Abstract
Several studies have investigated the associations between X‐ray repair cross‐complementing group 3 (XRCC3) Thr241Met polymorphism and the susceptibility to lung cancer and bladder cancer, but results have been inconclusive. In order to derive a more precise estimation of the relationship, a meta‐analysis was performed. A total of 22 case control studies, including 2976 cases and 4495 controls for lung cancer, and 3445 cases and 4599 controls for bladder cancer, met the inclusion criteria and were selected. Overall, there was no evidence showing a significant association between XRCC3 Thr241Met polymorphism and lung cancer risk. Furthermore, the results for bladder cancer showed that significant decreased risk was found for the additive model (odds ratio [OR] = 0.959, 95% confidence interval [CI], 0.924–0.996) and dominant model (OR = 0.982, 95% CI, 0.963–1.000) but not for the recessive model (OR = 0.958, 95% CI, 0.905–1.014). In summary, our meta‐analysis indicates that XRCC3 Thr241Met polymorphism may be weakly associated with the risk of bladder cancer. (Cancer Sci 2010)
Lung cancer is one of the most common cancers worldwide, and it is the leading cause of cancer‐related deaths in the world.( 1 ) The role of genetic susceptibility in lung cancer has shown that the relatives of patients with lung cancer had an increased risk of the disease.( 1 , 2 ) Only a fraction of smokers and a low number of non‐smokers develop lung cancer, which implies influence of host factors in individual susceptibility. This inter‐individual difference in susceptibility may be attributed to genetic polymorphisms in critical genes, including those involved in DNA repair.( 3 , 4 )
Bladder cancer is among the most frequent diagnosed cancer in the developed world.( 5 ) Although development of bladder cancer is associated with exposure to tobacco and occupational exposure,( 6 ) only a small proportion of exposed individuals will develop cancer, suggesting the involvement of genetic factors.
DNA repair systems play an critical role in maintaining genomic integrity.( 3 ) If DNA damage is unrepaired, mutations are propagated during subsequent cellular replication and ultimately result in activation of oncogenes or inactivation of tumor suppressor genes. So mutations on these genes which alter the function of these proteins may predispose an individual to cancer. Increasing molecular epidemiologic evidence has shown that polymorphisms in various DNA repair genes are associated with an increased risk of cancer.( 7 , 8 )
The X‐ray repair cross‐complementing group 3 (XRCC3) belongs to a family of genes responsible for repairing DNA double strand breaks caused by normal metabolic processes and/or exposure to ionizing radiation. The XRCC3 is involved in homologous recombination repair (HRR) and chromosomal double‐strand breaks repair processes, and it is necessary to maintain genomic integrity. It was demonstrated that cell lines defective in XRCC3 had a 25‐fold decrease in homology directed repair of DNA double‐strand breaks.( 9 ) Shen et al. ( 10 ) identified a C to T substitution in exon 7 at position 18067 of XRCC3, which results in a amino acid substitution (threonine to methionine) at codon 241. Carriers of the variant allele of XRCC3 Thr241Met had different DNA adduct levels in lymphocyte DNA; and the Met variant was significantly associated with higher DNA adduct levels, indicating that this polymorphism was associated with the DNA repair capacity.( 11 ) For this reason, we thought that the Met allele of the polymorphism should increase the risk of cancer.
Although the association between the risk of lung and bladder cancer and XRCC3 Thr241Met polymorphism had been widely investigated, the results were inconsistent; and most studies included only small numbers of cases and controls. To determine the effects of this polymorphism on the risk of lung and bladder cancer, we have undertaken a meta‐analysis.
Materials and Methods
Identification of studies. To identify all studies that examined the association of XRCC3 Thr241Met polymorphisms with lung and bladder cancer, we conducted a literature search of the PubMed database, without a language limitation, covering all papers published up to March 2010, using the following keywords and subject terms: X‐ray repair cross‐complementing group 3, XRCC3, polymorphism, lung neoplasms, lung, neoplasms, and cancer; or X‐ray repair cross‐complementing group 3, XRCC3, polymorphism, urinary bladder neoplasms, bladder, neoplasms, and cancer. We evaluated potentially relevant publications by checking their titles and abstracts and then obtained the most relevant publications for a detailed examination. Moreover, the reference lists of the selected papers were also screened for other potential articles that may have been missed in the initial search.
Selection criteria. The following criteria were used for selection of reports for the meta‐analysis: (i) studies concerning the association of the XRCC3 Thr241Met polymorphism with lung cancer or bladder cancer; (ii) case‐control studies; and (iii) studies with available genotype frequency, and genotype distribution of control population had to be in Hardy–Weinberg equilibrium (HWE). Accordingly, the following exclusion criteria were also used: (i) the design and the definition of the experiments were obviously different from those of the selected papers; (ii) the source of cases and controls and other essential information were not provided; and (iii) reviews and duplicated publications. After searching, we reviewed all papers in accordance with the criteria defined above for further analysis.
