Abstract
To evaluate the impact of smoking cessation on individuals and populations, we examined the decrease in risk of lung cancer death in male ex‐smokers by age at quitting by pooling the data from three large‐scale cohort studies in Japan. For simplicity, subjects were limited to male never smokers and former or current smokers who started smoking at ages 18–22 years, and 110 002 men aged 40–79 years at baseline were included. During the mean follow‐up of 8.5 years, 968 men died from lung cancer. The mortality rate ratio compared to current smokers decreased with increasing attained age in men who stopped smoking before age 70 years. Among men who quit in their fifties, the cohort‐adjusted mortality rate ratios (95% confidence interval) were 0.57 (0.40–0.82), 0.44 (0.29–0.66) and 0.36 (0.13–1.00) at attained ages 60–69, 70–79 and 80–89 years, respectively. The corresponding figures for those who quit in their sixties were 0.81 (0.44–1.48), 0.60 (0.43–0.82) and 0.43 (0.21–0.86). Overall, the mortality rate ratio for current smokers, relative to non‐smokers, was 4.71 (95% confidence interval 3.76–5.89) and those for ex‐smokers who had quit smoking 0–4, 5–9, 10–14, 15–19, 20–24 and ≥25 years before were 3.99 (2.97–5.35), 2.55 (1.80–3.62), 1.87 (1.23–2.85), 1.21 (0.66–2.22), 0.76 (0.33–1.75) and 0.67 (0.34–1.32), respectively. Although earlier cessation of smoking generally resulted in a lower rate of lung cancer mortality in each group of attained age, the absolute mortality rate decreased appreciably after stopping smoking even in men who quit at ages 60–69 years. (Cancer Sci 2007; 98: 584–589)
Tobacco smoking is the best‐established risk factor for lung cancer.( 1 , 2 ) For smokers, smoking cessation is the most effective measure to decrease the risk of lung cancer. In most epidemiological studies,( 3 , 4 , 5 , 6 , 7 , 8 ) the risk by years since cessation has been estimated for all ex‐smokers.
To estimate the decrease in risk by stopping smoking in individuals and populations, however, more detailed data, including the risk reduction according to the age at quitting, are required. Some studies have reported the decrease in risk by age at stopping smoking.( 9 , 10 , 11 ) However, most of the studies were conducted in Western countries. The association between smoking and lung cancer risk is not so strong in Japanese as in Western populations.( 6 , 7 , 12 ) The relative risk of current smokers versus never smokers is much smaller in Japan than in Western countries, where the relative risk is far beyond 10 in men. The information from Western countries on the decrease in risk of lung cancer after quitting smoking therefore may not be readily applicable to Japanese.
In Japan, Sobue et al. estimated lung cancer risk by age at quitting smoking using data from the national vital statistics, and from a case‐control study and a previous cohort study.( 13 ) Wakai and coworkers conducted a similar estimation by using the data from a large‐scale cohort study (the Japan Collaborative Cohort [JACC] Study).( 14 ) Nevertheless, these two studies were limited by the small number of lung cancer cases by age at stopping smoking and duration after quitting or attained age after quitting, and had to assume a mathematical model for never and current smokers.
In the present study therefore we describe the decrease in risk of lung cancer by age at quitting smoking and attained age among male ex‐smokers by pooling the data from three large prospective studies in Japan, namely, the Japan Public Health Center‐Based Prospective (JPHC) Study,( 15 ) the Three‐Prefecture Cohort Study,( 12 ) and the JACC Study.( 16 , 17 )
Subjects and methods
Study subjects. We analyzed the dataset derived from the JPHC Study, the Three‐Prefecture Cohort Study, and the JACC Study. The design of these cohort studies is described in detail elsewhere.( 12 , 15 , 16 , 17 , 18 ) In brief, the JPHC Study started in 1990 for the first group (JPHC Study I) and in 1993 for the second group (JPHC Study II). Study subjects, aged 40–59 years for JPHC Study I and 40–69 years for JPHC Study II, were recruited mainly from all of the residents in the target areas served by selected public health centers in Japan. Eventually, 50 217 eligible individuals (23 571 men and 26 646 women) participated in JPHC Study I, and 63 192 individuals (29 780 men and 33 412 women) participated in JPHC Study II. We treated JPHC Study I and Study II as two independent cohorts in the analyses because of the different age ranges of the two populations.
