Abstract
Using a novel monoclonal anti‐pan human leukocyte antigen (HLA) class I heavy chain antibody (EMR 8‐5) reacting with paraffin‐embedded sections, we examined the prognostic significance of HLA class I molecules in muscle‐invasive bladder cancer patients who underwent radical cystectomy. Immunohistochemical staining for HLA class I molecules with monoclonal antibody EMR 8‐5 was performed on specimens from 65 clinically muscle‐invasive bladder cancer patients who underwent radical cystectomy and pelvic lymph node dissection without neoadjuvant chemotherapy. We analyzed the clinicopathological and prognostic significance of HLA class I expression. Immunohistochemical analysis revealed HLA class I down‐regulation in 22 (33.8%) invasive bladder cancers. This down‐regulation had no correlation with clinicopathological parameters such as pathologic stage, nodal status, and grade. The recurrence‐free survival of patients with HLA class I–positive tumors was significantly better than that of those with down‐regulation (log rank, P = 0.0337). Multivariate analysis revealed that HLA class I expression was a significant factor influencing the recurrence‐free survival of bladder cancer patients after cystectomy (P = 0.0155). Our data demonstrate that HLA class I down‐regulation in tumor cells was clearly observed in about one‐third of the patients. HLA class I expression could be a prognostic marker for muscle‐invasive bladder cancer patients after cystectomy. (Cancer Sci 2009; 100: 2331–2334)
Radical cystectomy with pelvic lymph node dissection is a standard surgical procedure for muscle‐invasive bladder cancer, with a 5‐year survival rate of approximately 60%. However, one‐third of the patients treated by cystectomy die of the disease, mostly of metastatic spread. Pathologic stage and nodal involvement are reported to be the most important prognostic factors in bladder cancer.( 1 ) However, there are few other clinicopathological parameters predicting the survival of bladder cancer patients after cystectomy.
Human leukocyte antigen (HLA) class I molecules have a central role in the cell‐mediated immune system, especially as antigen‐presenting molecules for cytotoxic T lymphocytes (CTLs). CTL can recognize antigenic peptides presented on the cell surface with HLA class I molecules, and kill the target cell.( 2 , 3 ) Down‐regulation of HLA class I was found to be implicated in the immune escape of malignant tumors.( 2 , 3 ) It is reported that this phenomenon is observed in malignant tumors such as malignant melanoma, colorectal, lung, and ovarian cancers,( 4 , 5 , 6 , 7 ) and affects survival of a limited number of patients with these diseases.
We have recently established a novel anti‐pan HLA class I heavy chain mAb, EMR8‐5, which can detect HLA‐A, B, and C antigens in formalin‐fixed paraffin‐embedded tissue sections.( 8 , 9 , 10 ) Using this mAb, we previously reported that HLA class I down‐regulation was observed in about one‐third of superficial bladder cancers and that HLA class I expression contributed significantly to the therapeutic effect of Bacillus Calmette‐Guérin (BCG) immunotherapy.( 9 )
In this study, we immunohistochemically examined the expression profiles of HLA class I molecules in patients with muscle‐invasive bladder cancer who underwent radical cystectomy, and analyzed the prognostic significance of the expression.
Materials and Methods
Patients and tissues. We reviewed the clinical pathology archives of consecutive patients with muscle‐invasive bladder cancer who underwent radical cystectomy and regional pelvic lymph node dissection without neoadjuvant chemotherapy from January 1991 to December 2002 at Sapporo Medical University Hospital. The study protocol was approved by the Clinical Institutional Ethical Review Board of the Medical Institute of Bioregulation, Sapporo Medical University, Japan. Bladder cancer was histopathologically diagnosed by transurethral resection (TUR) in all patients before cystectomy. No patients had distant metastasis at the time of the initial diagnosis. Radical cystectomy and regional pelvic lymph node dissection were performed using a standard technique.( 11 ) Pelvic lymph node dissection included the internal iliac, external iliac, and obturator lymph nodes. Boundaries of dissection included the circumflex iliac vein inferiorly, pelvic side wall laterally, bladder wall medially and common iliac bifurcation superiorly. Concurrent urethrectomy was performed for male patients with a histologically proven cancer on the prostatic urethra. Anterior pelvic exenteration was done in women who selected urinary diversion other than an orthotopic ileal neobladder.
