation of APC in uterine tumors are limited, and the level of promoter hypermethylation varied from 22% to 46.6%.( 14 , 20 , 21 ) The aberrant protein expression of E‐cadherin, in some cancers, has also been attributed to methylation of its promoter region.( 21 , 22 , 23 , 24 ) The occurrence of E‐cadherin promoter hypermethylation in human ECs has been reported to be 14% and 15.6%.( 21 , 24 ) However, Pijnenborg et al. ( 25 ) detected E‐cadherin promoter methylation in none of their 28 studied patients at FIGO stage I of the disease. Nevertheless, most of these studies assayed the methylation status of different genes upon cancer tissue only and did not compare it to normal tissue or precursor lesions. To identify the role of APC and E‐cadherin in EC tumorigenesis, as key mediators of the Wnt signaling pathway, we studied the methylation status of the promoter region of both genes in normal endometrial tissue, endometrial atypia, and EC and compared it with the clinical/pathologic variables of the cancer and proliferation index, assessed by Ki‐67 immunostaining. Additionally, the methylation pattern of both genes in primary ECs (n = 24) and corresponding metastatic lesions were analyzed.
Materials and Methods
Patients and tissue samples. Formalin‐fixed, paraffin‐embedded tissues from NE (n = 43), endometrial hyperplasia (SH, n = 21 and AH, n = 17) and EC (n = 86) were collected from patients who had been admitted to the Department of Obstetrics and Gynecology, Otto‐von‐Guericke University (Magdeburg, Germany), the St. Salvator Hospital (Halberstadt, Germany), Staedtisches Klinikum (Magdeburg, Germany), or the Second Department of Gynecology, Lublin Medical University (Lublin, Poland), between 1995 and 2007. Mean patient age was 63 years (range, 35–86 years). Samples were obtained after providing informed consent of the patients.
Surgically removed tissues were forwarded for pathological assessment and DNA isolation was carried out at the Department of Pathology, Otto‐von‐Guericke University. Hormonal therapy, chemotherapy, or radiotherapy was not done before surgery. Twenty‐eight of the ECs revealed metastases, and 24 of them could be prepared simultaneously. In addition, a group of 58 ECs without metastasis were investigated. There were 15 non‐endometrioid and 71 endometrioid‐type tumors. Twenty‐five (29%) were well differentiated (G1), 43 (50%) were moderately differentiated (G2), and 18 (21%) were poorly differentiated (G3). The clinical staging of the disease was classified according to FIGO guidelines. Fifty‐three (61.6%) were of stages I–II, and 33 (38.4%) were of stages III–IV. The surgical protocol consisted of total abdominal hysterectomy/bilateral salpingo‐oophorectomy and pelvic/para‐aortic lymph nodes dissection. Palpation of abdominal organs and collection of peritoneal washings for cytological evaluation were also carried out. In selected cases, omentumectomy, appendectomy, tumor cytoreduction, and/or resection of distant metastases was done.
The clinical records were carefully reviewed to obtain information regarding patient age, menopausal status, symptoms of the disease, surgical treatment protocols, and follow‐up. Follow‐up was calculated on the basis of data obtained between primary diagnosis and last contact or death of the patient. Data on survival were collected from medical records, information obtained from general practitioners, and from parents and their families.
DNA isolation and methylation‐specific PCR. The methylation status of APC and E‐cadherin promoter region was determined by MSP.( 7 ) Tumor tissues were marked and prepared by highly experienced pathologists, from areas with a minimum of 70% tumor cells. Necrotic areas were excluded. Depending on the AH or tumor area, respectively, between two and four 10 μm paraffin slices were used for DNA isolation (NucleoSpin DNA extraction kit; Macherey‐Nagel, Dueren, Germany). The initial bisulfite reaction of conversion of unmethylated cytosines to uracils was achieved using the CpG genome DNA Modification Kit (Chemicon International, Temecula, CA, USA). The MSP was carried out using primers specific to either the modified unmethylated DNA or the methylated DNA. The primers used for APC and E‐cadherin promoters were described originally by Dong et al. ( 26 ) and Herman et al.,( 27 ) respectively. The primer sequences, PCR conditions, size of the amplified region, and the genomic position of the primers are shown in Table 1. The quality of the prepared DNA was checked by PCR quality control using the amplification of the housekeeping gene phenylalanin hydroxylase. The bisulfite reaction was carried out only if the control gene was amplified.( 28 ) The DNA amount was not measured. Positive (methylated standard DNA) and negative controls (placenta DNA from paraffin embedded tissue) were always included. Gels were interpreted only in case of working positive controls. We considered a case as methylated if a methylation‐specific PCR product was visible (Fig. 1). We did not quantify methylation from the silver‐stained poly acrylic acid (PAA) gels. Only a yes/no decision was made, which was interpreted as a pool of cells with methylated promotors. In general, both methylated and unmethylated bands were detectable. We never observed only a methylated band.
