Abstract
Docetaxel has come into wide use recently for the treatment of breast cancer in neoadjuvant, adjuvant and metastatic settings. Docetaxel binds to β‐tubulin and causes kinetic abnormalities in the dynamics of microtubules by increasing their polymerization and inhibiting their depolymerization, resulting in elevated levels of microtubule formation. During metaphase, defective spindle formation induced by docetaxel activates the mitotic checkpoint and leads to cell cycle arrest, culminating in apoptosis. However, docetaxel is not effective for all breast cancers. For example, in metastatic settings, the response rate to docetaxel reportedly ranges from 30 to 50%. It is therefore very important to develop a diagnostic method with high accuracy for the prediction of sensitivity to docetaxel in order to avoid unnecessary treatment. Currently it is impossible to identify, before the initiation of therapy, the patients for whom docetaxel will be effective. Various biological parameters have been studied clinically for their ability to predict response to docetaxel, such as parameters related to: (1) efflux (p‐glycoprotein) and metabolism (CYP3A4); (2) β‐tubulin (somatic mutation of β‐tubulin and changes in β‐tubulin isotypes levels); (3) cell cycle (HER2, BRCA1 and Aurora‐A); and (4) apoptosis (p53, BCL2 and thioredoxin). More recently, gene expression profiling techniques have been used for the development of a prediction model for response to docetaxel. In the present paper, clinical studies that have been conducted recently to identify predictive factors for response to docetaxel are reviewed together with a presentation of our recent work in this field. (Cancer Sci 2006; 97: 813–820)
Docetaxel is a second‐generation taxane derived from the needles of the European yew tree and acts on a variety of human tumors, including breast cancer. Several in vitro studies have shown that the cytotoxicity of docetaxel is 1.3–12 times higher than that of paclitaxel, another taxane, and recently a clinical trial comparing docetaxel with paclitaxel in a metastatic setting demonstrated that docetaxel is more effective than paclitaxel, which is consistent with the results of preclinical studies. Docetaxel, like paclitaxel, binds to β‐tubulin and causes kinetic abnormalities in the dynamics of microtubules by increasing their polymerization and inhibiting their depolymerization, resulting in elevated levels of microtubule formation (Fig. 1). During metaphase, moreover, defective spindle formation induced by docetaxel activates the mitotic checkpoint and leads to cell cycle arrest, culminating in apoptosis.
Figure 1.
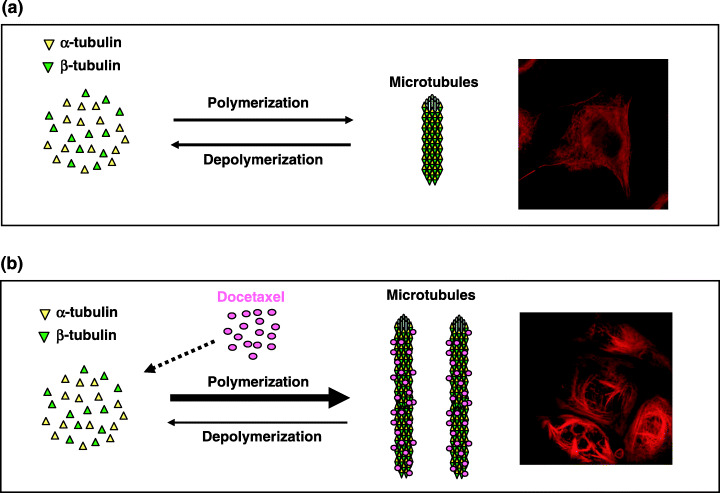
Mechanism of action of docetaxel. The levels of microtubules are regulated by the balance between polymerization of α‐tubulin and β‐tubulin and the depolymerization of microtubules. Compared with tumor cells without docetaxel treatment (a), tumor cells with docetaxel treatment (b) show increased polymerization and reduced depolymerization, leading to enhanced synthesis of microtubules. Immunocytochemical staining of microtubules showed enhanced microtubule formation in breast cancer cells (MCF‐7 cells) with docetaxel present (b, inset) compared with those with docetaxel absent (a, insert).
Docetaxel has been used widely in the treatment of metastatic breast cancer. In addition, recent studies have clearly shown that the use of docetaxel subsequent to that of doxorubicin and cyclophosphamide (AC) increases the pathological response rate of primary breast tumors in the neoadjuvant setting.( 1 ) Moreover, the use of docetaxel subsequent to that of epirubicin, 5‐FU, and cyclophosphamide (FEC) improves disease‐free and overall survival in the adjuvant setting. In addition, the combined use of docetaxel and doxorubicin plus cyclophosphamide (TAC) also improves disease‐free and overall survival in the adjuvant setting.( 2 ) These results have encouraged the use of docetaxel in both neoadjuvant and adjuvant settings.
Docetaxel is not effective for all breast cancers; the response rate to docetaxel in metastatic tumors reportedly ranges from 30 to 50%. It is therefore very important to develop a diagnostic method with high accuracy for the prediction of sensitivity to docetaxel in order to avoid unnecessary treatment. Currently it is impossible to identify, before the initiation of therapy, the patients for whom docetaxel will be effective.
Various biological parameters have been examined for their ability to predict the response to docetaxel, including β‐tubulin, HER2, p53, BRCA1, estrogen receptor (ER), BCL‐2, P‐glycoprotein, Ki‐67 and CYP3A4 (Fig. 2). More recently, gene expression profiling has been used for predicting response to docetaxel.( 3 , 4 ) The present paper comprises a review of the current status of studies of predictive factors for response to docetaxel as well as a presentation of our recent work in this field.
