Abstract
The combination of docetaxel, cisplatin, and fluorouracil significantly enhances the survival of head and neck cancer patients compared to cisplatin and fluorouracil. We hypothesized that docetaxel may affect invasiveness of the head and neck cancer cells in addition to its tumor‐killing effect. Two different head and neck cancer cell lines (HEp‐2 and Ca9‐22) were treated with docetaxel at IC10 and IC50 concentrations. Cell migration and invasive growth was evaluated by wound healing assay and three‐dimensional (3D) culture of multicellular tumor spheroids, respectively. Expression levels of possible downstream effectors for cell migration/invasiveness were measured by immunoblotting in conditions with or without docetaxel. Docetaxel, but not cisplatin, suppressed filopodia formation compared with no treatment (control) condition. Consistent with this, docetaxel suppressed two‐dimensional (2D) cell migration and 3D cell invasion compared with control or cisplatin. Only docetaxel treated cells exhibited thick tubulin bundle and had lower activity of Cdc42, a member of the Rho family of small GTPases. In conclusion, Docetaxel treatment suppressed migration and invasiveness of head and neck cancer cells in vitro, which is likely to be mediated by regulating Cdc42 activity. (Cancer Sci 2010; 00: 000–000)
Squamous cell carcinoma (SCC) of the head and neck accounts for 5% of cancers in adults worldwide.( 1 ) The disease is potentially curable at an early stage, but most patients present with locally advanced disease. Only 30–50% of patients with locally advanced disease live for 3 years after surgery and radiation therapy. Locoregional recurrences or distant metastases develop in 40–60% of them.( 2 , 3 , 4 , 5 ) Moreover, radical resection of the tumor, which results in loss of the larynx, severely compromises quality of life in such patients. In order to increase the survival rate and preserve voice and swallowing function over surgery and radiation alone, various supplementary chemotherapy regimens have been developed.( 6 , 7 , 8 ) Recently it was reported that the combination of docetaxel, cisplatin, and fluorouracil (TPF) followed by chemoradiotherapy significantly enhances survival of head and neck cancer patients compared to cisplatin and fluorouracil (PF) followed by chemoradiotherapy.( 9 ) Estimates of overall survival at 3 years were 62% in the TPF and 48% in the PF groups. It was also reported that incidence of distant metastasis is lower in the TPF (5%vs 9%), although it does not reach statistical significance. We therefore hypothesized that docetaxel may affect migration and invasiveness of the head and neck cancer cells. The mechanism of antitumor effect of the taxanes, such as docetaxel is thought to be the stabilization of microtubules during mitosis, which leads to blockade of cell division and eventually cell death. However, the effect of docetaxel on cell invasiveness has not been evaluated in head and neck cancers. We demonstrate here that docetaxel inhibited migration of head and neck cancer cells in vitro, suggesting that microtubule plays an important role in tumor cell motility.
Material and Methods
Cell culture. Two human head and neck SCC cell lines were used. HEp‐2 cells derived from larynx was obtained from the American Type Culture Collection (Manassas, VA, USA). Ca9‐22 cells derived from gingiva was obtained from the Japanese Collection of Research Bioresources (Osaka, Japan). HEp‐2 and Ca9‐22 cells were cultured in Eagle’s Minimum essential medium with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Proliferation assay. The anti‐proliferative effects of cisplatin (Wako, Osaka, Japan) and docetaxel (Toronto Research Chemicals, Toronto, ON, Canada) were analyzed in water soluble tetrazolium assay (a modified MTT assay). Cells were plated in 96‐well plates at 1000 cells per well. After 48 h of incubation, the cells were exposed to a drug for 1 h. After washing with PBS, the cells were grown in culture medium for an additional 96‐h period. The number of viable cells was determined by addition of WST‐8 (Dojindo laboratories, Kumamoto, Japan) as per the manufacturer’s instructions. The IC10 and IC50 values, being the drug concentrations at 10% and 50% cell growth inhibition compared with control cell growth, were used in all further experiments.