The Three‐Prefecture Cohort Study collected data from 1983 to 1985 in selected geographic areas of three prefectures (Miyagi, Aichi and Osaka) in Japan. An additional study population was sampled in 1990 in one city in Osaka Prefecture. The study population included all residents aged 40 years or older, but those aged 80 years or over were excluded in our study. A total of 104 877 eligible persons (49 114 men and 55 763 women) responded to the baseline survey. The JACC Study cohort was established from 1988 to 1990, when 110 792 inhabitants (46 465 men and 64 327 women) aged 40–79 years filled in a baseline questionnaire. They were enrolled in 45 study areas throughout Japan, mostly from health checkups provided by the municipalities or from all residents living in a given target area.
At baseline, the study participants of these cohort studies completed a self‐administered questionnaire including smoking habits. It elucidated the smoking status (never, former or current), the age at starting smoking and the average number of cigarettes smoked per day for former and current smokers, and the age at quitting smoking or the number of years since cessation for former smokers. The response rate was 81% for the JPHC Study,( 18 ) 83% for the Three‐Prefecture Cohort Study,( 12 ) and 83% for the JACC Study (for 17 of 22 areas, which included all living residents as eligible subjects and where the response rate was available).( 17 )
In the present study, subjects were restricted to men because female former smokers were too few (n = 3709) to compute the mortality rate of lung cancer by age at quitting and years after quitting smoking. Of the 148 930 male participants in total, we excluded 7230 with missing information on smoking status and 57 with inappropriate data on smoking habits or follow‐up. From the JACC Study, 1673 men were omitted because they lived in a study area also covered by the Three‐Prefecture Cohort Study. Further, because attained age, age at starting smoking, and duration of smoking are mutually dependent, we limited ever smokers to those who initiated smoking at ages 18–22 years for simplicity.( 13 , 14 ) In Japan, because those aged under 20 years have been prohibited to smoke by law since 1900, most smokers start smoking regularly at around 20 years of age: 73.7% of the ever smokers started smoking at ages 18–22 and 29 242 men were excluded. Ex‐smokers with missing age at quitting (n = 726) were also omitted. Consequently, 110 002 men comprising 28 715 never smokers, 25 081 former smokers and 56 206 current smokers were eligible for the present analysis. In the analysis by age at smoking cessation, men who stopped smoking at under 40 years (n = 8411) were also left out due to the small number of lung cancer deaths in that category (n = 11). Our study was approved by the institutional review board of the National Cancer Center, Tokyo, Japan.
Definitions of never, ex‐ and current smokers. In JPHC Study I, participants were asked whether they had ever smoked. If not, they were classified as never smokers. Ever smokers were further asked if they smoked at the time of baseline survey. Those with positive and negative responses to the question were categorized into current smokers and ex‐smokers, respectively. In JPHC Study II, participants were first asked whether they smoked at baseline. Subjects who reported smoking at baseline were defined as current smokers. Those who had quit smoking and did not smoke at baseline were requested to indicate the age at cessation, the number of cigarettes smoked per day during the smoking period, and the age at starting smoking. Those with or without responses to these questions were classified as ex‐smokers or never smokers, respectively. In the JACC Study, subjects were asked about their smoking habits with three possible responses: ‘smoked at baseline’, ‘had quit smoking before baseline’ or ‘had never smoked.’ They were grouped into current, ex‐ or never smokers according to the answer to this question. Most participants in the Three‐Prefecture Cohort Study were similarly categorized as in the JACC Study. In one study area of the Three‐Prefecture Cohort Study, the possible response for never smokers was ‘had little or never smoked.’ In another area of this study, subjects selected one response from four possible choices, that is, ‘smoked at baseline daily’, ‘sometimes smoked at baseline’, ‘had previously smoked’ and ‘had never smoked.’ Those who gave the former two responses were defined as current smokers, whereas those giving the latter two responses were defined as ex‐smokers and never smokers, respectively. Thus, no more detailed definition of never smokers was described in the questionnaire in any of the cohort studies.
Follow‐up. In each cohort study, subjects who died or moved out of the study areas were identified by using population registries. For those who died, death by lung cancer was ascertained by reviewing death certificates (in the JPHC Study and the JACC Study) or by searching the population‐based cancer registries (in the Three‐Prefecture Cohort Study). Study participants were followed‐up through to the end of 1999 (in the JPHC Study and the JACC Study) or for exactly 10 years (in the Three‐Prefecture Cohort Study).