Figure 2.
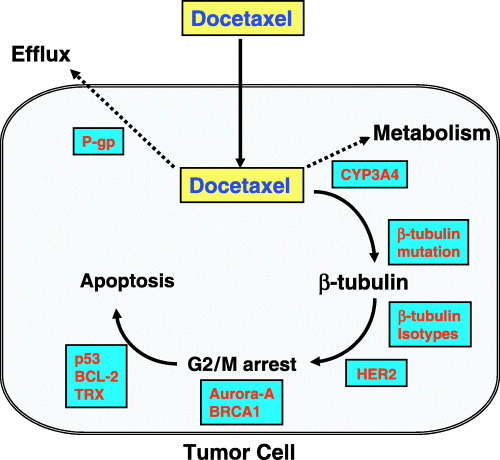
Possible parameters associated with docetaxel resistance. Intracellular docetaxel concentration can be reduced by an increase in P‐glycoprotein (p‐gp) levels because docetaxel is a substrate of p‐gp. The intracellular concentration of docetaxel can also be reduced by an increase in the level of CYP3A4, which metabolizes docetaxel into inactive metabolites. Somatic mutation of β‐tubulin as well as changes in β‐tubulin isotype levels, which are target molecules of docetaxel, may affect the antitumor activity of docetaxel. Moreover, HER2, BRCA1 and Aurora‐A may be implicated in docetaxel resistance through modification of the cell cycle or spindle check point, and p53, BCL2 and thioredoxin (TRX) in docetaxel resistance through modification of the apoptotic pathway.
β‐Tubulin
It has been reported that mutations in β‐tubulin induce resistance to paclitaxel in some cancer cells by altering microtubule dynamics, not by interfering with the binding of taxanes to microtubules. Monzo et al. observed somatic mutation of class I β‐tubulin in as many as 33% of human non‐small cell lung cancers, and found that it plays a significant role in resistance to paclitaxel.( 5 ) This prompted us to conduct a mutational analysis of class I β‐tubulin in human breast cancers but, contrary to expectation, we found that somatic mutation of this gene is very rare (1/62, 1.6%).( 6 ) Moreover, Maeno et al. did not detect any somatic mutations of class I β‐tubulin in 82 breast cancers.( 7 ) In fact, Monzo et al.'s findings have been contradicted by later studies( 8 , 9 ) (Table 1). Thus, it seems highly unlikely that resistance to docetaxel can be explained by somatic mutation of class I β‐tubulin in breast cancers.
Table 1.
Somatic mutations of class I β‐tubulin in human lung cancers and breast cancers
| Authors | Type of cancer | No. tumors | Frequency of mutation (%) |
|---|---|---|---|
| Monzo et al. ( 5 ) | NSCLC | 49 | 32.6 (16/49) |
| Kelley et al. ( 8 ) | NSCLC | 42 | 2.4 (1/42) |
| Tsurutani et al. ( 9 ) | NSCLC | 42 | 2.4 (1/42) |
| Maeno( 7 ) | Breast cancer | 82 | 0 (0/82) |
| Hasegawa et al. ( 6 ) | Breast cancer | 62 | 1.6 (1/62) |
NSCLC, non‐small‐cell lung cancer.
Another possible mechanism for docetaxel resistance is altered expression of β‐tubulin isotypes.( 10 ) In humans, expression of at least eight distinct β‐tubulin isotypes (classes I, II, III, IVa, IVb, V, VI and VII) has been reported, with their expression profiles differing from tissue to tissue. Of the eight identified β‐tubulin isotypes, class III β‐tubulin seems to be unique in that it destabilizes microtubules. This has led to speculation that the antitumor action of docetaxel can be affected by the expression level of class III β‐tubulin because the latter may counteract the stabilizing effect of docetaxel on microtubules. In fact, it is reported that a high level of class III β‐tubulin expression is associated with paclitaxel resistance in several human cancer cell lines (lung cancer, ovarian cancer, prostate cancer and breast cancer), and this has been demonstrated clinically in ovarian cancer.( 10 ) In addition, several studies have established an association between a high level of class I β‐tubulin expression and paclitaxel resistance in human non‐small cell lung cancer cell lines and ovarian tumors, although class I β‐tubulin, unlike class III β‐tubulin, does not destabilize microtubules.
These results indicate that expression levels of class I and III β‐tubulin may be a clinically useful predictor of response to taxanes in human breast cancers. Although most studies of the relationship between taxane resistance and β‐tubulin isotype mRNA expression have focused on paclitaxel, recent studies have reported similar results for docetaxel, including an association between high expression of class III β‐tubulin and docetaxel resistance in human pancreatic cancer cell lines.( 11 ) We have examined the relationship between the expression levels of class I and III β‐tubulin isotype mRNA and response to docetaxel in breast cancers. Consistent with results reported previously, which were mostly based on in vitro studies, we showed that not only high‐level expression of class III β‐tubulin isotype mRNA but also that of its class I counterpart is significantly associated with a poor response to docetaxel in breast cancers.( 6 ) Tumors with high levels of both class I and class III β‐tubulin isotype mRNA expression showed a lower response rate (15%) than did those with high expression levels of either class I or class III β‐tubulin isotype mRNA (50%). In addition, tumors with low‐level expression of both class I and class III β‐tubulin isotype mRNA showed the highest response rate (75%). These findings strongly suggest that both class I and class III β‐tubulin isotypes are implicated in docetaxel resistance in breast cancers. However, the ratio of class III to class I β‐tubulin isotype mRNA levels did not show any significant association with a response to docetaxel. This indicates that an absolute increase in mRNA expression levels of class I or class III β‐tubulin isotypes, but not an increase in class III relative to class I β‐tubulin isotype mRNA levels, is important in generating resistance to docetaxel. In support of this hypothesis, Kavallaris et al. reported that mRNA of both class I and class III β‐tubulin isotypes is upregulated in human ovarian tumors resistant to paclitaxel.( 12 ) Very recently, we conducted an immunohistochemical study of class I and class III β‐tubulin isotypes and were able to show that class III isotype expression is significantly associated with resistance to docetaxel (Table 2). This finding suggests the future possibility of immunohistochemical studies of class III β‐tubulin isotype for the selection of patients to be treated with docetaxel.