Migration assay. HEp‐2 and Ca9‐22 cells were seeded in 90‐mm dishes at 5 × 105 cells per dish. At 48 h of incubation, cells were treated with culture medium without drug (control), cisplatin, or docetaxel for 1 h. After washing, cells were maintained in culture medium for an additional 72‐h period, and a scratch wound was applied with a sterile 200‐μL pipette tip. Wound width was measured in four areas at time 0 (immediately after wounding) and after 12 or 24 h of incubation. At the end of the incubation, the cells were fixed with 4% paraformaldehyde for 10 min and stained for α‐tubulin (1:20; Calbiochem, La Jolla, CA, USA) and phalloidin (200 units/mL; Invitrogen, Carlsbad, CA, USA) (see Immunocytochemistry).
Three‐dimensional (3D)‐gel culture assay. HEp‐2 cells were seeded in a 96‐well low attachment plate (Smilon celltite spheroid U; Sumitomo Bakelite, Tokyo, Japan) at 2 × 103 cells per well. Multicellular tumor spheroids were observable around 48 h after plating. The spheroids were placed into the extracellular matrix (Matrigel; BD Biosciences, San Jose, CA, USA) in Transwell filters (Corning, Corning, NY, USA). After 48 h of incubation in the Transwell, the spheroids were treated with cisplatin, docetaxel, or culture medium (control) for 1 h at IC10 and IC50. After washing, cells were grown in culture medium for an additional 96‐h period.
Western blot analysis. HEp‐2 cells and Ca9‐22 cells were seeded at 1 × 106 cells in a 90‐mm dish, allowed 2 days for attachment, and treated with docetaxel and cisplatin at IC10 for 1 h. At 0, 24, and 48 h of culture (only immediately after treatment in the evaluation of Rho GTPases), cells were washed twice with ice‐cold PBS and were harvested by scraping in cell lysis buffer (50 mM Tris‐HCl, 150 mM NaCl, 0.5 mM EDTA, 1% Triton X‐100) with protease inhibitors (Roche Applied Science, Mannheim, Germany). After homogenization and centrifugation for 30 min at 13 000 g , the supernatants (30 μg of protein) were subjected to SDS–PAGE, followed by transferring to Hybond‐P PVDF membrane (GE Healthcare, Little Chalfont, UK). The membrane was incubated with either anti‐phospho Ezrin/Radixin/Moesin (ERM), cofilin, LIM domain kinase 1 (LIMK1) (Cell Signaling Technology, Danvers, MA, USA), Cdc42 (1:1000; Cytoskeleton), Rac1 (1:250; Cytoskeleton, Denver, CO, USA), or RhoA (1:500; Cytoskeleton). After washing, it was incubated with horseradish peroxidase‐conjugated antirabbit IgG or antimouse IgG (Jackson Immuno Research Laboratories, West Grove, PA, USA). The images were obtained with Fujifilm LAS‐4000 mini (Fujifilm, Tokyo, Japan).
Immunocytochemistry. HEp‐2 cells were seeded on glass coverslips in a 12‐well plate at 3 × 104 cells per well. After 48 h of culture, the cells were treated with docetaxel or cisplatin at IC10 for 1 h. After washing, the cells were fixed with cold methanol for 10 min. The fixed cells were made permeable with 0.2% Triton X‐100 (Sigma‐Aldrich, St. Louis, MO, USA) in PBS and incubated with a monoclonal antibody against Cdc42 (1:200; Cytoskeleton), Rac1 (1:20; Cytoskeleton), or RhoA(1:100; Cytoskeleton) in 10% goat serum. After washing, the coverslips were incubated with FITC‐labeled antimouse IgG antibodies (1:200; Invitrogen) and DAPI (1:500; Roche). After washing, coverslips were mounted in Dakocytomation Fluorescent Mounting Medium (Dako, Carpinteria, CA, USA) and were observed with a laser confocal microscope FV1000‐D (Olympus, Tokyo, Japan).
Rho GTPases activity assay. A G‐LISA Cdc42, Rac, and Rho‐A activation assay biochemistry kit (Cytoskeleton) was used for measuring Cdc42, Rac, and Rho‐A activity. HEp‐2 cells and Ca9‐22 cells were seeded at 5 × 105 cells per dish in 90‐mm dish, allowed 2 days for attachment and treated with docetaxel or cisplatin at IC10 for 1 h. Immediately after washing twice with ice‐cold PBS, the cells were harvested by scraping in lysis buffer and cells were processed rapidly on ice. The lysates were clarified by centrifugation at 7300–17 800g at 4°C for 2 min. Protein concentration was determined according to the manufacturer’s protocol, and the cell extracts were adjusted to 0.25 mg/mL for Cdc42 assay and 1.5 mg/mL for Rac/RhoA assay.