We finally selected 65 patients, based on the availability of sufficient material for immunohistochemical evaluation, before obtaining information on their clinical outcomes. All hematoxylin–eosin‐stained slides were reviewed, and clinical stage was assigned using the 1997 TNM classification( 12 ) and the World Health Organization system.( 13 )
Immunohistochemistry by EMR 8‐5. Immunohistochemical staining with the monoclonal anti‐pan HLA class I (HLA‐A, B, and C) antibody EMR8‐5, which was established at our laboratory, was performed as previously described.( 14 ) Human tonsil sections were used as positive controls for HLA class I. Staining of vascular endothelial cells and lymphocytes in sections were used as an internal positive control for immunostaining. Negative controls were done by omitting the primary antibody. All specimens were reviewed independently using light microscopy in at least five areas at ×400 magnification by investigators who were blinded to clinicopathological data (TT). The membrane immunoreactivity level for HLA class I was categorized from undetectable to +2. A score of zero was defined as undetectable staining. A score of +1 was defined as faint, incomplete membrane staining in more than 20% of the tumor cells, or as moderate to complete staining in cytoplasm but negative membrane staining in the tumor cells (Fig. 1a,b). Finally, a score of +2 was defined as complete membrane staining in more than 80% of the tumor cells (Fig. 1c,d). HLA class I expression was then classified as down‐regulated (scores 0 and 1) or positive (score 2).
Figure 1.
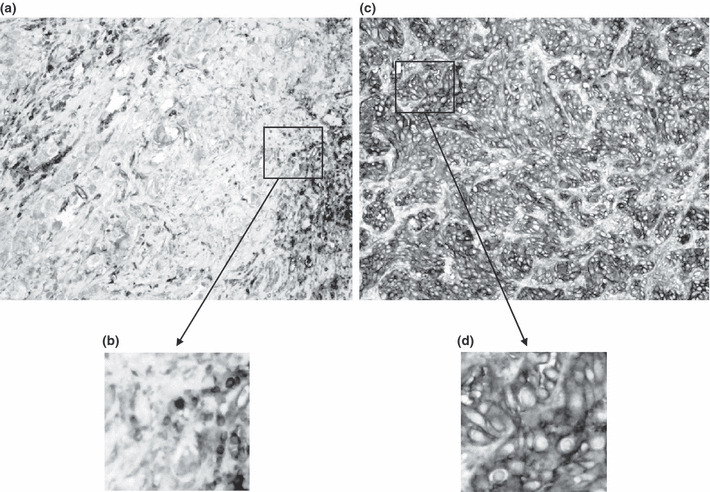
Representative pictures of immunohistochemical staining with mAb EMR 8‐5 reacting to human leukocyte antigen (HLA) class I molecules in invasive bladder cancer. (a) Stained tumor cells are seen in cytoplasm but not in the cell membrane, demonstrating down‐regulation of HLA class I molecules. (b) Magnified view of the box in panel A. (c) Cytoplasmic portion and cell membranes of tumor cells are also completely stained. (d) magnified view of the box in panel C.
Statistical analysis. Differences in clinicopathological characteristics between HLA class I positive and down‐regulated tissues were assessed using the Mann–Whitney test. Survival time was analyzed from the date of surgery. The end points of univariate and multivariate analyses were recurrence‐free survival. Survival estimates were constructed using the Kaplan–Meier method. The log‐rank test was used to evaluate the significance of differences in the univariate analysis. For multivariate analysis, Cox’s proportional hazards model was used. A value of P < 0.05 was considered to indicate statistical significance.