Table 2.
Relationship between various parameters and clinical response to docetaxel in human breast cancers
| Parameter | Category | No. patients † | Response rate (%) ‡ | P‐value |
|---|---|---|---|---|
| ER | Positive | 32 | 56 | 0.862 |
| Negative | 37 | 57 | ||
| HER2 | Positive | 13 | 69 | 0.193 |
| Negative | 49 | 49 | ||
| P‐glycoprotein | Positive | 26 | 54 | 0.987 |
| Negative | 37 | 54 | ||
| MIB‐1 | Positive | 39 | 49 | 0.354 |
| Negative | 23 | 60 | ||
| BRCA1 | Positive | 47 | 53 | 0.794 |
| Negative | 14 | 57 | ||
| BCL2 | Positive | 17 | 70 | 0.144 |
| Negative | 46 | 50 | ||
| p53 | Positive | 29 | 55 | 0.961 |
| Negative | 33 | 54 | ||
| β‐Tubulin III | Positive | 14 | 28 | <0.05 |
| Negative | 42 | 59 | ||
| CYP3A4 | Positive | 16 | 19 | <0.01 |
| Negative | 15 | 67 | ||
| Thioredoxin | Positive | 14 | 21 | 0.018 |
| Negative | 49 | 63 |
Patients with large primary breast cancers or locally recurrent breast cancers.
‡ Patients who showed a complete or partial clinical response were considered to be responders.
Another recent study found that the microtubule‐associated protein tau is the most differentially expressed gene in responders and non‐responders to neoadjuvant chemotherapy with paclitaxel, and that low tau expression is associated with a good response.( 13 ) This has resulted in speculation that low tau expression renders microtubules more vulnerable to paclitaxel and breast cancer cells hypersensitive to this drug. Tau thus seems to have the potential to function as a predictor of response to docetaxel as well, but no relevant findings have been reported.
HER2
Overexpression of HER2 is observed in approximately 20–30% of breast cancers, and has been suggested to induce resistance against docetaxel in vitro, as concomitant treatment with the anti‐HER2 antibody (trastuzumab) results in the sensitization of breast cancer cells to docetaxel.( 14 ) In fact, combination therapy consisting of docetaxel plus trastuzumab has been shown to be more efficacious than docetaxel alone in the metastatic setting in breast cancers.( 15 ) The exact mechanism of resistance to docetaxel in HER2‐overexpressing tumor cells is currently unknown but it has been suggested that HER2 overexpression induces docetaxel resistance by inducing CDK1‐inhibiting p21, resulting in the delay of docetaxel‐mediated entry into mitosis and apoptosis.( 16 )
Estevez et al. reported that the majority of pathological complete responses are observed in patients with HER2‐negative tumors treated with taxanes in the neoadjuvant setting.( 17 ) In addition, Learn et al. have suggested that the addition of docetaxel to the standard neoadjuvant treatment (AC) may not have an appreciably beneficial effect on clinical responses in patients with HER2‐overexpressing tumors.( 18 ) Although these results seem to imply some involvement of HER2 overexpression in resistance to docetaxel, no findings have been reported that demonstrate a statistically significant association between HER2 overexpression and resistance to docetaxel. Our study, too, could not establish such an association in the neoadjuvant setting (Table 2). Sjostrom et al. have reported similar response rates to docetaxel in HER2‐positive (53%) and HER2‐negative (53%) tumors in the neoadjuvant setting.( 19 ) In contrast, Durbecq et al. found that HER2‐positive tumors show a better response rate (67%) than HER2‐negative tumors (31%) in the metastatic setting.( 20 ) The clinical significance of HER2 overexpression as a predictor of response to docetaxel is therefore far from clear.
In contrast, HER2 overexpression has been reported to be associated with a good response to doxorubicin, an association that is explained not by HER2 overexpression itself but by the concurrent overexpression of topisomerase IIα (TOPO‐IIα), which is often coamplified with HER2. Durbecq et al. studied the relationship between TOPO‐IIα expression and response to docetaxel or doxorubicin, and found that high TOPO‐IIα expression is associated with a good response to doxorubicin but not docetaxel.( 20 ) TOPO‐IIα expression is thus considered a potential candidate as a predictor of doxorubicin but not of docetaxel.
p53
Only a few reported studies, including our own (Table 2), have examined the immunostaining status of p53 and its relationship to response to docetaxel in breast cancers.( 18 , 21 ) However, all of them have failed to demonstrate any significant association. As genomic DNA analysis is a more reliable method than immunohistochemical analysis, because the latter cannot identify nonsense mutations and frameshift mutations, we very recently used the former to conduct a study of the relationship between p53 genomic DNA mutation status and response to docetaxel in breast cancers. Of 50 breast tumors thus analyzed, we identified 16 tumors with p53 mutations and found that the response rate of p53‐mutated tumors (7/16, 44%) was not significantly different (P = 0.23) from that of p53‐non‐mutated tumors (21/34, 62%). This suggests that the mutational loss of p53 function does not confer resistance against docetaxel. These findings appear to be consistent with the in vitro finding that p53 mutation is not associated with resistance to paclitaxel,( 22 ) indicating that p53 mutation status evaluated either immunohistochemically or by genomic DNA analysis does not appear to be a promising predictor of response to docetaxel.