Statistical analysis. Welch’s t‐test was used to compare all experimental results. A P‐value less than 0.05 was considered significant.
Results
Determination of IC10 and IC50 in HEp‐2 and Ca9‐22 cells. The antiproliferative effects of cisplatin and docetaxel in HEp‐2 and Ca9‐22 cells were determined by a modified MTT assay (WST assay). Both drugs inhibited cell growth in a dose‐dependent manner (Fig. 1). HEp‐2 cells were more sensitive to cisplatin than Ca9‐22 cells, while Ca9‐22 cells were more sensitive to docetaxel. The IC10 and IC50 concentrations were used in the following experiments (Table 1).
Figure 1.
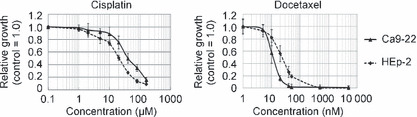
Growth curve after 1‐h drug exposure followed by a 96‐h incubation in cisplatin or docetaxel treatment. Control cell growth = 1.0. Each data point represents mean ± SE.
Table 1.
IC10 or IC50 values in two cell lines
| Cell line | Anticancer agent | IC10 | IC50 |
|---|---|---|---|
| HEp‐2 | Cisplatin | 2 μM | 20 μM |
| Docetaxel | 5 nM | 23 nM | |
| Ca9‐22 | Cisplatin | 10 μM | 40 μM |
| Docetaxel | 6 nM | 11 nM |
IC10 or IC50 values after 1‐h drug exposure followed by a 96‐h incubation in two head and neck cell lines.
Docetaxel inhibits the migration of head and neck cancer cells. Migration was assessed in a wound healing assay. In HEp‐2 cells, wound closure relative to no treatment condition was 100.8 ± 3.5% and 71.4 ± 7.8% (mean ± SE) after cisplatin and docetaxel treatment at IC10, respectively (P < 0.001). In Ca9‐22 cells, wound closure was 102.3 ± 3.1% and 58.7 ± 5.6% after cisplatin and docetaxel treatment at IC10, respectively (P < 0.001) (Fig. 2). Docetaxel significantly inhibited the migration as compared to cisplatin treatment or no treatment in both cell lines.
Figure 2.
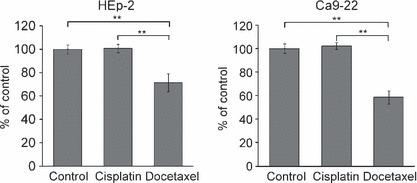
Migration assay at IC10 in two head and neck cancer cell lines. Migration rate compared to control cell migration rate. Each data point represents mean ± SE. **P < 0.001.
Docetaxel inhibits the invasiveness of multicellular tumor spheroids. Because 3D multicellular tumor spheroids provide a microenvironment that mimics tumors in vivo,( 10 ) spheroid culture has been used to evaluate tumor cell invasiveness and/or its responsiveness to anticancer drugs.( 11 , 12 , 13 , 14 , 15 ) At IC10 determined in monolayer culture, both cisplatin or docetaxel did not affect filopodia formation. However, at IC50 also determined in monolayer culture, docetaxel, but not cisplatin, significantly decreased filopodia formation in HEp‐2 cells in spheroid culture (Fig. 3). It has been shown that tumor cells in spheroid culture require higher concentrations of chemotherapeutic agents to inhibit their growth than those in monolayer culture. Therefore, it is not surprising that a higher concentration of docetaxel was required to suppress cell invasion in spheroid than in monolayer culture. In fact, the maximum plasma concentration of docetaxel in cancer patients treated by this drug is reported to be 1000‐fold higher than the concentration used in our experiment.( 16 ) Thus, we do not think that the docetaxel concentration in our experiment was high enough to non‐specifically affect various cell functions. Because Ca9‐22 cells formed cysts instead of spheroids under the culture condition, we could not evaluate the effect of docetaxel or cisplatin on the invasiveness of this cell line. Taken together with the result of wound healing assay, docetaxel, but not cisplatin, treatment inhibited head and neck cancer cell motility and invasiveness.
Figure 3.