Table 1.
Primer sequences, fragment size, annealing temperatures, and number of PCR cycles used for methylation‐specific PCR
| 5′ → 3′ | Size (bp) | Annealing temperature (°C) | Number of PCR cycles | |
|---|---|---|---|---|
| APC unmethylated | F: AATTTGTTGGATGTGGATTAGGGT R: AACCTCATATCAATCACATACA | 89 | 61 | 35 |
| APC methylated | F: CGTTGGATGCGGATTAGGGC R: CCTCATATCGATCACGTACG | 84 | 61 | 35 |
| E‐cadherin unmethylated | F: TAATTTTAGGTTAGAGGGTTATTGT R: CACAACCAATCAACAACACA | 97 | 59 | 37 |
| E‐cadherin methylated | F: TTAGGTTAGAGGGTTATCGCGT R: TAACTAAAAATTCACCTACCGAC | 116 | 61 | 37 |
APC, adenomatous polyposis coli; E‐cadherin, epithelial cadherin; F, forward; R, reverse.
Figure 1.
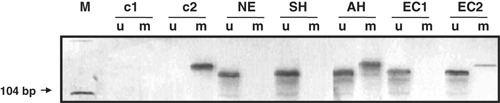
Methylation‐specific PCR with bisulfite‐treated samples of endometrial tissue. AH, atypical endometrial hyperplasia; c1, negative control; c2, positive control; EC1, endometrial carcinoma with absence of methylation of adenomatous polyposis coli (APC) promoter gene; EC2, endometrial carcinoma with methylation of APC promoter gene; m, reactions using primers specific for the methylated CpG sites; M, DNA ladder; NE, normal endometrial tissue; SH, simple endometrial hyperplasia; u, reactions using specific primers for the unmethylated CpG sites.
Immunohistochemistry. To investigate the expression of APC and Ki‐67 proliferation protein, tissue slices of paraffin‐embedded specimens of NE, SH, AH, and EC underwent immunostaining as previously described.( 28 ) Representative regions of SH, AH, and EC were selected on H&E stained slices. Samples were dewaxed by xylene and rehydrated in descending concentrations of ethanol before antigen retrieving in EDTA (pH 8.0) using a DakoCytomation Pascal pressure chamber (120°C, 30 s) (DakoCytomation, Glostrup, Denmark). The process of immunostaining was done with the help of a NexES immunostainer (Ventana Medical Systems, Tucson, AZ, USA). Incubation was carried out overnight at 4°C with mAb against APC protein (Calbiochem, Darmstadt, Germany) in dilution (1:200). The monoclonal mouse antihuman Ki‐67 antibody (dilution, 1:50; DakoCytomation) was incubated for 32 min at 37°C. Detection was carried out using a DAB detection kit (Ventana Medical Systems). All samples were counterstained with hemalum and dehydrated in ascending concentrations of ethanol.
Immunostaining of APC and Ki‐67 was assessed by two independent observers blinded to patient clinical outcome and local staging, displaying negligible inter‐observer differences. An immunoreactive score was compiled semiquantitatively. The immunoreactive score was calculated by multiplying staining intensity and the percentage of positivity. The possible maximum that could be reached was 12 points. Staining intensity was classified as: 0, no staining; 1, weak; 2, moderate; and 3, strong. Percentage of positivity was recorded between 0 and 4 (0, 0%; 1, 1–9%; 2, 10–50%; 3, 51–80%; and 4, 81–100%).
Statistical analysis. The comparisons of groups were made using the χ2‐test or Fisher’s exact test. Survival rates were calculated using the Kaplan–Meier method. Patients who died of other causes were censored at the date of death. The log–rank test was used to compare the survival curves for groups of patients defined by categories of each variable. Tests for interactions were carried out for the variables, with independent impact on survival in the multivariate Cox regression analysis. Data were analyzed using the SPSS 11.0 software package (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Promoter hypermethylation. To evaluate the role of APC and E‐cadherin in primary human endometrial carcinogenesis the methylation status of APC promoter 1A and E‐cadherin gene promoter were investigated by MSP. Promoter hypermethylation of the APC gene was absent in all of the investigated cases of NE and SH (Fig. 2). Four of 17 (23.5%) AH and 49 of 86 (56.9%) EC cases had methylation of APC. APC methylation in EC varied according to the FIGO stage and was expressed in maximal level in stage II and decreased stepwise in stages III and IV (Fig. 2). There was a significant increase of APC promoter methylation from SH (0%) to AH (23.5%; P = 0.040) and from AH to EC stage I (76.3%; P = 0.016), followed by significant decrease from EC stage II (80.0%) to EC stage III (26.1%) (Fig. 2; P = 0.003). Hypermethylation of E‐cadherin was observed in only 19 of 86 (22.1%) cases of EC (Fig. 2) and was absent in NE, SH, and AH.