BRCA1
BRCA1 is a tumor‐suppressor gene implicated in the maintenance of genomic stability through DNA repair and in the regulation of centrosome duplication. It has been shown recently that the induction of BRCA1 expression enhances paclitaxel‐induced apoptosis,( 23 ) indicating a possible involvement of BRCA1 in cellular sensitivity to docetaxel. Except for our own study,( 21 ) no data have been published on the relationship between BRCA1 expression and response to docetaxel or paclitaxel in breast cancers in the clinical setting. Our investigation could not determine any significant association between BRCA1 expression and response to docetaxel (Table 2). The role of BRCA1 expression in docetaxel resistance thus remains to be clarified.
ER
In the neoadjuvant setting, studies by Estevez et al.( 17 ) and us (Table 2) have found that ER status is not significantly associated with response to docetaxel, but Tham et al. reported that the response rate of ER‐positive tumors is higher (90%) than that of ER‐negative tumors (50%).( 24 ) In addition, Learn et al. suggested that ER‐positive tumors are more likely to respond clinically to docetaxel than ER‐negative tumors in the neoadjuvant setting by evaluating the effect of the addition of docetaxel to AC.( 18 ) The fact that docetaxel suppresses ovarian function indicates that it also functions as a hormonal therapy, thus confounding our understanding of the relationship between ER status and response to docetaxel. In addition, we recently found that docetaxel can downregulate intratumoral aromatase mRNA expression, which may thus represent an additional mechanism of docetaxel as hormonal therapy.( 25 )
In contrast, Henderson et al. conducted a CALGBC 9344 trial in the adjuvant setting, where the addition of adjuvant paclitaxel to AC was evaluated in early breast cancers. They reported improved disease‐free survival as a result of adding paclitaxel for patients with ER‐negative tumors, but not for those with ER‐positive tumors.( 26 ) However, in the NSABP Protocol B‐28 study, which also evaluated the addition of paclitaxel to AC, Mamounas et al. failed to confirm the observation of Henderson et al.( 27 ) Consequently, the predictive value of ER status for response to docetaxel or paclitaxel remains to be established.
BCL‐2
Several studies have demonstrated that overexpression of BCL‐2, which is an anti‐apoptotic protein, confers resistance against apoptosis induced by paclitaxel.( 28 ) In MCF‐7 cells, estrogens were found to upregulate BCL‐2 levels and confer resistance to paclitaxel but treatment with tamoxifen (anti‐estrogen) was found to reduce BCL‐2 levels and restore sensitivity to paclitaxel.( 29 ) In addition, it has been reported that paclitaxel‐induced mitotic arrest induces the phosphorylation of BCL‐2, resulting in the inactivation of BCL‐2 and promotion of apoptosis.( 30 ) These results suggest that there is some correlation between BCL‐2 expression and response to docetaxel. However, Sjostrom et al. have demonstrated that BCL‐2 is of no value for the prediction of response to docetaxel in breast cancers.( 31 ) Neither were we able to find any significant correlation between BCL‐2 expression and a response to docetaxel (Table 2). Interestingly, however, a recent study has indicated that docetaxel induces apoptosis and cell death through a BCL‐2‐independent mechanism.( 32 )
P‐glycoprotein
Acceleration of drug efflux via the overexpressed P‐glycoprotein has been proposed as one of the mechanisms of resistance to docetaxel, as docetaxel is a substrate of P‐glycoprotein.( 33 ) In vitro studies have indicated that overexpression of P‐glycoprotein is indeed associated with resistance to docetaxel.( 34 ) Our study of the relationship between P‐glycoprotein expression and response to docetaxel in breast cancers, however, could not find any significant correlation (Table 2). No other data are currently available on the relationship between P‐glycoprotein and response to docetaxel in the clinical setting.
Ki‐67
Highly proliferative tumors and response to chemotherapy appear to generally correlate with each other. Several studies have demonstrated a positive relationship between a high proliferation rate and tumor response to anthracycline‐based regimens.( 35 ) However, Estevez et al.,( 17 ) Tham et al.( 24 ) and Sjostrom et al.( 36 ) could find no relationship between Ki‐67 labeling index and response to docetaxel. The proliferation rate, determined by measuring Ki‐67, proved not to be predictive in our study either (Table 2). The reason for the association of high proliferation with anthracycline‐based regimens but not with docetaxel is currently unknown. Tumor cells in S‐phase are most sensitive to anthracycline‐containing regimens and those in G2/M‐phase are most sensitive to docetaxel. Proliferation rates expressed as Ki‐67 indices may thus constitute a more accurate representation of the proportion of cells in S‐phase than that of cells in G2/M phase.