Data extraction. Data were carefully extracted from all eligible publications independently by two of the authors according to the inclusion criteria mentioned above. The following information was extracted from each article: first author, year of publication, country of origin, genotyping methods, genotype frequency, and the design of experiment for XRCC3 Thr241Met polymorphism genotyping information. For conflicting evaluations, a final consensus was obtained following a discussion.
Statistical analysis. The odds ratio (OR) of XRCC3 Thr241Met polymorphisms and lung cancer or bladder cancer risk was estimated for each study. The effect of association was indicated as OR with the corresponding 95% confidence interval (CI). The pooled ORs were performed for an additive model (C/C vs T/T), a dominant model (C/C+C/T vs T/T), and a recessive model (C/C vs C/T+T/T). The chi square‐based Q statistical test was performed to assess heterogeneity among studies.( 12 ) A P‐value >0.05 for the Q‐test indicated a lack of heterogeneity among studies, so the pooled OR estimate of the each study was calculated by the fixed‐effects model (Mantel–Haenszel method.( 13 )) Otherwise, the random‐effects model (DerSimonian and Laird method( 14 )) was used. Subgroup analyses were performed by ethnicity, study design, and smoking habits. Sensitivity analysis was performed to assess the stability of the results. A single study involved in the meta‐analysis was deleted each time to reflect the influence of the individual data set to the pooled ORs. An estimate of potential publication bias was assessed by visual inspection of funnel plots,( 15 ) in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot indicates a possible publication bias. The symmetry of the funnel plot was further evaluated by Egger’s linear regression test (P < 0.05 was considered indicative of significant publication bias).( 16 ) To test for population stratification, the distribution of genotypes in control subjects of each individual population was tested for departure from the Hardy–Weinberg equilibrium using the chi square‐test. Statistical analysis was performed using STATA version 10.1 (Stata Corporation, College Station, TX, USA).
Results
Study characteristics. Through literature search and selection based on the inclusion criteria, 39 studies were found, but only 22 studies met our inclusion criteria, as listed in Table 1. Seventeen studies were excluded for the following reasons: five studies did not contain exact genotype distribution information;( 17 , 18 , 19 , 20 , 21 ) four studies were reviews;( 22 , 23 , 24 , 25 ) four studies were not case‐control studies;( 26 , 27 , 28 , 29 ) and in three studies,( 30 , 31 , 32 ) genotype distributions in control population deviated from the Hardy–Weinberg equilibrium. Furthermore, one study of bladder cancer( 33 ) in which the variant allele frequency was extremely lower than expected, which may reflect wrong allele counting or poor genotyping quality, was also excluded from our meta‐analysis. Among the 22 studies, two populations (Caucasians and African) were included in one study,( 34 ) so we divided the relevant data into two studies; and two studies( 35 , 36 ) were just included in the recessive model because they provided the genotype of C/T+T/T as a whole. The data for this analysis were derived from 22 studies, including 2976 cases and 4495 controls for lung cancer from 13 studies, and 3445 cases and 4599 controls for bladder cancer from nine studies. Table 1 lists the identified studies and their main characteristics.
Table 1.