Figure 2.
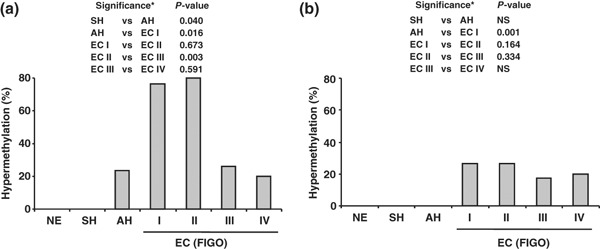
Frequency of adenomatous polyposis coli (APC) (a) and epithelial cadherin (E‐cadherin) (b) promoter hypermethylation in normal endometrial tissue (NE), simple endometrial hyperplasia (SH), atypical endometrial hyperplasia (AH) and endometrial carcinoma (EC) stages I–IV. The International Federation of Gynecology and Obstetrics (FIGO) staging system was applied. *Analyzed by Fisher’s exact test. NS, not statistically significant.
When the methylation status of investigated ECs was compared with their clinical and pathological characteristics (Table 2), APC promoter methylation was observed predominantly in the early stages of EC. Forty‐one out of 53 (77.4%) tumors in stages I and II and only 8 out of 33 (24.2%) specimens in stages III and IV revealed APC methylation (P < 0.0001). There was no significant relationship between APC hypermethylation and histological type of cancer. Although the relationship between histological grading and APC methylation did not reach statistical significance, the hypermethylation increased with loss of tumor differentiation (Table 2). There was a significant reverse correlation between APC methylation and development of metastases. Forty of 58 (69.0%) tumors without metastases and only 9 of 28 (32.1%) EC with metastases revealed an APC methylation (P = 0.002). It is worth noting that APC methylation was found more frequently in elderly patients. The mean age of the patients with aberrant APC methylation was 68.8 years, significantly higher compared to the mean age (60.9 years) of patients without methylation (P = 0.02). There was no significant difference in patient age between E‐cadherin methylated and unmethylated groups (65.2 and 67.3 years, respectively).
Table 2.
Adenomatous polyposis coli (APC) and epithelial cadherin (E‐cad) promoter methylation and Ki‐67 expression in relation to clinical and pathological variables of endometrial carcinoma patients
| Variables | No. of cases, n (86) | APC methylation Methylation, n (%) | E‐cad methylation Methylation, n (%) | Ki‐67 (IRS score) |
|---|---|---|---|---|
| FIGO stages | ||||
| I | 38 | 29 (76.3) | 9 (23.6) | 4.74 |
| II | 15 | 12 (80.0) | 4 (26.7) | 5.02 |
| III | 23 | 6 (26.1) | 4 (17.4) | 4.38 |
| IV | 10 | 2 (20.0) | 2 (20.0) | 4.99 |
| P < 0.0001 | NS | NS | ||
| Histological type | ||||
| Endometrioid | 71 | 42 (59.2) | 18 (25.4) | 4.52 |
| Non‐endometrioid | 15 | 7 (46.7) | 1 (6.7) | 4.86 |
| NS | NS | NS | ||
| Histological grading | ||||
| G1 | 25 | 11 (44.0) | 6 (24.0) | 4.60 |
| G2 | 43 | 24 (55.8) | 9 (20.9) | 4.93 |
| G3 | 18 | 14 (77.8) | 4 (22.2) | 6.94 |
| NS | NS | NS | ||
| Metastases | ||||
| PTs without M | 58 | 40 (68.9) | 13 (22.4) | 4.27 |
| PTs with M | 28 | 9 (32.1) | 6 (21.4) | 4.87 |
| P = 0.002 | NS | NS | ||
FIGO, International Federation of Gynecology and Obstetrics; IRS score, median value of immunohistochemical score of Ki‐67 expression; M, metastases; NS, not significant; PTs, primary tumors.
There was no relationship between aberrant methylation of E‐cadherin promoter and the clinicopathologic features of cancer (Table 2). Survival analysis revealed no prognostic impact for promoter hypermethylation of APC or E‐cadherin genes during the follow‐up. By determining the Pearson correlation coefficient and P value there was no significant correlation between APC and E‐cadherin promoter methylation status.