Results
Of the 65 patients, 51 were men and 14 were women. They ranged in age from 38 to 79 years (mean, 65.2 years). Clinically, 42 patients (64.6%) were diagnosed as having extravesical disease (T3 or more) before cystectomy (Table 1). Preoperative computed tomography demonstrated suspected metastasis to the pelvic lymph nodes in five patients. Pathologically, 28 patients (43.1%) had tumors confined to the bladder (pT2 or less) and 33 patients (50.8%) had tumors penetrating the bladder wall into perivesical fat or adjacent structures (pT3 or more). Pathological pelvic lymph node metastasis was found in 11 patients (16.9%). Histology of pure urothelial carcinoma was found in 49 patients (75.4%) and other histological components such as squamous cell carcinoma and adenocarcinoma in 16 (24.6%). Adjuvant chemotherapy was done for five patients (7.7%) with pathological T3 or T4 disease and/or nodal involvement according to the preferences of both the patients and urologists.
Table 1.
Clinical characteristics, pathological and nodal status, and tumor histology
| Clinical and pathological characteristics | HLA class I positive | HLA class I down‐regulation | P‐values |
|---|---|---|---|
| No. of patients | 43 | 22 | |
| Clinical stage | |||
| cT2N0 | 18 (40.0) | 5 (26.1) | 0.1188 |
| cT3N0 | 14 (33.3) | 8 (34.8) | |
| cT4N0 | 8 (20.0) | 7 (30.4) | |
| cT2‐4N+ | 3 (6.7) | 2 (8.7) | |
| Pathological stage | |||
| pT1‐2N0 | 18 (42.3) | 10 (43.5) | 0.8837 |
| pT3N0 | 11 (24.4) | 4 (21.7) | |
| pT4N0 | 8 (20.0) | 3 (13.1) | |
| pT1‐4N+ | 6 (13.3) | 5 (21.7) | |
| Histology | |||
| Pure UC | 32 (73.3) | 17 (73.9) | 0.8020 |
| Aberrant differentiation with or without UC | 11 (26.7) | 5 (26.1) | |
| Grade | |||
| 1–2 | 10 (20.0) | 7 (30.4) | 0.4608 |
| 3 | 33 (80.0) | 15 (69.6) | |
HLA, human leukocyte antigen; UC, urothelial carcinoma.
As shown in Table 1, of the 65 bladder cancer specimens, 43 (66.2%) were graded as having high expression of HLA class I molecules. HLA class I down‐regulation was found in 22 specimens (33.8%), with 19 having low and three having negative expression. In organ‐confined disease (pathological T2 or less disease with negative nodal involvement) and extravesical disease (pathological T3 or T4 disease and/or nodal involvement), HLA class I down‐regulation was observed in 10 specimens (35.7%) of 28 and 12 specimens (32.4%) of 37, respectively. There was no correlation between HLA class I down‐regulation and pathological stage. Moreover the status of HLA class I expression had no correlation with other clinicopathological parameters such as clinical stage, nodal status, histology, and grade (Table 1).
The median follow‐up period of the 65 patients was 35 months, ranging from 3 to 172 months. The median follow‐up of the 37 survivors was 52.5 months. Distant metastases and/or local recurrence developed in 33 of the 65 patients (50.8%) at a median of 11 months (range, 3–74 months) after surgery. The 5‐year recurrence‐free survival was 46.0% for all patients. The 5‐year survivals were 55.0% and 30.3% in the HLA class I positive and down‐regulated arms, respectively. Patients with HLA class I positive expression had significantly longer recurrence‐free survival than those with down‐regulated expression (log rank, P = 0.0337) (Fig. 2a). Although there was no significance because of the small number of patients, in extravesical disease (pathological T3 or T4 disease and/or nodal involvement), in particular, HLA class I expression seemed to be an important factor for predicting prognosis (Fig. 2b,c). As shown in Table 2, multivariate analysis revealed that, in addition to pathological stage, HLA class I expression was a significant factor influencing the disease‐free survival of bladder cancer patients after cystectomy (P = 0.0155).