CYP3A4
Docetaxel is metabolized by CYP3A4 in the liver into four major metabolites with minimal or no antitumor activity, so that metabolism of docetaxel depends on the enzyme activity of CYP3A4 in the liver of individual patients. In fact, measurement of the urinary metabolites of exogenous cortisol (6‐beta‐hydroxycortisol [6‐beta‐OHF]) is reportedly useful for estimating CYP3A4 activity in the liver, so that an individualized dosing method based on the total amount of urinary 6‐beta‐OHF after cortisol administration can reduce the pharmacokinetic variability of docetaxel.( 37 ) These results provide a strong indication of the importance of metabolism of docetaxel by CYP3A4 as a host factor in the determination of the efficacy and toxicity of docetaxel. In addition, recent studies have disclosed that human breast cancer tissues express CYP3A4,( 38 ) suggesting that docetaxel may be metabolized to its inactive forms in tumor tissues and that CYP3A4 activity in tumor tissues may therefore affect the antitumor activity of docetaxel. For this reason, we studied CYP3A4 mRNA expression levels in tumor tissues and their relationship with the response to docetaxel and were able to demonstrate that breast tumors with low expression of CYP3A4 mRNA show a significantly higher response rate (71%) than those with high expression of CYP3A4 mRNA (11%).( 39 ) Interestingly, CYP3A4 mRNA expression in tumor tissues showed no association with response to epirubucin‐based regimens.( 39 ) Subsequent to this study, we also investigated the relationship between immunohistochemically determined CYP3A4 expression and response to docetaxel and found that the response rate of CYP3A4‐positive breast tumors is lower (19%) than that of CYP3A4‐negative breast tumors (67%) (Table 2). These results taken together suggest that intratumoral expression of CYP3A4 mRNA or CYP3A4 protein determined by immunohistochemistry could serve as a predictor of resistance to docetaxel. The role of CYP3A4 in the acquisition of such resistance thus appears to merit further basic and clinical studies.
Aurora‐A
Aurora‐A, a serine/threonine kinase localized in the centrosome, plays an essential role in the progression of mitosis. Recently, it has been shown that high expression of Aurora‐A interferes with the Mad2–Cdc20 signal and overrides the mitotic checkpoint even in the presence of defective spindle formation,( 40 ) suggesting that high‐level expression of Aurora‐A may attenuate the antitumor activity of docetaxel. In fact, an association between high Aurora‐A expression and resistance to docetaxel has been reported in pancreatic cancer cell lines in vitro.( 41 ) These results indicate that high Aurora‐A expression may be associated with resistance to docetaxel in breast cancers too. For this reason, we very recently investigated Aurora‐A mRNA expression levels in breast tumors and their relationship with response to docetaxel. First, we studied the influence of Aurora‐A expression on the formation of aneuploid tumors and found that high Aurora‐A mRNA expression is significantly associated with chromosomal instability. This is consistent with the hypothesis that a high level of Aurora‐A expression disrupts the mitotic checkpoint, thus causing the cell cycle to proceed without rendering the cells apoptotic, and culminating in the appearance of anueploid cells (high chromosomal instability).( 42 ) Next, we studied the relationship between Aurora‐A mRNA levels and response to docetaxel in breast cancers, and our preliminary results indicate that breast tumors with high Aurora‐A mRNA levels are associated with a lower response rate (41%) than are tumors with low Aurora‐A mRNA levels (71%). Interestingly enough, such an association is seen only in ER‐negative tumors (33% vs 83%) but not in ER‐positive tumors (46% vs 56%). These observations seem to indicate that Aurora‐A mRNA levels may be clinically useful for predicting the response to docetaxel in ER‐negative, but not in ER‐positive, tumors. We also found that high levels of Aurora‐A mRNA are associated with high chromosomal instability in ER‐negative tumors but not in ER‐positive tumors. This suggests that high‐level Aurora‐A expression overrides the mitotic check point and induces chromosomal instability in ER‐negative tumors but not in ER‐positive tumors. We speculate that, in ER‐negative tumors, tumor cells with defective spindle formation induced by docetaxel are rendered apoptotic due to activation of the mitotic check point if Aurora‐A mRNA levels are not overexpressed, but that in the case of overexpression, such tumor cells can override the mitotic check point. In contrast, in ER‐positive tumors Aurora‐A appears not to play such an important role as neither docetaxel sensitivity nor chromosome instability is associated with Aurora‐A mRNA levels. The reason for this difference in the role of Aurora‐A in ER‐negative and ER‐positive tumors remains to be studied.
Gene expression profiling
High‐throughput simultaneous measurement of the expression of a large number of genes in tumor tissues is being explored as a potential diagnostic tool for prediction of response to chemotherapy, based on the hypothesis that a combination of multiple genes will be more accurately predictive for response than any single gene. In one study, investigators analyzed gene expression profiles by means of DNA microarray using core needle biopsies from 24 patients with locally advanced breast cancer who had undergone preoperative treatment with single‐agent docetaxel.( 3 ) The investigators identified 92 genes that correlated significantly with response, and with these genes developed a predictive model. Cross‐validation analysis of the same data confirmed the correct classification of 10 of the 11 sensitive tumors (91% specificity) and 11 of the 13 resistant tumors (85% sensitivity), yielding an overall response prediction accuracy of 88%.