APC hypermethylation and immunostaining. In order to determine whether APC gene hypermethylation will affect protein expression, we analysed APC protein expression in AH and EC. In all investigated cases of AH and EC, we observed no significant difference among the tissues with methylation of the APC promoter versus cases without methylation (Fig. 3a,b). As mentioned above, the highest level of APC methylation was observed in FIGO stages I and II. Therefore, we investigated the correlation between APC methylation and protein expression in the groups of early and advanced ECs. Interestingly, the level of APC protein expression correlated significantly with APC gene methylation only in the group of early stage carcinoma FIGO I and II (P = 0.039), but not in the advanced stage of the diseases, FIGO III and IV (P = 0.101) (Fig. 3c,d). These results indicate that promoter methylation might be an important mechanism of inactivation of APC in early stage endometrial carcinoma.
Figure 3.
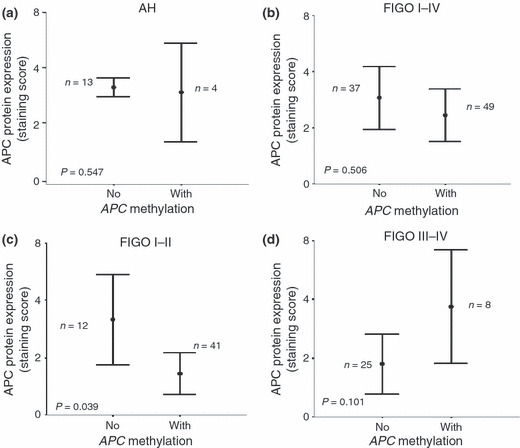
Correlation between adenomatous polyposis coli (APC) hypermethylation and protein expression. (a) APC protein expression did not correlate significantly with the occurrence of APC promoter methylation in atypical endometrial hyperplasia (P = 0.547). (b) No significant correlation was observed between APC immunoexpression and APC promoter hypermethylation in endometrial carcinoma (EC) stages I–IV (P = 0.506). The International Federation of Gynecology and Obstetrics (FIGO) staging system was applied. (c) APC protein expression correlated significantly with the occurrence of APC promoter methylation in FIGO stages I and II of EC (P = 0.039). (d) No significant correlation was observed between APC immunoexpression and APC promoter hypermethylation in advanced‐stage EC, FIGO III and IV (P = 0.101).
Hypermethylation status and Ki‐67 expression. To determine the possible role of APC promoter methylation in cell proliferation, the expression of Ki‐67, a routinely used marker of cell cycling and proliferation, was analyzed immunohistochemically and compared with APC promoter metylation. The expression of Ki‐67 gradually increased from NE through AH to EC. There was no statistically significant difference between Ki‐67 expression and APC methylation in AH (Fig. 4a; P = 0.222), nor between Ki‐67 expression and the clinical/pathologic features of EC (Table 2). Interestingly, there was a highly significant relationship between Ki‐67 immunostaining and methylation of APC promoter only in the early clinical stages of EC (FIGO I and II) (Fig. 4b; P = 0.006). As shown in Figure 4(c), this phenomenon was not observed in the advanced stage uterine neoplasms (P = 0.797). Expression of Ki‐67 did not correlate with the E‐cadherin methylation status in the study group (Fig. 4d; P = 0.437).
Figure 4.
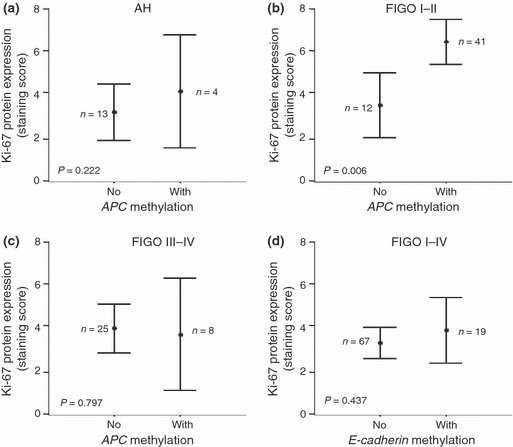
Correlation between hypermethylation and Ki‐67 expression. (a) Ki‐67 protein expression did not correlate significantly with the occurrence of adenomatous polyposis coli (APC) promoter methylation in atypical endometrial hyperplasia (AH) (P = 0.222). (b) Ki‐67 protein expression correlated significantly with the occurrence of APC promoter methylation in stage I and II endometrial carcinomas ECs (P = 0.006). The International Federation of Gynecology and Obstetrics (FIGO) staging system was applied. (c) No significant correlation was observed between Ki‐67 immunoexpression and APC promoter hypermethylation in advanced stage ECs, FIGO III and IV (P = 0.797). (d) No significant correlation was observed between Ki‐67 immunoexpression and epithelial cadherin (E‐cadherin) promoter hypermethylation in ECs (P = 0.437).