Figure 2.
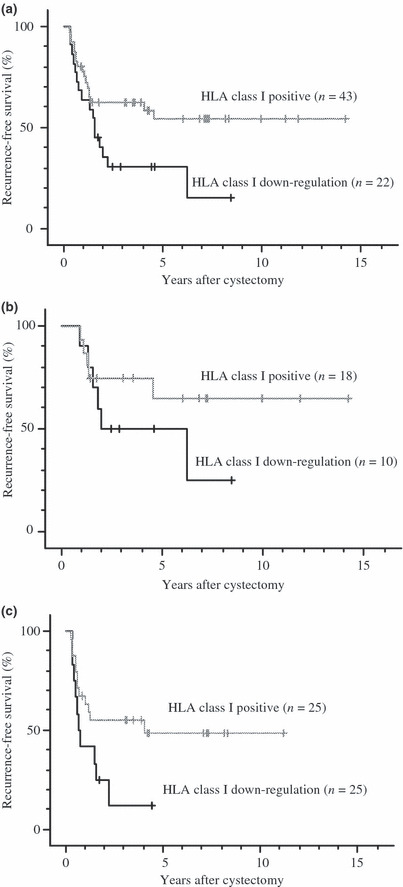
Recurrence‐free survival 65 patients stratified by HLA class I expression. (a) Comparison of HLA class I positive findings (n = 43) and HLA class I down‐regulation (n = 22) in all 65 patients (P = 0.0337). (b) Comparison of HLA class I positive findings (n = 18) and HLA class I down‐regulation (n = 10) in 28 patients with pT1‐2 node‐negative disease (P = 0.2274). (c) Comparison of HLA class I positive findings (n = 25) and HLA class I down‐regulation (n = 12) in 39 patients with pT3‐4 and/or node‐positive disease (P = 0.0566). All P‐values were determined by the log‐rank test.
Table 2.
Univariate and multivariate analyses of parameters predicting disease‐specific survival
| Factor | Univariate P‐values | Multivariate P‐values | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Histology: pure UCversus aberrant differentiation | 0.9696 | 0.8981 | 1.052 | 0.484–2.285 |
| Pathological stage:pT1‐2 versus pT3‐4 | 0.0095 | 0.0093 | 2.604 | 1.266–5.358 |
| Nodal involvement: no versus yes | 0.2053 | 0.1364 | 1.947 | 0.810–4.679 |
| HLA class I: positive versus down‐regulation | 0.0337 | 0.0155 | 2.392 | 1.180–4.848 |
CI, confidence interval; HLA, human leukocyte antigen; UC, urothelial carcinoma.
Discussions
Down‐regulation of HLA class I expression has been demonstrated in various types of solid tumors and is considered to be one of the mechanisms of tumor immune escape.( 2 , 3 ) Some studies have reported that this down‐regulation could be a significant prognostic factor for various malignant tumors.( 4 , 5 , 6 , 7 ) An anti‐HLA class I mAb detecting HLA antigens in formalin‐fixed paraffin‐embedded histological biopsy and surgically resected tissue specimens would be highly useful in determining the exact characteristics of the HLA class I expression in tumors. As such mAb HC‐10 which reacted with HLA‐B and C alleles was established.( 15 )
We have recently established a novel monoclonal anti‐pan HLA class I heavy chain antibody, EMR8‐5, which can detect HLA‐A, B, and C antigens in paraffin‐embedded sections.( 8 , 9 , 10 ) Using this monoclonal antibody, we examined the expression profiles of HLA class I molecules immunohistochemically in muscle‐invasive bladder cancer, and analyzed the prognostic significance of HLA class I expression.
Previous studies of HLA class I antigen expression in bladder cancers have reported that HLA class I down‐regulation was observed in between 27% and 44% of patients,( 16 , 17 , 18 ) results similar to those observed in our previous study.( 9 , 14 ) In this study HLA class I down‐regulation was observed in 33.8% of muscle‐invasive bladder cancers.