Statistical methods. For never and current smokers, we calculated the mortality rate of lung cancer by attained age (40–49, 50–59, 60–69, 70–79 and 80–89 years). For ex‐smokers, the rate was computed by age at quitting smoking (40–49, 50–59, 60–69 and 70–79 years) and attained age after quitting (40–49, 50–59, 60–69, 70–79 and 80–89 years). Person‐years for attained age 90 years or over were omitted due to the sparse data. The rate difference between ex‐ and current smokers in the same 10‐year category of attained age ([the rate in ex‐smokers] − [the rate in current smokers]) was also computed. The 95% confidence interval (CI) of the mortality rate and the rate difference was estimated assuming that the number of lung cancer deaths in a group has a Poisson distribution.( 19 ) The rates in former smokers by age at cessation of smoking and attained age were depicted in a log plot versus age with the rates in never and current smokers to illustrate changes in the mortality rates after quitting smoking.
To assess the reduction of lung cancer risk by smoking cessation, the mortality rate ratio with the 95% CI compared to current or continuing smokers was computed by attained age and age at which they stopped smoking. The rate ratio was adjusted for cohort (JPHC Study I, JPHC Study II, Three‐Prefecture Cohort Study or JACC Study) (mortality rate ratio 1) or cohort and number of cigarettes smoked per day (0–19, 20–39, 40–59, ≥60 or unknown [0.9% of the ever smokers]) (mortality rate ratio 2) using the Poisson regression model.( 20 ) We adopted the Poisson model instead of the proportional hazards model because the data were distributed as aggregated data to the members of our study group from its secretariat to strictly protect the privacy of participants.
In addition, the overall risk reduction according to the duration of abstinence from smoking at baseline (0–4, 5–9, 10–14, 15–19, 20–24 and ≥25 years) was evaluated with the mortality rate ratio adjusted for attained age (40–49, 50–59, 60–69, 70–79 or 80–89 years) and cohort (JPHC Study I, JPHC Study II, Three‐Prefecture Cohort Study or JACC Study) also applying the Poisson regression model.( 20 ) The decreasing trend in lung cancer risk with increasing duration of smoking cessation was tested statistically by scoring current smokers as 0, and ex‐smokers with 0–4, 5–9, 10–14, 15–19, 20–24 and ≥25 years of abstinence as 1, 2, 3, 4, 5 and 6, respectively, and including the score in the regression model.
All P‐values were two‐sided. The Poisson regression analysis was conducted using the Statistical Analysis System.( 21 )
Results
A total of 968 men died from lung cancer during the follow‐up of 930 004 person‐years (mean follow‐up period, 8.5 years). When the mortality rate of lung cancer among current smokers was compared to that among never smokers, the gap in the rate between the two groups rapidly increased with increasing attained age (Table 1).
Table 1.
Lung cancer mortality rates (per 100 000 person‐years) among male never smokers and current smokers who started smoking at age 18–22 years by attained age (attained age, 40–89 years)
| Attained age (years) | Never smokers | Current smokers | ||||
|---|---|---|---|---|---|---|
| Person‐years | No. lung cancer deaths | Lung cancer mortality rate (95% CI) | Person‐years | No. lung cancer deaths | Lung cancer mortality rate (95% CI) | |
| 40–49 | 48 311.6 | 5 | 10.3 (1.3–19.4) | 117 444.4 | 17 | 14.5 (7.6–21.4) |
| 50–59 | 95 595.2 | 13 | 13.6 (6.2–21.0) | 172 110.1 | 81 | 47.1 (36.8–57.3) |
| 60–69 | 66 781.6 | 30 | 44.9 (28.8–61.0) | 128 517.5 | 258 | 200.8 (176.3–225.3) |
| 70–79 | 27 207.1 | 28 | 102.9 (64.8–141.0) | 48 321.8 | 261 | 540.1 (474.6–605.7) |
| 80–89 | 7817.8 | 11 | 140.7 (57.6–223.9) | 6951.4 | 67 | 963.8 (733.0–1194.6) |
| Total | 245 713.3 | 87 | 35.4 (28.0–42.8) | 473 345.2 | 684 | 144.5 (133.7–155.3) |
CI, confidence interval.