Taken together, our results indicate a subset of early‐staged tumors, characterized by APC promoter methylation, which leads to a remarkable increase in cell proliferation activity assessed by Ki‐67 immunostaining.
APC and E‐cadherin hypermethylation in primary ECs and corresponding metastases. As shown in Table 2, PTs without metastases posses significantly higher levels of APC promoter methylation compared to PTs with metastases (P = 0.002). Interestingly, in the subset of PTs without metastases, there was also a significant relationship between APC methylation and Ki‐67 expression (Fig. 5; P < 0.01). This phenomenon was not observed for the subgroup of PTs with metastases (Fig. 5).
Figure 5.
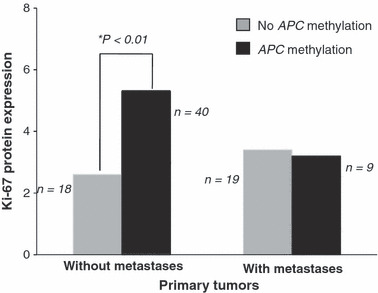
Ki‐67 expression correlated significantly with adenomatous polyposis coli (APC) methylation in primary tumors without metastases (P < 0.01), but did not correlate in primary tumors with metastases.
To assess the potential role of APC and E‐cadherin promoter methylation in the development of EC metastases, we directly compared the methylation patterns of 24 primary uterine carcinomas with the corresponding metastatic lesions. Promoter hypermethylation of APC gene was reported in nine of 24 (37.5%) PTs and in six of 24 (25%) corresponding metastases (Fig. 6). Hypermethylation of E‐cadherin was noted in three of 24 (12.5%) PTs and in eight of 24 (33.3%) metastases (Fig. 6). There was no correlation between the methylation rate of both genes in PTs and corresponding metastatic lesions. There was no significant difference in Ki‐67 protein expression between metastatic tissues with or without promoter methylation of one or both genes.
Figure 6.

Patterns of epigenetic promoter hypermethylation of adenomatous polyposis coli (APC) and epithelial cadherin (E‐cadherin) genes among primary tumors (P) and their corresponding metastases (M). Patients are indicated by their case number in the series. Black boxes indicate a promoter hypermethylation. There was no significant correlation between the methylation rate of APC and E‐cadherin promoters.
Discussion
This investigation shows that APC promoter methylation might be an early and critical event in the development of EC. We found that APC methylation was more common in early stage tumors than in those at advanced stage. Aberrant APC methylation was observed in AH (23.5%) and EC (56.9), but not in NE or SH. Promoter methylation of the E‐cadherin gene was present in 22.1% of EC cases, but not in NE, SH, or AH.
The high frequency of APC aberrant methylation is in line with the limited published data, in which the hypermethylation of the APC gene has been reported to range between 22% and 46.6%.( 14 , 17 , 20 , 25 ) These results clearly show that promoter hypermethylation is an important mechanism of inactivation of APC in human ECs, which has already been shown for colorectal cancer.( 17 , 18 ) A significant and progressive increase of APC promoter methylation was observed between SH and AH, and AH and EC FIGO stage I and II cancers (Fig. 2). The highest percentage of promoter methylation was observed in clinical FIGO stages I and II. This finding confirms the results of Pijnenborg et al.,( 25 ) who assessed high aberrant methylation of APC in FIGO stage I uterine tumors, although their patient cohort was small. In contrast, a stepwise decrease of APC promoter methylation was observed from FIGO stage II to stages III and IV. Moreover, APC gene hypermethylation correlated significantly in the early stage with decreased protein expression of APC and increased expression of the proliferative marker Ki‐67. Therefore, APC promoter hypermethylation might be an important mechanism of APC gene inactivation, with a consequent increase in the proliferative potential of the tumor cells. Considering these results we can speculate that APC promoter hypermethylation might have a role in early stage EC. However, other mechanisms might be also responsible for induction of the EC progression to a late stage. Recent results have indicated that APC methylation decreases with an increase in the clinical stage and with a relative increase in APC methylation from AH to EC.( 21 ) Zysman et al. have also suggested the role of APC promoter methylation in endometrial tumorigenesis.( 20 ) However, further investigations are needed to answer this important question. Our results revealed only a specific correlation between APC hypermethylation and the early stage of EC. This fact can also explain the missing correlation between APC methylation and patient survival in Kaplan–Meier curves, revealing worse outcome in late stages of disease (data not shown). The fact that APC promoter methylation was observed most frequently in PTs without metastases suggested the possibility that some primary tumors, which lack hypermethylation of APC promoter, selectively progress to a later stage of the disease. However, the inactivation of APC promoter gene by hypermethylation might also be an effective and necessary mechanism in the early stage of EC with a consequent increase of the potentially proliferative activity of the cancer cells and tumor progression. This heterogeneity might increase the capability of the tumor to answer to different environmental challenges and most likely is a feature of selective advantage in tumor progression.