Main characteristics of all studies included in the meta‐analysis
| Author | Year | Country (Racial descent) | Design | Methods | Case | Control | Case | Control | HWE (P) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/C | C/T | T/T | C/C | C/T | T/T | ||||||||
| Lung cancer | |||||||||||||
| Matullo( 42 ) | 2006 | Europe (Caucasian) | PB | TaqMan | 116 | 1094 | 44 | 56 | 16 | 383 | 544 | 167 | 0.249 |
| David‐Beabes( 34 ) | 2001 | USA (Caucasian) | PB | PCR‐RFLP | 178 | 453 | 76 | 78 | 24 | 175 | 210 | 68 | 0.701 |
| David‐Beabes( 34 ) | 2001 | USA (African) | PB | PCR‐RFLP | 153 | 234 | 90 | 54 | 9 | 136 | 88 | 10 | 0.365 |
| Misra( 43 ) | 2003 | Finland (Caucasian) | PB | TaqMan | 313 | 306 | 160 | 124 | 29 | 149 | 134 | 23 | 0.339 |
| López‐Cima( 44 ) | 2007 | Spain (Caucasian) | HB | PCR‐RFLP | 403 | 434 | 168 | 185 | 50 | 178 | 196 | 60 | 0.607 |
| Zienolddiny( 45 ) | 2006 | Norway (Caucasian) | PB | TaqMan | 220 | 250 | 114 | 90 | 16 | 115 | 111 | 24 | 0.709 |
| Improta( 46 ) | 2008 | Italy (Caucasian) | HB | PCR‐RFLP | 94 | 121 | 31 | 33 | 30 | 67 | 46 | 8 | 0.978 |
| Zhang( 47 ) | 2007 | China (Asian) | HB | TaqMan | 291 | 273 | 259 | 30 | 2 | 244 | 28 | 1 | 0.837 |
| Popanda( 48 ) | 2004 | Germany (Caucasian) | HB | PCR‐RFLP | 462 | 459 | 175 | 201 | 86 | 168 | 222 | 69 | 0.756 |
| Jacobsen( 49 ) | 2004 | Denmark (Caucasian) | Cohort | TaqMan | 246 | 269 | 95 | 123 | 28 | 113 | 113 | 43 | 0.105 |
| Xia( 50 ) | 2008 | China (Asian) | PB | TaqMan | 103 | 139 | 91 | 12 | 0 | 118 | 21 | 0 | 0.335 |
| Harms( 51 ) | 2004 | USA (Caucasian) | HB | PCR‐RFLP | 110 | 119 | 61 | 37 | 12 | 61 | 49 | 9 | 0.845 |
| Rky( 35 ) | 2006 | Sweden (Caucasian) | PB | TaqMan | 175 | 154 | 79 | 96 | 56 | 98 | NA | ||
| Wang( 36 ) | 2003 | USA (Mixed) | PB | PCR‐RFLP | 112 | 190 | 69 | 43 | 119 | 71 | NA | ||
| Bladder cancer | |||||||||||||
| Figueroa( 52 ) | 2007 | Spain (Caucasian) | HB | TaqMan | 1083 | 1010 | 392 | 524 | 167 | 398 | 468 | 144 | 0.733 |
| Stern( 53 ) | 2002 | USA (Caucasian) | HB | PCR‐RFLP | 233 | 209 | 90 | 110 | 33 | 94 | 91 | 24 | 0.781 |
| Broberg( 54 ) | 2005 | Sweden (Caucasian) | PB | MALDI‐TOF | 61 | 153 | 23 | 33 | 5 | 60 | 72 | 21 | 0.935 |
| Matullo( 42 ) | 2006 | Europe (Caucasian) | PB | TaqMan | 131 | 1094 | 46 | 61 | 17 | 383 | 544 | 167 | 0.248 |
| Matullo( 55 ) | 2005 | Italy (Caucasian) | HB | TaqMan/PCR‐RFLP | 317 | 317 | 99 | 155 | 63 | 117 | 148 | 52 | 0.652 |
| Sanyal( 56 ) | 2004 | Sweden (Caucasian) | PB | PCR‐RFLP | 311 | 246 | 131 | 129 | 51 | 107 | 109 | 30 | 0.782 |
| Andrew( 57 ) | 2008 | USA and Italy (Caucasian) | PB | TaqMan/PCR‐RFLP | 1046 | 1275 | 397 | 477 | 172 | 482 | 617 | 176 | 0.335 |
| Gangwar( 58 ) | 2009 | India (Asian) | HB | PCR‐RFLP | 212 | 250 | 135 | 68 | 9 | 159 | 80 | 11 | 0.816 |
| Fontana( 59 ) | 2008 | France (Caucasian) | HB | TaqMan | 51 | 45 | 8 | 28 | 15 | 4 | 23 | 18 | 0.376 |
HB, hospital‐based study; HWE, Hardy–Weinberg equilibrium; NA, not available; PB, population‐based study.
Meta‐analysis results. To summarize the published data, we did a comprehensive meta‐analysis. The overall data showed that the individuals who carried the C/C genotype did not have significantly increased lung cancer risk compared with those who carried T/T genotype (additive model: OR = 0.888, 95% CI, 0.646–1.222; Fig. 1); and no significant association was found in the dominant model (OR = 0.871, 95% CI, 0.644–1.178) or recessive model (OR = 1.016, 95% CI, 0.968–1.066). Then, the 13 studies were analyzed by stratification based on ethnicity, study design, and smoking habits. In the subgroup analysis of ethnicity and smoking habits, there was no significant association between polymorphism and lung cancer risk. In the stratified analysis of study design, significant increased risk was found in population‐based study for the dominant model (OR = 1.071, 95% CI, 1.003–1.144). The details are listed in Table 2.
Figure 1.