Table 2 summarizes the lung cancer mortality rates among ex‐smokers by age at quitting smoking and attained age with the mortality rate difference and rate ratio to current smokers. The mortality rate ratio decreased with increasing attained age in men who stopped smoking before age 70 years. Even among men who stopped at age 60–69 years, a 40% (mortality rate ratio 1; 95% CI, 18–57%) decrease in lung cancer mortality was observed at age 70–79 years compared to current or continuing smokers. On the contrary, those who quit smoking in their seventies did not demonstrate a reduction in the risk of lung cancer death. The adjustment for number of cigarettes smoked per day generally lowered the rate ratio. In each category of attained age, the rate ratio tended to increase as the age at quitting increased. Furthermore, the earlier the cessation of smoking, the lower the lung cancer mortality rate was at a given attained age, as can be clearly seen in Fig. 1. The only exception was the rate at attained age of 80–89 years among men who quit smoking in their forties. However, the rate was based on an observation of only 744 person‐years.
Table 2.
Lung cancer mortality rates (per 100 000 person‐years), mortality rate differences and rate ratios among male ex‐smokers who started smoking at age 18–22 years by age at quitting smoking and attained age (attained age, 40–89 years)
| Age at quitting smoking (years) | Attained age (years) | Person‐years | No. lung cancer deaths | Lung cancer mortality rate (95% CI) | Crude mortality rate difference compared with current smokers (95% CI) | Mortality rate ratio 1 † compared with current smokers (95% CI) | Mortality rate ratio 2 ‡ compared with current smokers (95% CI) |
|---|---|---|---|---|---|---|---|
| 40–49 | 40–49 | 9087.5 | 1 | 11.0 (0.0–32.6) | −3.5 (−26.1–19.2) | 0.78 (0.10–5.86) | 0.79 (0.10–5.92) |
| 50–59 | 31 465.2 | 4 | 12.7 (0.3–25.2) | −34.4 (−50.5 to −18.2) | 0.27 (0.10–0.74) | 0.25 (0.09–0.68) | |
| 60–69 | 19 530.9 | 9 | 46.1 (16.0–76.2) | −154.7 (−193.5 to −115.9) | 0.23 (0.12–0.44) | 0.21 (0.11–0.41) | |
| 70–79 | 5183.7 | 2 | 38.6 (0.0–92.1) | −501.5 (−586.1 to −417.0) | 0.07 (0.02–0.29) | 0.07 (0.02–0.27) | |
| 80–89 | 744.2 | 3 | 403.1 (0.0–859.3) | −560.7 (−1072.0 to −49.5) | 0.42 (0.13–1.33) | 0.33 (0.10–1.08) | |
| 50–59 | 50–59 | 7643.8 | 5 | 65.4 (8.1–122.8) | 18.3 (−39.9–76.6) | 1.40 (0.57–3.46) | 1.33 (0.54–3.28) |
| 60–69 | 29 008.9 | 33 | 113.8 (74.9–152.6) | −87.0 (−132.9 to −41.1) | 0.57 (0.40–0.82) | 0.51 (0.35–0.74) | |
| 70–79 | 10 612.0 | 25 | 235.6 (143.2–327.9) | −304.5 (−417.8 to −191.3) | 0.44 (0.29–0.66) | 0.39 (0.26–0.60) | |
| 80–89 | 1146.8 | 4 | 348.8 (7.0–690.6) | −615.0 (−1027.5 to −202.6) | 0.36 (0.13–1.00) | 0.30 (0.11–0.83) | |
| 60–69 | 60–69 | 7082.8 | 11 | 155.3 (63.5–247.1) | −45.5 (−140.4–49.5) | 0.81 (0.44–1.48) | 0.74 (0.40–1.37) |
| 70–79 | 13 393.2 | 43 | 321.1 (225.1–417.0) | −219.1 (−335.3 to −102.9) | 0.60 (0.43–0.82) | 0.54 (0.39–0.76) | |
| 80–89 | 2188.8 | 9 | 411.2 (142.5–679.8) | −552.7 (−906.8 to −198.5) | 0.43 (0.21–0.86) | 0.35 (0.17–0.72) | |
| 70–79 | 70–79 | 1881.1 | 16 | 850.6 (433.8–1267.3) | 310.4 (−111.5–732.3) | 1.58 (0.95–2.62) | 1.45 (0.87–2.42) |
| 80–89 | 1670.3 | 21 | 1257.3 (719.5–1795.0) | 293.4 (−291.7–878.6) | 1.31 (0.80–2.14) | 1.10 (0.66–1.83) | |
| Total | 140 639.0 | 186 | 132.3 (113.2–151.3) |
Adjusted for cohort (JPHC Study I, JPHC Study II, Three‐Prefecture Cohort Study or JACC Study).