To check the proliferation activity of the tumor, Ki‐67 protein expression was investigated and correlated with the aberrant methylation of APC in AH and EC. Ki‐67 antigen is a prototypic cell cycle‐related nuclear protein, expressed by proliferating cells in all phases of the active cell cycle (G1, S, G2, and M) and being absent in resting (G0 and early G1) cells.( 29 ) This protein represents a proliferation marker establishing the growing fraction of cells in neoplasms.( 30 ) Previously, Ki‐67 expression was related to histological grading and myometrial invasion of ECs,( 31 ) and has been shown to increase from NE tissue through hyperplasia to EC.( 32 ) This was confirmed in our tumor cohort, in which we found an increase in Ki‐67 immunoexpression with an increasing histological grade (Table 2). However, this correlation did not reach a significant level. As shown in Figure 4, we revealed a significant positive correlation between Ki‐67 expression and aberrant methylation of the APC gene. Interestingly, this applied only to early stage ECs (FIGO stages I and II; P = 0.006). We can speculate that the APC promoter hypermethylation might lead to a stimulation of the proliferation potential of endometrial carcinoma cells.
E‐cadherin is an epithelial‐specific cell adhesion molecule and is considered to play a significant role in the process of development of metastases in different human neoplasms. The role of E‐cadherin in the metastatic potential of EC has already been reported.( 24 , 33 , 34 ) In the current study, hypermethylation of E‐cadherin promoter region was observed in 22.1% of primary uterine neoplasms and did not correlate with any of the clinicopathologic characteristics of the tumors (Table 2). The low rate of E‐cadherin promoter methylation is comparable to that reported by others.( 21 , 24 ) However, Blechschmidt et al. described reduced immunoreactivity of this cell adhesion protein in 44.8% of primary ECs and in 65.4% of metastases.( 33 ) Scholten and co‐workers found a loss of E‐cadherin adhesion expression in 44% of uterine tumors.( 35 ) Given the fact that molecular mechanisms, other than methylation, can cause protein loss, these results should definitively not be interpreted as diametrically opposed to ours. The small sample size in our analysis compromises the applicability of the results. Similar to the results published by Saito et al. ( 24 ) we did not find any methylation of E‐cadherin promoter in NE, SH, or AH. Thus, in contrast to APC promoter hypermethylation, the methylation of E‐cadherin promoter may be an intermediate and/or late event in the multistep endometrial carcinogenesis, playing an important role in the progression of EC.
As shown in Table 2, PTs without metastases posses a significantly higher level of APC promoter methylation compared to PTs with metastases (P = 0.002). The direct comparison of APC and E‐cadherin promoter methylation in 24 PTs and their corresponding metastatic lesions did not reveal any relationship between the methylation status of either gene and the development of metastases (Fig. 6). However, the fact that nine of 24 (37.5%) cases showed APC methylation in primary tumors/metastases does indicate a possible role of APC inactivation in the spread of primary ECs. Even more, APC inactivation by promoter hypermethylation leads to a significant increase in the proliferative activity of primary EC without metastases (Fig. 5). Increased expression of Ki‐67 in EC is a well‐known predictive factor for tumor aggressiveness and unfavorable prognosis.( 29 , 36 ) Among the different histological subgroups, with an increase of the histological tumor grade, an increase in the immunoexpression of Ki‐67 was detected, without reaching a significant level (Table 2).
Additionally, we found a significant increase in APC methylation with increased age of EC patients, which was not observed by others.( 21 ) That the risk of developing cancer increases with age is probably due to the fact that progenitor cells from mature organisms accumulate a critical number of molecular alterations, such as genetic and/or epigenetic ones.( 37 , 38 ) Epigenetic modifications, including DNA methylation and histone deacetylation, might be essential not only for carcinogenesis, but also for normal development to evade the homeostatic control. It has been proposed that aging leads to predisposition of the cells to acquire further genetic and epigenetic changes.( 38 , 39 ) It might also differ between different organs, suggesting that the relationship between hypermethylation and aging in cancer is not simple, and might explain the different study results.( 38 , 39 )
In conclusion, the hypermethylation of APC promoter occurs more often in early stages of EC and is associated with decreased APC protein expression and increased expression of the proliferation marker Ki‐67.