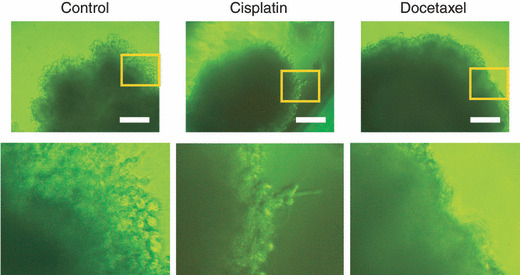
Three‐dimensional‐Gel culture of HEp‐2 cell spheroids at IC50 concentration. HEp‐2 cell spheroids were treated with cisplatin or docetaxel at IC50 followed by 96‐h incubation. Bars, 250 μm.
Docetaxel suppresses filopodia formation. By phalloidin staining, the filamentous actin structure of HEp‐2 cells and Ca9‐22 cells was visualized (Fig. 4). Filopodia formation was suppressed when cells were treated with docetaxel. But no gross abnormality was found in the actin filament structure. Ezrin/radixin/moesin (ERM) proteins link the cortical cytoskeleton to the plasma membrane. In their active conformation (i.e. phosphorylated ERM), the N‐terminal ERM domain binds to the cytoplasmic tails of transmembrane proteins, and the C‐terminal ERM association domain binds to actin filaments.( 17 ) Using a p‐ERM antibody, the levels of active ERM proteins were evaluated by western blotting after no treatment, cisplatin, or docetaxel treatment at IC10. There was no significant difference in the level of p‐ERM among cisplatin, docetaxel, and no treatment over a time course up to 48 h (data not shown). We also investigated the cofilin pathway as a regulator of the actin cytoskeleton. Cofilin is a small protein that is able to bind both G‐actin and F‐actin, and regulated by LIM kinase 1 and its related kinases. The levels of cofilin and LIMK1 were evaluated over a time course of treatment at IC10. The levels of these proteins were also not significantly different among cisplatin, docetaxel, and no treatment (data not shown).
Figure 4.
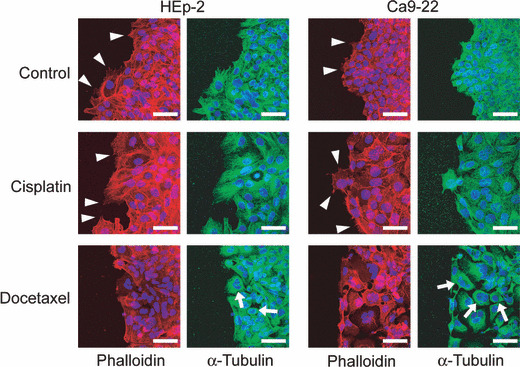
Staining of α‐tubulin (anti‐α‐tubulin), F‐actin (phalloidin), and nucleus (DAPI) in HEp‐2 cells and Ca9‐22 cells treated by cisplatin, docetaxel, or no treatment at IC50. Arrows, thick tubulin bundle; arrow heads, filopodia. Bars, 50 μm.
Tubulin bundle is formed by docetaxel treatment. Docetaxel acts as a microtubule stabilizer so that tubulin monomers cannot break apart from microtubules for remodeling.( 18 ) Cytotoxic effect is believed to be due to inhibition of centrosome‐associated microtubule turnover that eventually affect cell division.( 19 ) But docetaxel may exert similar effect on cytosolic, non‐centrosome associated microtubules. We therefore examined the structure of microtubules. Tubulin bundle formation was noted in docetaxel treatment (Fig. 4), as has been previously demonstrated with docetaxel or paclitaxel,( 20 , 21 ) but not in cisplatin treatment. This deformed microtubular structure may be sufficient to inhibit cell movement. However, the fact that docetaxel treatment inhibited filopodia formation suggests that there might be a connection between microtubular deformity and the actin remodeling process.
Docetaxel inhibits Cdc42 activity and its subcellular local‐ization. Rho GTPases are molecular switches that regulate many essential cellular processes, including actin dynamics, gene transcription, cell‐cycle progression, cell adhesion, tumor progression, and invasiveness.( 22 , 23 , 24 ) Of Rho‐GTPases, Cdc42 has been previously implicated in connecting microtubular input to actin filament organization.( 25 ) Cdc42 also promotes leading‐edge extension through activation of Rac. Rac, and Rho are implicated in the formation of lamellipodia and stress fiber, respectively.( 26 ) Thus, we examined the activity of Cdc42, Rac, and RhoA in the cells that underwent cisplatin or docetaxel treatment or no treatment. At IC10 concentration, docetaxel significantly decreased Cdc42 and Rac activity in HEp‐2 cells, but not RhoA activity (Fig. 5a). The total amount of Cdc42, Rac, and RhoA was not significantly different among these three conditions (Fig. 5b).