Adjusted for cohort and number of cigarettes smoked per day (0–19, 20–39, 40–59, ≥60 or unknown). CI, confidence interval.
Figure 1.
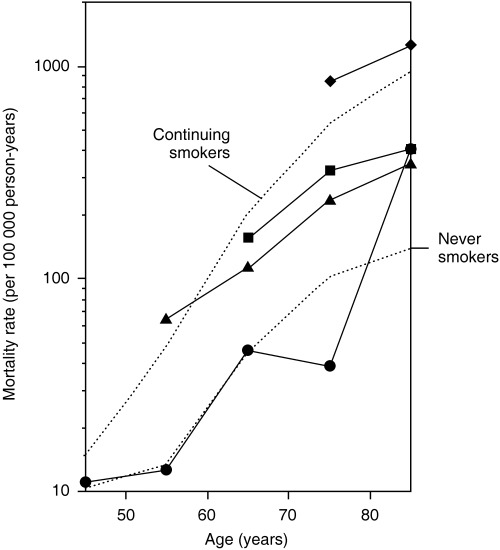
Log plot of lung cancer mortality rate versus age among never, former and continuing (current) smokers who started smoking at age 18–22 years. •, ▴, ▪ and ◆ denote ex‐smokers who quit at ages 40–49, 50–59, 60–69 and 70–79 years, respectively.
In contrast, the absolute reduction or the rate difference in the mortality rate of lung cancer among ex‐smokers compared with current smokers after 10–20 years of smoking cessation was considerably greater in the older men who quit up to their sixties than in the younger group because of the high mortality rate in the elderly (Table 2).
We also estimated the overall mortality rate ratio for death from lung cancer according to years since quitting smoking and found a decreasing trend in the ratio with increasing years after smoking cessation (Table 3; trend P = 6 × 10−26 for ex‐ and current smokers). It took more than 15 years for the risk in ex‐smokers to approach the level in non‐smokers; the mortality rate ratio was 1.21 (95% CI, 0.66–2.22) for abstinence of 15–19 years and 0.76 (0.33–1.75) for abstinence of 20–24 years. When former or current smokers were not restricted to those who initiated smoking at ages 18–22 years, the values of rate ratio for current smokers decreased somewhat (4.23; 95% CI, 3.40–5.28), but the decreasing pattern of risk was not essentially altered; the rate ratios (95% CI) for men who had quit 0–4, 5–9, 10–14, 15–19, 20–24 and ≥25 years before, relative to never smokers, were 3.68 (2.79–4.86), 2.52 (1.84–3.45), 1.87 (1.29–2.71), 1.24 (0.75–2.07), 1.09 (0.60–1.99) and 0.59 (0.32–1.11) (trend P = 1 × 10−29 for ex‐ and current smokers), respectively.
Table 3.
Mortality rate ratios for death from lung cancer according to years since cessation of smoking among male current and ex‐smokers who started smoking at age 18–22 years (attained age, 40–89 years)
| Status | Person‐years | No. lung cancer deaths | Mortality rate ratio † compared with current smokers (95% CI) |
|---|---|---|---|
| Never smokers | 245 713.3 | 87 | 1.00 |
| Current smokers | 473 345.2 | 684 | 4.71 (3.76–5.89) |
| Ex‐smokers (years since cessation of smoking | |||
| 0–4 | 58 730.7 | 91 | 3.99 (2.97–5.35) |
| 5–9 | 51 366.0 | 50 | 2.55 (1.80–3.62) |
| 10–14 | 40 776.2 | 29 | 1.87 (1.23–2.85) |
| 15–19 | 24 576.1 | 12 | 1.21 (0.66–2.22) |
| 20–24 | 16 750.7 | 6 | 0.76 (0.33–1.75) |
| ≥25 | 18 746.1 | 9 | 0.67 (0.34–1.32) |
| Trend | P = 6 × 10−26 ‡ | ||
Adjusted for attained age (40–49, 50–59, 60–69, 70–79 or 80–89 years) and cohort (JPHC Study I, JPHC Study II, Three‐Prefecture Cohort Study or JACC Study).
‡ Trend for ex‐ and current smokers. CI, confidence interval.