Acknowledgments
We thank H. Scharfenort and A. Schinlauer, from the molecular genetics laboratory, and the whole team at the laboratory of immunohistochemistry for their excellent technical assistance. We are grateful to Dr. E. Erbstößer, Dr. K. Hellwig, and Dr. M. Löttge for providing the paraffin‐embedded specimens. This work was supported by NBL3‐2 of the BMBF (Förderkennzeichen 01ZZ0407).
References
- 1. Amant F, Moerman P, Neven P et al. Endometrial cancer. Lancet 2005; 366: 491–505. [DOI] [PubMed] [Google Scholar]
- 2. Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long‐term study of “untreated” hyperplasia in 170 patients. Cancer 1985; 56: 403–12. [DOI] [PubMed] [Google Scholar]
- 3. Sherman AI, Brown S. The precursors of endometrial carcinoma. Am J Obstet Gynecol 1979; 135: 947–56. [DOI] [PubMed] [Google Scholar]
- 4. Kendall BS, Ronnett BM, Isacson C et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well‐differentiated carcinoma. Am J Surg Pathol 1998; 22: 1012–9. [DOI] [PubMed] [Google Scholar]
- 5. Mutter GL, Baak JP, Crum CP et al. Endometrial precancer diagnosis by histopathology, clonal analysis, and computerized morphometry. J Pathol 2000; 190: 462–9. [DOI] [PubMed] [Google Scholar]
- 6. Deligdisch L, Kase NG, Bleiweiss IJ. Endometrial cancer in elderly women: a histologic and steroid receptor study. Gerontology 2000; 46: 17–21. [DOI] [PubMed] [Google Scholar]
- 7. Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 2003; 349: 2042–54. [DOI] [PubMed] [Google Scholar]
- 8. Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Hum Mol Genet 2001; 10: 721–33. [DOI] [PubMed] [Google Scholar]
- 9. Behrens J, Von Kries JP, Kuhl M et al. Functional interaction of beta‐catenin with the transcription factor LEF‐1. Nature 1996; 382: 638–42. [DOI] [PubMed] [Google Scholar]
- 10. Fujimoto J, Ichigo S, Hori M et al. Expressions of E‐cadherin and alpha‐ and beta‐catenin mRNAs in uterine endometrial cancers. Eur J Gynaecol Oncol 1998; 19: 78–81. [PubMed] [Google Scholar]
- 11. Christofori G, Semb H. The role of the cell‐adhesion molecule E‐cadherin as a tumour‐suppressor gene. Trends Biochem Sci 1999; 24: 73–6. [DOI] [PubMed] [Google Scholar]
- 12. Fodde R. The APC gene in colorectal cancer. Eur J Cancer 2002; 38: 867–71. [DOI] [PubMed] [Google Scholar]
- 13. Gamallo C, Palacios J, Moreno G et al. beta‐catenin expression pattern in stage I and II ovarian carcinomas : relationship with beta‐catenin gene mutations, clinicopathological features, and clinical outcome. Am J Pathol 1999; 155: 527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreno‐Bueno G, Hardisson D, Sanchez C et al. Abnormalities of the APC/beta‐catenin pathway in endometrial cancer. Oncogene 2002; 21: 7981–90. [DOI] [PubMed] [Google Scholar]
- 15. Sparks AB, Morin PJ, Vogelstein B et al. Mutational analysis of the APC/beta‐catenin/Tcf pathway in colorectal cancer. Cancer Res 1998; 58: 1130–4. [PubMed] [Google Scholar]
- 16. Stawerski P, Wagrowska‐Danilewicz M, Stasikowska O et al. Immunoexpression of beta‐catenin – E‐cadherin complex in primary serous ovarian tumors. Pol J Pathol 2008; 59: 27–32. [PubMed] [Google Scholar]
- 17. Esteller M, Corn PG, Baylin SB et al. A gene hypermethylation profile of human cancer. Cancer Res 2001; 61: 3225–9. [PubMed] [Google Scholar]
- 18. Virmani AK, Rathi A, Sathyanarayana UG et al. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter 1A in breast and lung carcinomas. Clin Cancer Res 2001; 7: 1998–2004. [PubMed] [Google Scholar]
- 19. Lambertz S, Ballhausen WG. Identification of an alternative 5′ untranslated region of the adenomatous polyposis coli gene. Hum Genet 1993; 90: 650–2. [DOI] [PubMed] [Google Scholar]
- 20. Zysman M, Saka A, Millar A et al. Methylation of adenomatous polyposis coli in endometrial cancer occurs more frequently in tumors with microsatellite instability phenotype. Cancer Res 2002; 62: 3663–6. [PubMed] [Google Scholar]