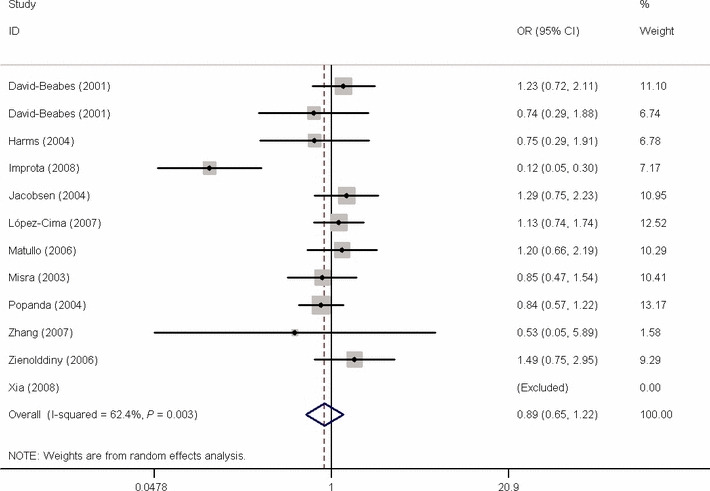
Forest plot of OR of lung cancer risk associated with X‐ray repair cross‐complementing group 3 (XRCC3) Thr241Met polymorphism by additive model. Studies are plotted according to the first author’s last name. Horizontal lines represent 95% CI. Each square represents the OR point estimate and its size is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with confidence interval given by its width. The unbroken vertical line is at the null value (RR = 1.0).
Table 2.
Summary of OR for XRCC3 Thr241Met polymorphism and lung cancer risk
| Subgroup | Number of comparisons | C/C vs T/T | (C/C+C/T) vs T/T | C/C vs (C/T+T/T) |
|---|---|---|---|---|
| Ethnicity | ||||
| Caucasian | 10 | 0.904 (0638–1.282)* | 0.886 (0.637–1.232)* | 1.031 (0.968–1.099) |
| Asian | 2 | 0.996 (0.983–1.010) | 0.997 (0.985–1.009) | 1.009 (0.960–1.060) |
| Design | ||||
| Hospital case‐control | 5 | 0.587 (0.291–1.181)* | 0.590 (0.313–1.112)* | 0.907 (0.676–1.218)* |
| Population case‐control | 8 | 1.018 (0.970–1.068) | 1.004 (0.977–1.031) | 1.071 (1.003–1.144)** |
| Smoking habits | ||||
| Never | 2 | NA | NA | 0.952 (0.851–1.065) |
| Heavy smokers | 4 | 0.975 (0.902–1.054) | 0.980 (0.938–1.024) | 0.835 (0.44–1.584) |
| Light smokers | 3 | NA | NA | 1.006 (0.854–1.186) |
| Overall | 12 | 0.888 (0.646–1.222)* | 0.871 (0.644–1.178)* | 1.016 (0.968–1.066) |
*Random effect estimate. **P = 0.041. OR, odds ratio; XRCC3, X‐ray repair cross‐complementing group 3.
For bladder cancer, the results showed that significant decreased risk was found for the additive model (OR = 0.959, 95% CI, 0.924–0.996; Fig. 2) and dominant model (OR = 0.982, 95% CI, 0.963–1.000), but not for the recessive model (OR = 0.958, 95% CI, 0.905–1.014). In the subgroup analysis by ethnicity, statistically significant decreased risk was found in Caucasians (additive model: OR = 0.954, 95% CI, 0.916–0.995 and dominant model: OR = 0.980, 95% CI, 0.960–1.000). When stratified by study design, statistically significant decreased risk was found in hospital‐based study (recessive model: OR = 0.920 95% CI, 0.852–0.994). The details are listed in Table 3.
Figure 2.
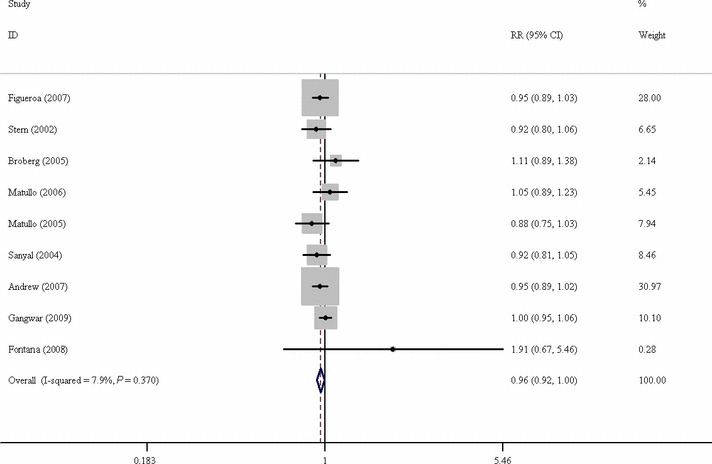
Forest plot of OR of bladder cancer risk associated with X‐ray repair cross‐complementing group 3 (XRCC3) Thr241Met polymorphism by additive model. CI, confidence interval.