Discussion
In each group of attained age, the earlier cessation of smoking generally resulted in a lower rate of lung cancer mortality, reflecting the shorter period of exposure. In terms of the mortality rate ratio compared with current smokers, however, a 40% decrease in the risk can be expected after 10 years of cessation among men who stopped smoking even in their sixties. The absolute reduction in the risk in a given period was largest in this generation because of the higher mortality rate in older current or continuing smokers. Discouraging smoking in this age group therefore will be quite useful for the population‐level prevention of lung cancer. In the assessment of the overall mortality rate ratio, it took more than 15 years after smoking cessation for the risk of death from lung cancer in ex‐smokers to approach the level in non‐smokers.
The overall decrease in lung cancer risk in males after quitting smoking has been investigated previously in Japan.( 3 , 4 , 5 , 6 , 7 , 8 ) The finding that more than 15 years of smoking cessation is required to decrease the risk of lung cancer in former smokers to the level in never smokers is generally in line with previous studies.( 3 , 4 , 5 , 6 , 7 , 8 ) Figure 2a displays the findings from the previous cohort( 3 , 7 , 8 ) and case‐control( 4 , 5 , 6 ) studies, together with ours. In a previous study, Hirayama reported a far more rapid decline in risk than other studies, including ours, in the Six‐Prefecture Cohort Study; the mortality rate of lung cancer decreased to 46% that in current smokers after only 1–4 years of abstinence.( 3 )
Figure 2.
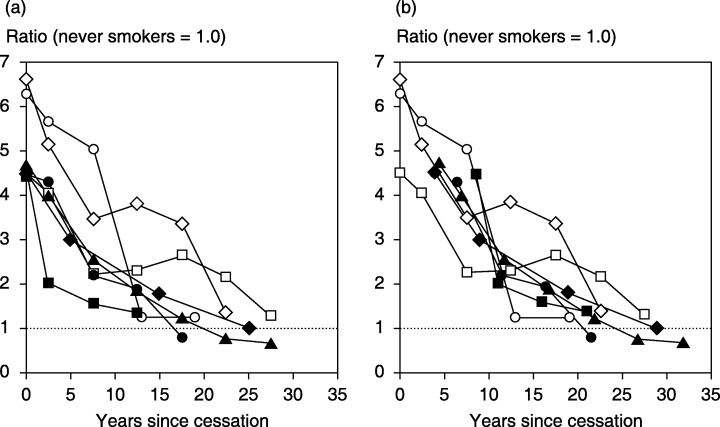
Decreasing patterns of lung cancer risk in men after quitting smoking in case‐control and cohort studies in Japan (a), and the graph redrawn adding half of the follow‐up period to years since cessation in the cohort studies (b). Odds ratios or mortality/incidence rate ratios compared with never smokers are displayed from published data: ▪, Hirayama;( 3 )◆, Sobue et al. (JPHC Study);( 7 )•, Ando et al. (JACC Study);( 8 )▴, the present study; □, Sobue et al.;( 4 )◊, Gao et al.;( 5 ) and ○, Stellman et al. (the Aichi portion with community controls).( 6 )
However, cohort studies may overrate the speed of reduction in risk because they usually fix the number of years since smoking cessation in former smokers at the baseline survey. The years actually increase during the follow‐up. If this assumption is the case, half of the follow‐up period, on average, should be added to years since cessation in prospective studies that fix the years at baseline. When half of the follow‐up period, that is, 8.5, 3.9, 4.0 and 4.3 years, is added to years since quitting in the Six‐Prefecture Cohort Study, the JPHC Study, the JACC Study and our study, respectively, the risk reduction after cessation of smoking appears fairly consistent among the seven studies (Fig. 2b). As a whole, stopping smoking seems to halve the risk of lung cancer in male smokers within 10–15 years.
Considering findings from recent long‐term follow‐up studies, a report by the US Surgeon General in 2004 concluded that even after many years of not smoking, the risk of lung cancer in former smokers remains higher than in persons who have never smoked.( 1 ) In Japan, however, as seen in Fig. 2b, lung cancer risk may decrease to the level of lifetime non‐smokers after abstinence of 20–30 years. In the present study, men who quit smoking at age 40–49 years demonstrated a mortality rate from lung cancer similar to that among never smokers, allowing for the large random error at attained ages of 70 years and over (Fig. 1). The lower relative risk for lung cancer among current smokers in Japan than in Western countries( 6 , 7 , 12 ) may result in different effects of smoking cessation to those observed among Westerners. Prospective studies with a longer follow‐up period are warranted to further address this question.