Adaptor‐tagged competitive (ATAC)‐polymerase chain reaction (PCR), invented by Kato et al.,( 43 ) is another technique for simultaneous high‐throughput measurement of the expression of a large number of genes. We have used this technique as, in the clinical setting, ATAC‐PCR has several advantages over DNA microarrays. These advantages include the need for only a very small amount of total RNA (0.8 ng total RNA) per reaction, some tolerance for RNA degradation, and easy conversion to a more common technique, real‐time PCR. ATAC‐PCR has very recently been used successfully for the analysis of gene expression profiles in various human tumors including breast carcinomas,( 4 , 44 ) hepatocellular carcinomas,( 45 , 46 ) colon carcinomas( 47 ) and thyroid tumors.( 48 ) This procedure has enabled us to develop a diagnostic system based on the expression of 85 genes, which can predict a patient's response to docetaxel with over 80% accuracy.( 4 )
These 85 genes, which are expressed differently in docetaxel‐sensitive and docetaxel‐resistant breast tumors, include several genes, such as thioredoxin, related to the regulation of the cellular redox system. Thioredoxin plays a crucial role in regulation of the cellular redox system and acts as a cytoprotector against various kinds of oxidative stress such as ultraviolet, X‐ray irradiation and viral infection. Many studies have suggested that a high level of thioredoxin expression in various cancers is associated with a biologically aggressive phenotype (i.e. increased proliferation and decreased apoptosis). Moreover, recent in vitro studies have demonstrated an association between high‐level thioredoxin expression in cancer cells and resistance to cisplatin, mitomycin C, adriamycin and etoposide.( 49 ) This prompted us to study the relationship between thioredoxin expression and response to docetaxel in breast cancers in the clinical setting.( 21 ) Immunohistochemical analysis of thioredoxin expression led to the finding that high thioredoxin expression is associated with a poor response to docetaxel (Table 2). Thioredoxin is expected to function as a protector of cancer cells against oxidative stress induced by anticancer drugs but it is yet to be determined whether docetaxel induces oxidative stress. However, Pae et al. have demonstrated recently that paclitaxel generates superoxide anion via the stabilization of microtubules in a murine cell line.( 50 ) Another possible mechanism of thioredoxin‐induced resistance to docetaxel is that thioredoxin may reduce the disulfide bridge in tubulin dimers and thus inhibit microtubule assembly.
Future perspectives
As discussed in this review, several biomarkers have been shown to be associated with resistance to docetaxel and might be useful in the prediction of response to docetaxel in human breast cancers. However, their clinical utility seems to be of limited value, necessitating the development of a more reliable diagnostic method. Under the hypothesis that a combination of multiple genes will predict response to chemotherapy with a higher accuracy than any single gene, recently, much effort has been put into the development of a diagnostic system for response to chemotherapy utilizing gene expression profiling technologies. Several encouraging results have been reported on the differentiation between responsive and non‐responsive breast tumors to some chemotherapeutic regimens through the analysis of gene expression profiles.( 51 ) These results, however, are still preliminary and are far from clinical application. Before acceptance of these diagnostic systems based on gene expression profiling as routine clinical tests, several important issues need to be addressed. These include: (1) standardization of gene expression profiling technologies (microarray technology is usually subject to wide variations); (2) optimization of tissue sampling methods (breast tumors often have a high degree of intersite variation of gene expression profile); (3) validation study (studies so far reported are still preliminary and need to be validated in a prospective study); and (4) standardization of data analysis (optimal statistical method to generate a diagnostic system needs to be determined). Another high‐throughput analytical method that is becoming increasingly popular is proteomics. With the advent of new technologies based on mass spectrometry, proteome analysis is becoming more and more sensitive and specific. Proteome analysis is expected to be superior to transcriptome analysis in the differentiation of tumor phenotypes, including drug resistance. However, proteome analysis is supposed to be more complex than transcriptome analysis as the 45 000 human genes are estimated to generate over 1.5 million proteins as a result of alternative splicing of mRNA and complex post‐translational processing. Currently, studies based on proteome analysis have rarely been reported on the prediction of response to chemotherapy in human breast cancers.( 52 , 53 )
Several well‐established guidelines for the selection of breast cancer patients who are candidates for adjuvant chemotherapy are now available. In clinical practice, the indication of adjuvant chemotherapy for a patient is usually decided according to such guidelines by using the various clinicopathological prognostic factors for evaluating the risk of relapse. Unfortunately, however, there are no guidelines for the selection of antitumor drugs that are most likely to be efficacious. Ideally, adjuvant chemotherapy should be given to the most suitable patients (those who are most likely to develop recurrence) with the proper regimens (antitumor drugs that are most effective for the tumors). Much more time and effort are needed for the development of predictive factors for response to chemotherapy in breast cancers. In order to attain this goal, well‐designed prospective clinical studies are indispensable.