Table 3.
Summary of OR for XRCC3 Thr241Met polymorphism and bladder cancer risk
| Subgroup | Number of comparisons | C/C vs T/T | (C/C+C/T) vs T/T | C/C vs (C/T+T/T) |
|---|---|---|---|---|
| Ethnicity | ||||
| Caucasian | 8 | 0.954 (0.916–0.995)* | 0.980 (0.960–1.000)** | 0.953 (0.895–1.013) |
| Design | ||||
| Hospital‐based study | 5 | 0.954 (0.907–1.003) | 0.985 (0.960–1.011) | 0.920 (0.852–0.994)*** |
| Population‐based study | 4 | 0.965 (0.913–1.021) | 0.978 (0.951–1.005) | 1.002 (0.921–1.090) |
| Overall | 9 | 0.959 (0.924–0.996)**** | 0.982 (0.963–1.000)***** | 0.958 (0.905–1.014) |
*P = 0.027; **P = 0.050; ***P = 0.034; ****P = 0.029; *****P = 0.050. OR, odds ratio; XRCC3, X‐ray repair cross‐complementing group 3.
Sensitivity analysis. Sensitivity analyses were conducted to determine whether modification of the inclusion criteria of the meta‐analysis affected the final results. These were carried out by limiting the meta‐analysis to studies conforming to HWE and altering corresponding statistic variables and analysis models. No results were materially altered (data not shown).
Publication bias. Begg’s funnel plots and Egger’s tests were performed to assess publication bias. The shapes of the funnel plots revealed no obvious asymmetry. Egger’s test was then used to statistically assess funnel plot symmetry. The results suggested no evidence of publication bias (lung cancer: P = 0.283 for additive model, P = 0.322 for dominant model, and P = 0.846 for recessive model; bladder cancer: P = 0.591 for additive model, P = 0.723 for dominant model, and P = 0.264 for recessive model). The results indicated that the results of these meta‐analyses are relatively stable and that publication bias is unlikely to affect the results of the meta‐analyses.
Discussion
Biological evidence has indicated that XRCC3 takes part in the homologous recombination repairs of DNA damage.( 9 ) Functional data had validated that XRCC3 Thr241Met polymorphism was associated with the capacity of DNA repair.( 11 ) Increasing molecular epidemiologic evidence has shown that this polymorphism was associated with an increased risk of different kinds of cancer.( 37 , 38 , 39 , 40 )
As it is known that individual studies with a small sample size may have not enough statistical power to detect a small risk factor, in this meta‐analysis, we involved a total of 2976 cases and 4495 controls for lung cancer and 3445 cases and 4599 controls for bladder cancer, and investigated the associations of the XRCC3 Thr241Met polymorphism with lung and bladder cancer risk.
We found that there were no significant associations between the XRCC3 Thr241Met polymorphism and lung cancer risk. However, in the subgroup analysis of study design, the individuals carrying the C/C genotype showed a higher lung cancer risk compared with those with the (C/T+T/T) genotype for population‐based study, but not for the hospital‐based studies. This may be due to the fact that the hospital‐based studies may have some biases when controls represent an ill‐defined reference population sample and are not truly representative of the general population, particularly when the genotypes under investigation are associated with the disease conditions that the hospital‐based controls may have. Therefore, using a proper and representative population‐based study is very important to reduce biases in such genetic association studies.
For bladder cancer, individuals who carried the C/C or C/T genotype had a significant smaller cancer risk compared with the T/T carriers. But in the subgroup of hospital‐based study, the C/C carriers had a decreased cancer risk compared with the individuals who carried the (C/T+T/T) genotype. This result is contradictory with the overall results and results from Caucasian patients. For the above‐mentioned reason, we thought that the association may be a false positive result. So it is necessary to take into account case‐control study design, especially for hospital‐based case control studies.( 41 )
The results showed that XRCC3 Thr241Met polymorphism plays different role in lung cancer and bladder cancer. It may not be uncommon for the same polymorphism to play different roles in cancer susceptibility across different tumor locations, because cancer is a complicated multi‐genetic disease and genetic heterogeneity exists in different tumor sites.
There are some limitations to this meta‐analysis. First, only published studies were included in the meta‐analysis. It is possible that some related unpublished studies that might meet the inclusion criteria were missed; therefore, publication bias may have been present, even though statistical analysis indicated this not to be the case. Second, our results were based on unadjusted estimates and a more precise analysis could have been conducted if individual data were available; this would allow for adjustment by other covariates such as age, ethnicity, environmental factors, and lifestyle. Third, in the subgroup analyses, the number of Asians was relatively small for lung cancer and there was no Asian study on bladder cancer with enough statistical power to explore the association of the polymorphism with cancer susceptibility. However, our meta‐analysis also had some advantages. First, a substantial number of cases and controls were pooled from different studies, which significantly increased the statistical power of the analysis. Second, no publication bias was detected, indicating that the pooled result should be reliable.