Figure 5.
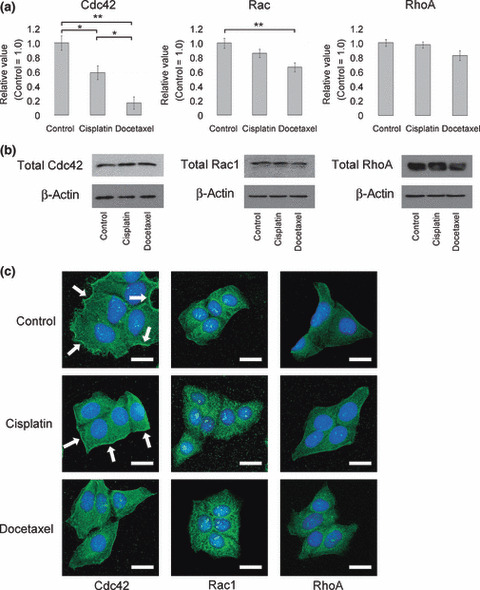
(a) Colorimetric assay of Cdc42, Rac, and RhoA activity in HEp‐2 cells. The levels of activated Cdc42, Rac, and RhoA in HEp‐2 cells were evaluated immediately after 1 h of indicated treatment. Each data point represents mean ± SE. *P < 0.05, **P < 0.01. (b) Western blot analysis of rho family GTPases. (c) Immunostaining of Cdc42, Rac1, and RhoA in HEp‐2 cells treated by cisplatin, docetaxel, or no treatment at IC10. Nucleus was stained by DAPI. Arrows, Cdc42 localized at plasma membrane of HEp‐2 cells. Bars, 25 μm.
It is reported that Cdc42 is activated in a thin band at cell edges extending filopodia.( 27 ) Consistent with the results of activity assay, Cdc42 localizing at the plasma membrane was decreased after docetaxel treatment at IC10 (Fig. 5c). Localization of Rac1 and RhoA had no apparent changes after treatment compared to control.
Discussion
We have shown that IC10 and/or IC50 concentration of docetaxel, but not cisplatin, inhibited migration of head and neck cancer cell lines both in 2D and 3D cultures. These results suggest that docetaxel inhibits local invasion of head and neck cancer cells. Although the effect of docetaxel on cell migration and invasiveness of ovary cancer cells,( 28 ) umbilical vein endothelial cells,( 29 ) and glioma cells( 30 ) has been described, its effect on head and neck cancer cells has not been evaluated.
Moreover, we also revealed that docetaxel treatment decreased Cdc42 activities as compared to cisplatin treatment or control in HEp‐2 cells. Actin polymerization and filament elongation at the front, coupled to actin filament contraction at the rear, are thought to provide the major driving forces for migration in animal cells. Persistent and efficient directed migration requires additional cellular changes involving polarization of the microtubule cytoskeleton and secretory pathway.( 31 , 32 ) Among Rho GTPases, Cdc42 was reported to control the polarity of actin and microtubule through distinct signal transduction pathways.( 25 ) On the other hand, microtubule or actin may direct Cdc42 activation to specific peripheral locations.( 33 , 34 , 35 , 36 ) Rac1/Cdc42 capture microtubule plus ends through IQ motif containing GTPase activating protein 1 (IQGAP1) and CAP‐GLY domain containing linker protein 1 (CLIP‐170 also known as CLIP‐1), and form a tripartite complex.( 37 ) This complex links microtubules and special cortical regions, which is essential for cell polarization or inducing multiple leading edges.( 37 ) Moreover, both upstream guanine‐nucleotide‐exchange‐factor activators of Rac1/Cdc42, including guanine nucleotide exchange factor‐H1 (GEF‐H1),( 38 ) Vav,( 39 ) and Lfc,( 40 ) and the downstream Rac1/Cdc42 effectors MLK2 and JNK,( 41 ) either bind to tubulin or localize to microtubules in cells.( 42 ) These mechanisms would explain how growing microtubule plus ends could provide active Rac1/Cdc42 to the leading cell edge. We propose that the abnormal tubulin bundle induced by docetaxel lead to suppression of Cdc42 activity by losing the ability to direct Cdc42 activation to intended locations of plasma membrane. Decreased Cdc42 localization at the plasma membrane after docetaxel treatment (Fig. 5) is consistent with this possibility. This decreased Cdc42 activity was likely to affect actin filament formation at the leading edge, resulting in decreased filopodia (Fig. 4).