References
- 1. Bear HD, Anderson S, Brown A et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B‐27. J Clin Oncol 2003; 21: 4165–74. [DOI] [PubMed] [Google Scholar]
- 2. Campone M, Fumoleau P, Bourbouloux E, Kerbrat P, Roche H. Taxanes in adjuvant breast cancer setting: which standard in Europe? Crit Rev Oncol Hematol 2005; 55: 167–75. [DOI] [PubMed] [Google Scholar]
- 3. Chang JC, Wooten EC, Tsimelzon A et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 2003; 362: 362–9. [DOI] [PubMed] [Google Scholar]
- 4. Iwao‐Koizumi K, Matoba R, Ueno N et al. Prediction of docetaxel response in human breast cancer by gene expression profiling. J Clin Oncol 2005; 23: 422–31. [DOI] [PubMed] [Google Scholar]
- 5. Monzo M, Rosell R, Sanchez JJ et al. Paclitaxel resistance in non‐small‐cell lung cancer associated with beta‐tubulin gene mutations. J Clin Oncol 1999; 17: 1786–93. [DOI] [PubMed] [Google Scholar]
- 6. Hasegawa S, Miyoshi Y, Egawa C et al. Mutational analysis of the class I beta‐tubulin gene in human breast cancer. Int J Cancer 2002; 101: 46–51. [DOI] [PubMed] [Google Scholar]
- 7. Maeno K, Ito K, Hama Y et al. Mutation of the class I beta‐tubulin gene does not predict response to paclitaxel for breast cancer. Cancer Lett 2003; 198: 89–97. [DOI] [PubMed] [Google Scholar]
- 8. Kelley MJ, Li S, Harpole DH. Genetic analysis of the beta‐tubulin gene, TUBB, in non‐small‐cell lung cancer. J Natl Cancer Inst 2001; 93: 1886–8. [DOI] [PubMed] [Google Scholar]
- 9. Tsurutani J, Komiya T, Uejima H et al. Mutational analysis of the beta‐tubulin gene in lung cancer. Lung Cancer 2002; 35: 11–16. [DOI] [PubMed] [Google Scholar]
- 10. Burkhart CA, Kavallaris M, Band Horwitz S. The role of beta‐tubulin isotypes in resistance to antimitotic drugs. Biochim Biophys Acta 2001; 1471: O1–9. [DOI] [PubMed] [Google Scholar]
- 11. Liu B, Staren E, Iwamura T, Appert H, Howard J. Taxotere resistance in SUIT Taxotere resistance in pancreatic carcinoma cell line SUIT 2 and its sublines. World J Gastroenterol 2001; 7: 855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kavallaris M, Kuo DY, Burkhart CA et al. Taxol‐resistant epithelial ovarian tumors are associated with altered expression of specific beta‐tubulin isotypes. J Clin Invest 1997; 100: 1282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rouzier R, Rajan R, Wagner P et al. Microtubule‐associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA 2005; 102: 8315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst 2004; 96: 739–49. [DOI] [PubMed] [Google Scholar]
- 15. Crown J, O’Leary M, Ooi WS. Docetaxel and paclitaxel in the treatment of breast cancer: a review of clinical experience. Oncologist 2004; 9 (Suppl. 2): 24–32. [DOI] [PubMed] [Google Scholar]
- 16. Yu D, Jing T, Liu B et al. Overexpression of ErbB2 blocks taxol‐induced apoptosis by upregulation of p21Cip1, which inhibits p34Cdc2 kinase. Mol Cell 1998; 2: 581–91. [DOI] [PubMed] [Google Scholar]
- 17. Estevez LG, Cuevas JM, Anton A et al. Weekly docetaxel as neoadjuvant chemotherapy for stage II and III breast cancer: efficacy and correlation with biological markers in a phase II, multicenter study. Clin Cancer Res 2003; 9: 686–92. [PubMed] [Google Scholar]
- 18. Learn PA, Yeh IT, McNutt M et al. HER‐2/neu expression as a predictor of response to neoadjuvant docetaxel in patients with operable breast carcinoma. Cancer 2005; 103: 2252–60. [DOI] [PubMed] [Google Scholar]
- 19. Sjostrom J, Collan J, Von Boguslawski K et al. C‐erbB‐2 expression does not predict response to docetaxel or sequential methotrexate and 5‐fluorouracil in advanced breast cancer. Eur J Cancer 2002; 38: 535–42. [DOI] [PubMed] [Google Scholar]
- 20. Durbecq V, Paesmans M, Cardoso F et al. Topoisomerase‐II alpha expression as a predictive marker in a population of advanced breast cancer patients randomly treated either with single‐agent doxorubicin or single‐agent docetaxel. Mol Cancer Ther 2004; 3: 1207–14. [PubMed] [Google Scholar]
- 21. Kim SJ, Miyoshi Y, Taguchi T et al. High thioredoxin expression is associated with resistance to docetaxel in primary breast cancer. Clin Cancer Res 2005; 11: 8425–30. [DOI] [PubMed] [Google Scholar]
- 22. Wahl AF, Donaldson KL, Fairchild C et al. Loss of normal p53 function confers sensitization to taxol by increasing G2/M arrest and apoptosis. Nat Med 1996; 2: 72–9. [DOI] [PubMed] [Google Scholar]
- 23. Mullan PB, Quinn JE, Gilmore PM et al. BRCA1 and GADD45 mediated G2/M cell cycle arrest in response to antimicrotubule agents. Oncogene 2001; 20: 6123–31. [DOI] [PubMed] [Google Scholar]
- 24. Tham YL, Gomez LF, Mohsin S et al. Clinical response to neoadjuvant docetaxel predicts improved outcome in patients with large locally advanced breast cancers. Breast Cancer Res Treat 2005; 94: 279–84. [DOI] [PubMed] [Google Scholar]
- 25. Miyoshi Y, Kim SJ, Akazawa K et al. Down‐regulation of intratumoral aromatase messenger RNA levels by docetaxel in human breast cancers. Clin Cancer Res 2004; 10: 8163–9. [DOI] [PubMed] [Google Scholar]
- 26. Henderson IC, Berry DA, Demetri GD et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node‐positive primary breast cancer. J Clin Oncol 2003; 21: 976–83. [DOI] [PubMed] [Google Scholar]
- 27. Mamounas EP, Bryant J, Lembersky B et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node‐positive breast cancer: results from NSABP B‐28. J Clin Oncol 2005; 23: 3686–96. [DOI] [PubMed] [Google Scholar]