In summary, our meta‐analysis indicates that XRCC3 Thr241Met polymorphism is weakly associated with the risk of lung and bladder cancer. However, it is necessary to conduct large sample studies using standardized unbiased genotyping methods and well‐matched controls.
Acknowledgment
The project was supported by the National Natural Science Foundation of China (no. 30671146).
References
- 1. Kiyohara C, Yoshimasu K, Shirakawa T, Hopkin JM. Genetic polymorphisms and environmental risk of lung cancer: a review. Rev Environ Health 2004; 19: 15–38. [DOI] [PubMed] [Google Scholar]
- 2. Lichtenstein P, Holm NV, Verkasalo PK et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000; 343: 78–85. [DOI] [PubMed] [Google Scholar]
- 3. Spitz MR, Wei Q, Dong Q, Amos CI, Wu X. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev 2003; 12: 689–98. [PubMed] [Google Scholar]
- 4. Yokota J, Kohno T. Molecular footprints of human lung cancer progression. Cancer Sci 2004; 95: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madeb R, Messing EM. Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol 2004; 22: 86–92. [DOI] [PubMed] [Google Scholar]
- 6. Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C. Mechanisms of disease: the epidemiology of bladder cancer. Nat Clin Pract Urol 2006; 3: 327–40. [DOI] [PubMed] [Google Scholar]
- 7. El‐Zein R, Monroy CM, Etzel CJ et al. Genetic polymorphisms in DNA repair genes as modulators of Hodgkin disease risk. Cancer 2009; 115: 1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krupa R, Synowiec E, Pawlowska E et al. Polymorphism of the homologous recombination repair genes RAD51 and XRCC3 in breast cancer. Exp Mol Pathol 2009; 87: 32–5. [DOI] [PubMed] [Google Scholar]
- 9. Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology‐directed repair of DNA damage in mammalian cells. Genes Dev 1999; 13: 2633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res 1998; 58: 604–8. [PubMed] [Google Scholar]
- 11. Matullo G, Palli D, Peluso M et al. XRCC1, XRCC3, XPD gene polymorphisms, smoking and (32)P‐DNA adducts in a sample of healthy subjects. Carcinogenesis 2001; 22: 1437–45. [DOI] [PubMed] [Google Scholar]
- 12. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Inter Med 1997; 127: 820–6. [DOI] [PubMed] [Google Scholar]
- 13. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22: 719–48. [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 15. Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta‐analysis. Psychiatry Res 2004; 129: 39–44. [DOI] [PubMed] [Google Scholar]
- 16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Butkiewicz D, Rusin M, Enewold L, Shields PG, Chorazy M, Harris CC. Genetic polymorphisms in DNA repair genes and risk of lung cancer. Carcinogenesis 2001; 22: 593–7. [DOI] [PubMed] [Google Scholar]
- 18. Covolo L, Placidi D, Gelatti U et al. Bladder cancer, GSTs, NAT1, NAT2, SULT1A1, XRCC1, XRCC3, XPD genetic polymorphisms and coffee consumption: a case‐control study. Eur J Epidemiol 2008; 23: 355–62. [DOI] [PubMed] [Google Scholar]
- 19. Stern MC, Conway K, Li Y, Mistry K, Taylor JA. DNA repair gene polymorphisms and probability of p53 mutation in bladder cancer. Mol Carcinog 2006; 45: 715–9. [DOI] [PubMed] [Google Scholar]
- 20. Andrew AS, Mason RA, Kelsey KT et al. DNA repair genotype interacts with arsenic exposure to increase bladder cancer risk. Toxicol Lett 2009; 187: 10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ricceri F, Guarrera S, Sacerdote C et al. ERCC1 haplotypes modify bladder cancer risk: a case‐control study. DNA Repair (Amst) 2010; 9: 191–200. [DOI] [PubMed] [Google Scholar]
- 22. Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev 2002; 11: 1513–30. [PubMed] [Google Scholar]
- 23. Han S, Zhang HT, Wang Z et al. DNA repair gene XRCC3 polymorphisms and cancer risk: a meta‐analysis of 48 case‐control studies. Eur J Hum Genet 2006; 14: 1136–44. [DOI] [PubMed] [Google Scholar]