In conclusion, these results suggest that docetaxel treatment has the benefit of reducing local invasion in addition to its tumor‐killing effect, giving experimental support for clinical data that TPF is better than PF for treating head and neck cancer.
Acknowledgments
This work was supported in part by grants from Yamada Bee Farm Grant for Honeybee Research and Setsuro Fujii Memorial, and the Osaka Foundation for Promotion of Fundamental Medical Research.
References
- 1. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106–30. [DOI] [PubMed] [Google Scholar]
- 2. Adelstein DJ, Li Y, Adams GL et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003; 21: 92–8. [DOI] [PubMed] [Google Scholar]
- 3. Denis F, Garaud P, Bardet E et al. Final results of the 94‐01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced‐stage oropharynx carcinoma. J Clin Oncol 2004; 22: 69–76. [DOI] [PubMed] [Google Scholar]
- 4. Bonner JA, Harari PM, Giralt J et al. Radiotherapy plus cetuximab for squamous‐cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567–78. [DOI] [PubMed] [Google Scholar]
- 5. Forastiere AA, Goepfert H, Maor M et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 2003; 349: 2091–8. [DOI] [PubMed] [Google Scholar]
- 6. Kohno N. The role of chemotherapy for advanced oro and hypopharyngeal cancer. Auris Nasus Larynx 2004; 31: 113–8. [DOI] [PubMed] [Google Scholar]
- 7. Hong WK, Bromer R. Chemotherapy in head and neck cancer. N Engl J Med 1983; 308: 75–9. [DOI] [PubMed] [Google Scholar]
- 8. Kogashiwa Y, Yamauchi K, Nagafuji H et al. Concurrent chemoradiotherapy for organ function preservation in advanced patients with hypopharyngeal and laryngeal cancer. Oncol Rep 2009; 22: 1163–7. [DOI] [PubMed] [Google Scholar]
- 9. Posner MR, Hershock DM, Blajman CR et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007; 357: 1705–15. [DOI] [PubMed] [Google Scholar]
- 10. Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 1988; 240: 177–84. [DOI] [PubMed] [Google Scholar]
- 11. Doillon CJ, Gagnon E, Paradis R, Koutsilieris M. Three‐dimensional culture system as a model for studying cancer cell invasion capacity and anticancer drug sensitivity. Anticancer Res 2004; 24: 2169–77. [PubMed] [Google Scholar]
- 12. Inoue S, Ohnuma T, Takaoka K et al. Effects of doxorubicin and cisplatin on multicellular tumor spheroids from human lung cancer. Cancer Drug Deliv 1987; 4: 213–24. [DOI] [PubMed] [Google Scholar]
- 13. Kohno N, Ohnuma T, Biller HF, Holland JF. Effects of cisplatin plus fluorouracil vs cisplatin plus cytarabine on head and neck squamous multicellular tumor spheroids. Arch Otolaryngol Head Neck Surg 1988; 114: 157–61. [DOI] [PubMed] [Google Scholar]
- 14. Kohno N, Ohnuma T, Holland JF, Biller H. Effects of anticancer agents on the shedding of cells from human multicellular tumor spheroids. Invasion Metastasis 1987; 7: 264–74. [PubMed] [Google Scholar]
- 15. Kohno N, Ohnuma T, Kaneko M, Holland JF. Interactions of doxorubicin and cis‐platin in squamous carcinoma cells in culture. Br J Cancer 1988; 58: 330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bruno R, Hille D, Riva A et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 1998; 16: 187–96. [DOI] [PubMed] [Google Scholar]
- 17. Fievet BT, Gautreau A, Roy C et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol 2004; 164: 653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gueritte‐Voegelein F, Guenard D, Lavelle F, Le Goff MT, Mangatal L, Potier P. Relationships between the structure of taxol analogues and their antimitotic activity. J Med Chem 1991; 34: 992–8. [DOI] [PubMed] [Google Scholar]
- 19. Hennequin C, Giocanti N, Favaudon V. S‐phase specificity of cell killing by docetaxel (Taxotere) in synchronised HeLa cells. Br J Cancer 1995; 71: 1194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst 1991; 83: 288–91. [DOI] [PubMed] [Google Scholar]