We found that HLA class I antigen down‐regulation affected survival in invasive bladder cancer patients. In contrast, some studies of malignant melanoma have shown a lack of prognostic significance for HLA class I expression.( 19 , 20 ) It should also be noted that total loss of HLA class I has been proposed as an indicator of good prognosis in breast cancer and non‐small‐cell lung cancer.( 21 , 22 ) In those reports, the authors mentioned that HLA class I antigen down‐regulation may make the tumors more susceptible to natural killer (NK) cell killing and result in a better prognosis. Our preliminary data from immunohistochemical staining using monoclonal antibodies for cluster of differentiation (CD)‐8 and CD56 seemed to indicate that infiltration of CD8‐positive cells was increased in HLA class I positive cases (data not shown). This implied that the HLA class I‐restricted CTLs were mobilized around HLA class I–positive cancer cells. However, CD56‐positive cells were rarely observed in either HLA class I–positive or down‐regulated cases. This finding suggested that the immune surveillance system via the HLA class I–restricted CTL pathway may play a role in the regulation of microscopic metastatic disease progression in advanced extravesical disease, in particular. However, NK cells might not act for tumor rejection.
In recent years there have been many studies of specific immunotherapies for various cancers.( 23 , 24 ) The rationale for such studies has been supported by strong cellular immune responses, that is introducing cancer‐specific CTLs from patients. The effectiveness of immunotherapy using tumor‐specific antigens largely depends on the expression of the appropriate HLA class I alleles on the tumor cells. In the future, when T cell‐based immunotherapy is available for the treatment of bladder cancer, it will be necessary to evaluate and clarify the defects in the antigen‐presentation machinery that are likely to generate cancer cells able to escape the host’s T‐cell control.
In this study, for accurate pathological evaluation, we excluded patients with pT0 and those who received neoadjuvant chemotherapy. Moreover, patients whose formalin‐fixed paraffin‐embedded tissue was incomplete were excluded. In this limited patient group with more high‐stage disease, nodal status did not affect the prognosis after cystectomy. Therefore this study may have some limitations; however, the results of the current study suggest that HLA class I expression may be one of the important prognostic factors in advanced extravesical disease, in particular. Moreover HLA class I expression may be one of the important factors to select proper patients for treatment with CTL‐based immunotherapy in the future. Further studies are required to elucidate the mechanisms of HLA class I gene down‐regulation and up‐regulation.
Conclusions
Our data demonstrated that HLA class I down‐regulation on tumor cells could be observed in about one‐third of patients and that it was an independent prognostic factor for muscle‐invasive bladder cancer, especially in patients with extravesical disease. This finding suggests that HLA class I expression could be a prognostic marker in muscle‐invasive bladder cancer patients after cystectomy.
Acknowledgments
Kumiko Shimozawa, Emiri Nakazawa, and Akari Takahashi provided technical assistance. This study was supported in part by a grant‐in‐aid for Clinical Cancer Research from the Ministry of Health, Labor and Welfare of Japan (2005–2008), and research grants from the Stiftelsen Japanese‐Swedish Research Foundation, and Gotaro Sugawara‐Memorial Research Fund for Urological Diseases.