- 24. Manuguerra M, Saletta F, Karagas MR et al. XRCC3 and XPD/ERCC2 single nucleotide polymorphisms and the risk of cancer: a HuGE review. Am J Epidemiol 2006; 164: 297–302. [DOI] [PubMed] [Google Scholar]
- 25. Hung RJ, Christiani DC, Risch A et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev 2008; 17: 3081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hill CE, Affatato AA, Wolfe KJ et al. Gender differences in genetic damage induced by the tobacco‐specific nitrosamine NNK and the influence of the Thr241Met polymorphism in the XRCC3 gene. Environ Mol Mutagen 2005; 46: 22–9. [DOI] [PubMed] [Google Scholar]
- 27. De Las Penas R, Sanchez‐Ronco M, Alberola V et al. Polymorphisms in DNA repair genes modulate survival in cisplatin/gemcitabine‐treated non‐small‐cell lung cancer patients. Ann Oncol 2006; 17: 668–75. [DOI] [PubMed] [Google Scholar]
- 28. Medina PP, Ahrendt SA, Pollan M, Fernandez P, Sidransky D, Sanchez‐Cespedes M. Screening of homologous recombination gene polymorphisms in lung cancer patients reveals an association of the NBS1‐185Gln variant and p53 gene mutations. Cancer Epidemiol Biomarkers Prev 2003; 12: 699–704. [PubMed] [Google Scholar]
- 29. Ryk C, Kumar R, Sanyal S et al. Influence of polymorphism in DNA repair and defence genes on p53 mutations in bladder tumours. Cancer Lett 2006; 241: 142–9. [DOI] [PubMed] [Google Scholar]
- 30. Narter KF, Ergen A, Agachan B, Gormus U, Timirci O, Isbir T. Bladder cancer and polymorphisms of DNA repair genes (XRCC1, XRCC3, XPD, XPG, APE1, hOGG1). Anticancer Res 2009; 29: 1389–93. [PubMed] [Google Scholar]
- 31. Shen M, Hung RJ, Brennan P et al. Polymorphisms of the DNA repair genes XRCC1, XRCC3, XPD, interaction with environmental exposures, and bladder cancer risk in a case‐control study in northern Italy. Cancer Epidemiol Biomarkers Prev 2003; 12: 1234–40. [PubMed] [Google Scholar]
- 32. Matullo G, Guarrera S, Carturan S et al. DNA repair gene polymorphisms, bulky DNA adducts in white blood cells and bladder cancer in a case‐control study. Int J Cancer 2001; 92: 562–7. [DOI] [PubMed] [Google Scholar]
- 33. Hao GY, Zhang YY, Zhang WD, Yang MS, Jia Q. Relationship between XRCC3 gene polymorphism and bladder cancer in the Han population. Journal OF Shandong University(Health Sciences) 2008; 46: 612–5. [Google Scholar]
- 34. David‐Beabes GL, Lunn RM, London SJ. No association between the XPD (Lys751G1n) polymorphism or the XRCC3 (Thr241Met) polymorphism and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2001; 10: 911–2. [PubMed] [Google Scholar]
- 35. Ryk C, Kumar R, Thirumaran RK, Hou SM. Polymorphisms in the DNA repair genes XRCC1, APEX1, XRCC3 and NBS1, and the risk for lung cancer in never‐ and ever‐smokers. Lung cancer 2006; 54: 285–92. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Liang D, Spitz MR et al. XRCC3 genetic polymorphism, smoking, and lung carcinoma risk in minority populations. Cancer 2003; 98: 1701–6. [DOI] [PubMed] [Google Scholar]
- 37. Burri RJ, Stock RG, Cesaretti JA et al. Association of single nucleotide polymorphisms in SOD2, XRCC1 and XRCC3 with susceptibility for the development of adverse effects resulting from radiotherapy for prostate cancer. Radiat Res 2008; 170: 49–59. [DOI] [PubMed] [Google Scholar]
- 38. Economopoulos KP, Sergentanis TN. XRCC3 Thr241Met polymorphism and breast cancer risk: a meta‐analysis. Breast Cancer Res Treat 2009; [E pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39. Huang WY, Olshan AF, Schwartz SM et al. Selected genetic polymorphisms in MGMT, XRCC1, XPD, and XRCC3 and risk of head and neck cancer: a pooled analysis. Cancer Epidemiol Biomarkers Prev 2005; 14: 1747–53. [DOI] [PubMed] [Google Scholar]
- 40. Stern MC, Siegmund KD, Conti DV, Corral R, Haile RW. XRCC1, XRCC3, and XPD polymorphisms as modifiers of the effect of smoking and alcohol on colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 2006; 15: 2384–90. [DOI] [PubMed] [Google Scholar]