- 21. Banno K, Yanokura M, Susumu N et al. Relationship of the aberrant DNA hypermethylation of cancer‐related genes with carcinogenesis of endometrial cancer. Oncol Rep 2006; 16: 1189–96. [PubMed] [Google Scholar]
- 22. Droufakou S, Deshmane V, Roylance R et al. Multiple ways of silencing E‐cadherin gene expression in lobular carcinoma of the breast. Int J Cancer 2001; 92: 404–8. [DOI] [PubMed] [Google Scholar]
- 23. Kallakury BV, Sheehan CE, Winn‐Deen E et al. Decreased expression of catenins (alpha and beta), p120 CTN, and E‐cadherin cell adhesion proteins and E‐cadherin gene promoter methylation in prostatic adenocarcinomas. Cancer 2001; 92: 2786–95. [DOI] [PubMed] [Google Scholar]
- 24. Saito T, Nishimura M, Yamasaki H et al. Hypermethylation in promoter region of E‐cadherin gene is associated with tumor dedifferention and myometrial invasion in endometrial carcinoma. Cancer 2003; 97: 1002–9. [DOI] [PubMed] [Google Scholar]
- 25. Pijnenborg JM, Kisters N, Van Engeland M et al. APC, beta‐catenin, and E‐cadherin and the development of recurrent endometrial carcinoma. Int J Gynecol Cancer 2004; 14: 947–56. [DOI] [PubMed] [Google Scholar]
- 26. Dong SM, Kim HS, Rha SH et al. Promoter hypermethylation of multiple genes in carcinoma of the uterine cervix. Clin Cancer Res 2001; 7: 1982–6. [PubMed] [Google Scholar]
- 27. Herman JG, Graff JR, Myohanen S et al. Methylation‐specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 1996; 93: 9821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ignatov A, Bischoff J, Schwarzenau C et al. P16 alterations increase the metastatic potential of endometrial carcinoma. Gynecol Oncol 2008; 111: 365–71. [DOI] [PubMed] [Google Scholar]
- 29. Cattoretti G, Becker MH, Key G et al. Monoclonal antibodies against recombinant parts of the Ki‐67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave‐processed formalin‐fixed paraffin sections. J Pathol 1992; 168: 357–63. [DOI] [PubMed] [Google Scholar]
- 30. Scholzen T, Gerdes J. The Ki‐67 protein: from the known and the unknown. J Cell Physiol 2000; 182: 311–22. [DOI] [PubMed] [Google Scholar]
- 31. Semczuk A, Skomra D, Cybulski M et al. Immunohistochemical analysis of MIB‐1 proliferative activity in human endometrial cancer. Correlation with clinicopathological parameters, patient outcome, retinoblastoma immunoreactivity and K‐ras codon 12 point mutations. Histochem J 2001; 33: 193–200. [DOI] [PubMed] [Google Scholar]
- 32. Horree N, Van Diest PJ, Van Der GP et al. Progressive derailment of cell cycle regulators in endometrial carcinogenesis. J Clin Pathol 2008; 61: 36–42. [DOI] [PubMed] [Google Scholar]
- 33. Blechschmidt K, Kremmer E, Hollweck R et al. The E‐cadherin repressor snail plays a role in tumor progression of endometrioid adenocarcinomas. Diagn Mol Pathol 2007; 16: 222–8. [DOI] [PubMed] [Google Scholar]
- 34. Sakuragi N, Nishiya M, Ikeda K et al. Decreased E‐cadherin expression in endometrial carcinoma is associated with tumor dedifferentiation and deep myometrial invasion. Gynecol Oncol 1994; 53: 183–9. [DOI] [PubMed] [Google Scholar]
- 35. Scholten AN, Aliredjo R, Creutzberg CL et al. Combined E‐cadherin, alpha‐catenin, and beta‐catenin expression is a favorable prognostic factor in endometrial carcinoma. Int J Gynecol Cancer 2006; 16: 1379–85. [DOI] [PubMed] [Google Scholar]
- 36. Gassel AM, Backe J, Krebs S et al. Endometrial carcinoma: immunohistochemically detected proliferation index is a prognosticator of long‐term outcome. J Clin Pathol 1998; 51: 25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agrawal A, Murphy RF, Agrawal DK. DNA methylation in breast and colorectal cancers. Mod Pathol 2007; 20: 711–21. [DOI] [PubMed] [Google Scholar]
- 38. Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet 2007; 23: 413–8. [DOI] [PubMed] [Google Scholar]
- 39. Shames DS, Minna JD, Gazdar AF. DNA methylation in health, disease, and cancer. Curr Mol Med 2007; 7: 85–102. [DOI] [PubMed] [Google Scholar]