Sobue et al. reported that ex‐smokers with a mean age at cessation of 44.3 years showed an odds ratio of 0.35 compared to current smokers, after 19.6 years cessation on average; this was also true for those with a mean age at cessation of 53.9 years who showed an odds ratio of 0.50 compared to current smokers, after an average abstinence of 19.4 years.( 13 ) These figures are somewhat greater but in fairly good agreement with ours when random error is taken into consideration; for males who quit smoking in their forties, the mortality rate ratio 2 in our study was 0.21 (95% CI, 0.11–0.41) at attained age of 60–69 years, and for those who quit in their fifties, the ratio was 0.39 (0.26–0.60) at attained age of 70–79 years. We here used the mortality rate ratio 2 for comparison because the number of cigarettes smoked per day was adjusted in the study by Sobue and coworkers.( 13 ) The fairly good agreement between the two studies based on different datasets may support the validity of our estimates. The initial decrease in the risk of lung cancer death in male ex‐smokers who quit smoking at age 50–59 years seems to be greater in the present study than in the study based exclusively on the JACC Study.( 14 ) The mortality rate ratios (mortality rate ratio 1) compared to current smokers were 1.40 (95% CI, 0.57–3.46), 0.57 (0.40–0.82) and 0.44 (0.29–0.66) at attained age 50–59, 60–69 and 70–79 years, respectively, in our study whereas they were 1.64, 0.86 and 0.38, respectively, in the JACC Study. The estimates in men who stopped smoking in their sixties are fairly similar between the two studies; they (mortality rate ratio 1) were 0.81 (95% CI, 0.44–1.48) and 0.60 (0.43–0.82) at attained ages 60–69 and 70–79 years, respectively, and were 0.57 and 0.53 in the JACC Study. The reduction in lung cancer risk by stopping smoking in their sixties has also been supported by studies in Western countries.( 9 , 10 )
We did not find a decrease in the risk of lung cancer death among those who quit smoking in their seventies, which is in line with a previous study based on the JACC Study.( 14 ) Although the present study focused on the decrease in lung cancer risk after smoking cessation, quitting smoking benefits smokers not only in reducing the risk of lung cancer, but also in a vast range of disease preventions and health promotions. A report by the US Surgeon General concluded that quitting smoking had immediate as well as long‐term benefits, reducing the risk of diseases caused by smoking and improving health in general, based on an extensive review of epidemiological studies.( 1 ) This conclusion was endorsed by 40 years’ follow‐up of male British doctors.( 22 ) In that study, even those who stopped smoking at ages 65–74 years (mean, 71 years) showed a lower mortality rate than continuing smokers at ages over 75 years.
In our pooled analysis, the mortality rate ratio for male current smokers compared with never smokers was 4.71, which is much lower than the corresponding relative risk in Western countries.( 7 ) This large gap in the relative risk has been discussed extensively by Sobue et al.( 7 ) and Marugame et al.( 12 ) and may be attributable to both the lower risk of lung cancer in current smokers and the higher risk in non‐smokers. The lower lifetime consumption of cigarettes in Japanese, due partly to the later start of smoking habits, the lower consumption per day, or the shortage of cigarettes during and shortly after World War II in Japan, may be one reason for the lower risk of lung cancer in Japanese smokers. However, the risk in never smokers may be higher due to environmental tobacco smoke in Japan compared with Western countries. The differences in other factors including ingredients and filters of cigarettes, lifestyle factors other than smoking such as diet, histological type of lung cancer and genetic susceptibility to the cancer between Japanese and Western populations, should also be taken into account when explaining the lower relative risk in Japanese.( 7 , 12 )
The strength of the present study is derived principally from its prospective design, which enabled us to directly compute the mortality rate of lung cancer after smoking cessation. We did not need to assume complicated mathematical models because of the large sample size obtained by pooling the data from the three large‐scale cohort studies. In addition, most of the subjects were enrolled from all residents in defined target areas with a high response rate (>80%).( 12 , 17 , 18 ) Our findings may be therefore reasonably generalizable to Japanese populations and would provide basic information for smoking cessation strategies.