- 41. Ruano‐Ravina A, Perez‐Rios M, Barros‐Dios JM. Population‐based versus hospital‐based controls: are they comparable? Gac Sanit 2008; 22: 609–13. [DOI] [PubMed] [Google Scholar]
- 42. Matullo G, Dunning AM, Guarrera S et al. DNA repair polymorphisms and cancer risk in non‐smokers in a cohort study. Carcinogenesis 2006; 27: 997–1007. [DOI] [PubMed] [Google Scholar]
- 43. Misra RR, Ratnasinghe D, Tangrea JA et al. Polymorphisms in the DNA repair genes XPD, XRCC1, XRCC3, and APE/ref‐1, and the risk of lung cancer among male smokers in Finland. Cancer Lett 2003; 191: 171–8. [DOI] [PubMed] [Google Scholar]
- 44. Lopez‐Cima MF, Gonzalez‐Arriaga P, Garcia‐Castro L et al. Polymorphisms in XPC, XPD, XRCC1, and XRCC3 DNA repair genes and lung cancer risk in a population of northern Spain. BMC cancer 2007; 7: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zienolddiny S, Campa D, Lind H et al. Polymorphisms of DNA repair genes and risk of non‐small cell lung cancer. Carcinogenesis 2006; 27: 560–7. [DOI] [PubMed] [Google Scholar]
- 46. Improta G, Sgambato A, Bianchino G et al. Polymorphisms of the DNA repair genes XRCC1 and XRCC3 and risk of lung and colorectal cancer: a case‐control study in a Southern Italian population. Anticancer Res 2008; 28: 2941–6. [PubMed] [Google Scholar]
- 47. Zhang ZL, Zhou CC, Zhang J, Tang L, Su B. [Relationship between polymorphisms of DNA repair gene XRCC3 and susceptibility to lung cancer]. Zhonghua Jie He He Hu Xi Za Zhi 2007; 30: 936–40. [PubMed] [Google Scholar]
- 48. Popanda O, Schattenberg T, Phong CT et al. Specific combinations of DNA repair gene variants and increased risk for non‐small cell lung cancer. Carcinogenesis 2004; 25: 2433–41. [DOI] [PubMed] [Google Scholar]
- 49. Jacobsen NR, Raaschou‐Nielsen O, Nexo B et al. XRCC3 polymorphisms and risk of lung cancer. Cancer Lett 2004; 213: 67–72. [DOI] [PubMed] [Google Scholar]
- 50. Xia W, Zhang Y, Su D, Shi F. Association of single nucleotide polymorphisms of DNA repair gene XRCC3‐241 with non‐small cell lung cancer. Zhejiang Medical Journal 2008; 30: 1291–3. [Google Scholar]
- 51. Harms C, Salama SA, Sierra‐Torres CH, Cajas‐Salazar N, Au WW. Polymorphisms in DNA repair genes, chromosome aberrations, and lung cancer. Environ Mol Mutagen 2004; 44: 74–82. [DOI] [PubMed] [Google Scholar]
- 52. Figueroa JD, Malats N, Rothman N et al. Evaluation of genetic variation in the double‐strand break repair pathway and bladder cancer risk. Carcinogenesis 2007; 28: 1788–93. [DOI] [PubMed] [Google Scholar]
- 53. Stern MC, Umbach DM, Lunn RM, Taylor JA. DNA repair gene XRCC3 codon 241 polymorphism, its interaction with smoking and XRCC1 polymorphisms, and bladder cancer risk. Cancer Epidemiol Biomarkers Prev 2002; 11: 939–43. [PubMed] [Google Scholar]
- 54. Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis 2005; 26: 1263–71. [DOI] [PubMed] [Google Scholar]
- 55. Matullo G, Guarrera S, Sacerdote C et al. Polymorphisms/haplotypes in DNA repair genes and smoking: a bladder cancer case‐control study. Cancer Epidemiol Biomarkers Prev 2005; 14: 2569–78. [DOI] [PubMed] [Google Scholar]
- 56. Sanyal S, Festa F, Sakano S et al. Polymorphisms in DNA repair and metabolic genes in bladder cancer. Carcinogenesis 2004; 25: 729–34. [DOI] [PubMed] [Google Scholar]
- 57. Andrew AS, Karagas MR, Nelson HH et al. DNA repair polymorphisms modify bladder cancer risk: a multi‐factor analytic strategy. Hum Hered 2008; 65: 105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gangwar R, Ahirwar D, Mandhani A, Mittal RD. Do DNA repair genes OGG1, XRCC3 and XRCC7 have an impact on susceptibility to bladder cancer in the North Indian population? Mutat Res 2009; 680: 56–63. [DOI] [PubMed] [Google Scholar]
- 59. Fontana L, Bosviel R, Delort L et al. DNA repair gene ERCC2, XPC, XRCC1, XRCC3 polymorphisms and associations with bladder cancer risk in a French cohort. Anticancer Res 2008; 28: 1853–6. [PubMed] [Google Scholar]