- 28. Bhalla KN. Microtubule‐targeted anticancer agents and apoptosis. Oncogene 2003; 22: 9075–86. [DOI] [PubMed] [Google Scholar]
- 29. Huang Y, Ray S, Reed JC et al. Estrogen increases intracellular p26Bcl‐2 to p21Bax ratios and inhibits taxol‐induced apoptosis of human breast cancer MCF‐7 cells. Breast Cancer Res Treat 1997; 42: 73–81. [DOI] [PubMed] [Google Scholar]
- 30. Haldar S, Jena N, Croce CM. Inactivation of Bcl‐2 by phosphorylation. Proc Natl Acad Sci USA 1995; 92: 4507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sjostrom J, Blomqvist C, Von Boguslawski K et al. The predictive value of bcl‐2, bax, bcl‐xL, bag‐1, fas, and fasL for chemotherapy response in advanced breast cancer. Clin Cancer Res 2002; 8: 811–16. [PubMed] [Google Scholar]
- 32. Kraus LA, Samuel SK, Schmid SM, Dykes DJ, Waud WR, Bissery MC. The mechanism of action of docetaxel (Taxotere) in xenograft models is not limited to bcl‐2 phosphorylation. Invest New Drugs 2003; 21: 259–68. [DOI] [PubMed] [Google Scholar]
- 33. Litman T, Druley TE, Stein WD, Bates SE. From MDR, to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci 2001; 58: 931–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hill BT, Whelan RD, Shellard SA, McClean S, Hosking LK. Differential cytotoxic effects of docetaxel in a range of mammalian tumor cell lines and certain drug resistant sublines in vitro . Invest New Drugs 1994; 12: 169–82. [DOI] [PubMed] [Google Scholar]
- 35. Vincent‐Salomon A, Rousseau A, Jouve M et al. Proliferation markers predictive of the pathological response and disease outcome of patients with breast carcinomas treated by anthracycline‐based preoperative chemotherapy. Eur J Cancer 2004; 40: 1502–8. [DOI] [PubMed] [Google Scholar]
- 36. Sjostrom J, Blomqvist C, Heikkila P et al. Predictive value of p53, mdm‐2, p21, and mib‐1 for chemotherapy response in advanced breast cancer. Clin Cancer Res 2000; 6, 3103–10. [PubMed] [Google Scholar]
- 37. Yamamoto N, Tamura T, Murakami H et al. Randomized pharmacokinetic and pharmacodynamic study of docetaxel: dosing based on body‐surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J Clin Oncol 2005; 23: 1061–9. [DOI] [PubMed] [Google Scholar]
- 38. Kapucuoglu N, Coban T, Raunio H et al. Expression of CYP3A4 in human breast tumour and non‐tumour tissues. Cancer Lett 2003; 202: 17–23. [DOI] [PubMed] [Google Scholar]
- 39. Miyoshi Y, Taguchi T, Kim SJ, Tamaki Y, Noguchi S. Prediction of response to docetaxel by immunohistochemical analysis of CYP3A4 expression in human breast cancers. Breast Cancer 2005; 12: 11–15. [DOI] [PubMed] [Google Scholar]
- 40. Anand S, Penrhyn‐Lowe S, Venkitaraman AR. AURORA‐A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to taxol. Cancer Cell 2003; 3: 51–62. [DOI] [PubMed] [Google Scholar]
- 41. Hata T, Furukawa T, Sunamura M et al. RNA interference targeting aurora kinase a suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Res 2005; 65: 2899–905. [DOI] [PubMed] [Google Scholar]
- 42. Miyoshi Y, Iwao K, Egawa C, Noguchi S. Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int J Cancer 2001; 92: 370–3. [DOI] [PubMed] [Google Scholar]
- 43. Kato K. Adaptor‐tagged competitive PCR: a novel method for measuring relative gene expression. Nucleic Acids Res 1997; 25: 4694–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iwao K, Matoba R, Ueno N et al. Molecular classification of primary breast tumors possessing distinct prognostic properties. Hum Mol Genet 2002; 11: 199–206. [DOI] [PubMed] [Google Scholar]
- 45. Kurokawa Y, Matoba R, Nagano H et al. Molecular prediction of response to 5‐fluorouracil and interferon‐alpha combination chemotherapy in advanced hepatocellular carcinoma. Clin Cancer Res 2004; 10: 6029–38. [DOI] [PubMed] [Google Scholar]
- 46. Kurokawa Y, Matoba R, Takemasa I et al. Molecular‐based prediction of early recurrence in hepatocellular carcinoma. J Hepatol 2004; 41: 284–91. [DOI] [PubMed] [Google Scholar]
- 47. Muro S, Takemasa I, Oba S et al. Identification of expressed genes linked to malignancy of human colorectal carcinoma by parametric clustering of quantitative expression data. Genome Biol 2003; 4: R21 (1–10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taniguchi K, Takano T, Miyauchi A et al. Differentiation of follicular thyroid adenoma from carcinoma by means of gene expression profiling with adapter‐tagged competitive polymerase chain reaction. Oncology 2005; 69: 428–35. [DOI] [PubMed] [Google Scholar]
- 49. Yokomizo A, Ono M, Nanri H et al. Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res 1995; 55: 4293–6. [PubMed] [Google Scholar]
- 50. Pae HO, Jun CD, Yoo JC et al. Enhancing and priming of macrophages for superoxide anion production by taxol. Immunopharmacol Immunotoxicol 1998; 20: 27–37. [DOI] [PubMed] [Google Scholar]
- 51. Pusztai L, Rouzier R, Wagner P, Symmans WF. Individualized chemotherapy treatment for breast cancer: is it necessary? Is it feasible? Drug Resist Update 2004; 7: 325–31. [DOI] [PubMed] [Google Scholar]
- 52. Goncalves A, Esterni B, Bertucci F et al. Postoperative serum proteomic profiles may predict metastatic relapse in high‐risk primary breast cancer patients receiving adjuvant chemotherapy. Oncogene 2006; 25: 981–9. [DOI] [PubMed] [Google Scholar]
- 53. Pusztai L, Gregory BW, Baggerly KA et al. Pharmacoproteomic analysis of prechemotherapy and postchemotherapy plasma samples from patients receiving neoadjuvant or adjuvant chemotherapy for breast carcinoma. Cancer 2004; 100: 1814–22. [DOI] [PubMed] [Google Scholar]