References
- 1. Stein JP, Lieskovsky G, Cote R et al. Radical cystectomy in the treatment of invasive bladder cancer: long‐term results in 1,054 patients. J Clin Oncol 2001; 19: 666–75. [DOI] [PubMed] [Google Scholar]
- 2. Bubenik J. MHC class I down‐regulation: tumour escape from immune surveillance? Int J Oncol 2004; 25: 487–91. [PubMed] [Google Scholar]
- 3. Khong HT, Restifo NP. Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat Immunol 2002; 3: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down‐regulation of HLA class I antigen‐processing molecules in malignant melanoma: association with disease progression. Am J Pathol 1999; 154: 745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watson NF, Ramage JM, Madjd Z et al. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer 2006; 118: 6–10. [DOI] [PubMed] [Google Scholar]
- 6. Kikuchi E, Yamazaki K, Torigoe T et al. HLA class I antigen expression is associated with a favorable prognosis in early stage non‐small cell lung cancer. Cancer Sci 2007; 98: 1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vitale M, Pelusi G, Taroni B et al. HLA class I antigen down‐regulation in primary ovary carcinoma lesions: association with disease stage. Clin Cancer Res 2005; 11: 67–72. [PubMed] [Google Scholar]
- 8. Sato N, Hirohashi Y, Tsukahara T et al. Molecular pathological approaches to human tumor immunology. Pathol Int 2009; 59: 205–17. [DOI] [PubMed] [Google Scholar]
- 9. Kitamura H, Torigoe T, Honma I et al. Effect of human leukocyte antigen class I expression of tumor cells on outcome of intravesical instillation of Bacillus Calmette‐Guerin immunotherapy for bladder cancer. Clin Cancer Res 2006; 12: 4641–4. [DOI] [PubMed] [Google Scholar]
- 10. Tsukahara T, Kawaguchi S, Torigoe T et al. Prognostic significance of HLA class I expression in osteosarcoma defined by anti‐pan HLA class I monoclonal antibody, EMR8‐5. Cancer Sci 2006; 97: 1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whitmore WF Jr. Management of invasive bladder neoplasms. Semin Urol 1983; 1: 34–41. [PubMed] [Google Scholar]
- 12. Sobin LH, Wittekind Ch. TNM Classification of Malignant tumors, 5th edn. New York: Wiley‐Liss Inc, 1997. [Google Scholar]
- 13. Mostofi FK, Davis CJ, Sesterhenn IA. Histological typing of urinary bladder tumours, 2nd edn. New York: Springer, 1999. [Google Scholar]
- 14. Kitamura H, Torigoe T, Honma I et al. Expression and antigenicity of survivin, an inhibitor of apoptosis family member, in bladder cancer: implications for specific immunotherapy. Urology 2006; 67: 955–9. [DOI] [PubMed] [Google Scholar]
- 15. Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA‐B locus heavy chains permit biochemical characterization of certain HLA‐C locus products. J Immuol 1986; 137: 2299–306. [PubMed] [Google Scholar]
- 16. Eryigit M, Kirkali Z. HLA antigens and transitional cell carcinoma of the bladder. Urol Int 1990; 45: 75–7. [DOI] [PubMed] [Google Scholar]
- 17. Levin I, Klein T, Goldstein J, Kuperman O, Kanetti J, Klein B. Expression of class I histocompatibility antigens in transitional cell carcinoma of the urinary bladder in relation to survival. Cancer 1991; 68: 2591–4. [DOI] [PubMed] [Google Scholar]
- 18. Witjes JA, Umbas R, Debruyne FM, Schalken JA. Expression of markers for transitional cell carcinoma in normal bladder mucosa of patients with bladder cancer. J Urol 1995; 154: 2185–9. [PubMed] [Google Scholar]
- 19. Kamarashev J, Ferrone S, Seifert B et al. TAP1 down‐regulation in primary melanoma lesions: an independent marker of poor prognosis. Int J Cancer 2001; 95: 23–8. [DOI] [PubMed] [Google Scholar]
- 20. Hofbauer GF, Burkhart A, Schüler G, Dummer R, Burg G, Nestle FO. High frequency of melanoma‐associated antigen or HLA class I loss does not correlate with survival in primary melanoma. J Immunother 2004; 27: 73–8. [DOI] [PubMed] [Google Scholar]
- 21. Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer 2005; 117: 248–55. [DOI] [PubMed] [Google Scholar]
- 22. Ramnath N, Tan D, Li Q et al. Is downregulation of MHC class I antigen expression in human non‐small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother 2005; 55: 891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenberg SA, Yang JC, Schwartzentruber DJ et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 1998; 4: 321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 1999; 10: 281–7. [DOI] [PubMed] [Google Scholar]