- 21. Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA 1980; 77: 1561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res 2000; 261: 1–12. [DOI] [PubMed] [Google Scholar]
- 23. Price LS, Collard JG. Regulation of the cytoskeleton by Rho‐family GTPases: implications for tumour cell invasion. Semin Cancer Biol 2001; 11: 167–73. [DOI] [PubMed] [Google Scholar]
- 24. Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279: 509–14. [DOI] [PubMed] [Google Scholar]
- 25. Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci 2005; 118: 2579–87. [DOI] [PubMed] [Google Scholar]
- 26. Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 2000; 348 (Pt 2): 241–55. [PMC free article] [PubMed] [Google Scholar]
- 27. Nalbant P, Hodgson L, Kraynov V, Toutchkine A, Hahn KM. Activation of endogenous Cdc42 visualized in living cells. Science 2004; 305: 1615–9. [DOI] [PubMed] [Google Scholar]
- 28. Bijman MN, Van Berkel MP, Van Nieuw Amerongen GP, Boven E. Interference with actin dynamics is superior to disturbance of microtubule function in the inhibition of human ovarian cancer cell motility. Biochem Pharmacol 2008; 76: 707–16. [DOI] [PubMed] [Google Scholar]
- 29. Bijman MN, Van Nieuw Amerongen GP, Laurens N, Van Hinsbergh VW, Boven E. Microtubule‐targeting agents inhibit angiogenesis at subtoxic concentrations, a process associated with inhibition of Rac1 and Cdc42 activity and changes in the endothelial cytoskeleton. Mol Cancer Ther 2006; 5: 2348–57. [DOI] [PubMed] [Google Scholar]
- 30. Terzis AJ, Thorsen F, Heese O et al. Proliferation, migration and invasion of human glioma cells exposed to paclitaxel (Taxol) in vitro . Br J Cancer 1997; 75: 1744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ridley AJ, Schwartz MA, Burridge K et al. Cell migration: integrating signals from front to back. Science 2003; 302: 1704–9. [DOI] [PubMed] [Google Scholar]
- 32. Bershadsky AD, Vaisberg EA, Vasiliev JM. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug‐induced microtubule depolymerization. Cell Motil Cytoskeleton 1991; 19: 152–8. [DOI] [PubMed] [Google Scholar]
- 33. Etienne‐Manneville S, Hall A. Integrin‐mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 2001; 106: 489–98. [DOI] [PubMed] [Google Scholar]
- 34. Waterman‐Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol 1999; 1: 45–50. [DOI] [PubMed] [Google Scholar]
- 35. Higashida C, Miyoshi T, Fujita A et al. Actin polymerization‐driven molecular movement of mDia1 in living cells. Science 2004; 303: 2007–10. [DOI] [PubMed] [Google Scholar]
- 36. Wedlich‐Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin‐based delivery of the Cdc42 GTPase. Science 2003; 299: 1231–5. [DOI] [PubMed] [Google Scholar]
- 37. Fukata M, Watanabe T, Noritake J et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP‐170. Cell 2002; 109: 873–85. [DOI] [PubMed] [Google Scholar]
- 38. Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF‐H1, a microtubule‐associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem 1998; 273: 34954–60. [DOI] [PubMed] [Google Scholar]
- 39. Fernandez JA, Keshvara LM, Peters JD, Furlong MT, Harrison ML, Geahlen RL. Phosphorylation‐ and activation‐independent association of the tyrosine kinase Syk and the tyrosine kinase substrates Cbl and Vav with tubulin in B‐cells. J Biol Chem 1999; 274: 1401–6. [DOI] [PubMed] [Google Scholar]
- 40. Glaven JA, Whitehead I, Bagrodia S, Kay R, Cerione RA. The Dbl‐related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J Biol Chem 1999; 274: 2279–85. [DOI] [PubMed] [Google Scholar]
- 41. Nagata K, Puls A, Futter C et al. The MAP kinase kinase kinase MLK2 co‐localizes with activated JNK along microtubules and associates with kinesin superfamily motor KIF3. EMBO J 1998; 17: 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995; 81: 53–62. [DOI] [PubMed] [Google Scholar]