However, some methodological limitations should be kept in mind when interpreting the findings. First, some smokers may have quit due to symptoms or diagnosis of lung cancer itself. We could not exclude those with a medical history of lung cancer because the questionnaire of the Three‐Prefecture Cohort Study did not elicit any history of cancer. The higher mortality rate immediately after smoking cessation may partly be explained by this ‘quitting‐ill’ effect.( 9 ) After 10 years of quitting, however, such an effect will almost disappear because the prognosis of lung cancer is rather poor: the 5‐year relative survival rate of patients diagnosed from 1993 to 1995 was reported to be 13.8% in the population‐based cancer registry of Osaka Prefecture in Japan, which followed cancer patients extensively.( 23 ) Second, the smoking habits of the participants could have changed during the observation period of cohort studies. Both current smokers who stopped smoking and ex‐smokers who resumed the habit would have led to an underestimate of the decrease in risk after smoking cessation. Because older current smokers at baseline would have been more likely to have become ex‐smokers than younger ones during the follow‐up,( 24 ) the benefit of smoking cessation may have been more underestimated in older age groups, namely, those who quit in their seventies in the present study. Third, the patterns observed at different ages may be partly the result of a birth cohort effect. Studies with a very long follow‐up period are required to address this issue.
The questions on age at starting smoking and cessation of smoking, and on the number of cigarettes smoked per day, were almost the same across the cohort studies pooled in our analysis. Those on smoking status (i.e. current, former or never), however, varied considerably among the cohorts. We did not validate directly the comparability of the different types of questionnaires, which might be another limitation of the present analysis. Nevertheless, the relative risks for male current versus never smokers for lung cancer were 4.5, 5.10 and 4.46 for the JPHC Study,( 7 ) the Three‐Prefecture Cohort Study( 12 ) and the JACC Study,( 8 ) respectively. The corresponding figures for former versus never smokers were 2.2, 2.60 and 2.38. These quite comparable values among cohorts suggest the compatibility of the definition of smoking status in men among the studies.
Finally, we could not adjust for confounding by factors other than the number of cigarettes smoked because the format of the questionnaires varied among the three cohort studies. The potential confounder may include dietary factors.( 25 ) If men who stop smoking at a younger age are health conscious and have dietary habits that reduce lung cancer death, the gap in the mortality rate between males who quit earlier and those who quit later would be exaggerated.
In conclusion, smoking cessation at a younger age will keep the risk of lung cancer death at a lower level. The mortality rate, however, can decline appreciably after stopping smoking, even in men who quit at age 60–69 years.
Acknowledgments
We sincerely thank the members and coworkers of the Japan Public Health Center‐Based Prospective Study Group, the Three‐Prefecture Cohort Study Group, and the Japan Collaborative Cohort Study Group. This work was supported by Grants‐in‐aid for Comprehensive Research on Cardiovascular Diseases, for Cancer Research, and for the Third‐Term Comprehensive Ten‐Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare, Japan, and also by Grants‐in‐aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. US Department of Health and Human Services. Introduction and approach to causal inference. In: The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004; 1–33. [Google Scholar]
- 2. Wakai K, Inoue M, Mizoue T et al. Tobacco smoking and lung cancer risk: an evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol 2006; 36: 309–24. [DOI] [PubMed] [Google Scholar]
- 3. Hirayama T. Smoking and mortality. In: Wahrendorf J, ed. Life‐Style and Mortality: A Large‐Scale Census‐Based Cohort Study in Japan. Basel: Karger, 1990; 28–59. [Google Scholar]
- 4. Sobue T, Suzuki T, Fujimoto I et al. Lung cancer risk among exsmokers. Jpn J Cancer Res 1991; 82: 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao CM, Tajima K, Kuroishi T, Hirose K, Inoue M. Protective effects of raw vegetables and fruit against lung cancer among smokers and ex‐smokers: a case‐control study in the Tokai area of Japan. Jpn J Cancer Res 1993; 84: 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stellman SD, Takezaki T, Wang L et al. Smoking and lung cancer risk in American and Japanese men: an international case‐control study. Cancer Epidemiol Biomarkers Prev 2001; 10: 1193–9. [PubMed] [Google Scholar]
- 7. Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, Tsugane S. Cigarette smoking and subsequent risk of lung cancer by histologic type in middle‐aged Japanese men and women: the JPHC Study. Int J Cancer 2002; 99: 245–51. [DOI] [PubMed] [Google Scholar]
- 8. Ando M, Wakai K, Seki N et al. Attributable and absolute risk of lung cancer death by smoking status: findings from the Japan Collaborative Cohort Study. Int J Cancer 2003; 105: 249–54. [DOI] [PubMed] [Google Scholar]
- 9. Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst 1993; 85: 457–64. [DOI] [PubMed] [Google Scholar]
- 10. Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case‐control studies. BMJ 2000; 321: 323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004; 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marugame T, Sobue T, Satoh H et al. Lung cancer death rates by smoking status: comparison of the Three‐Prefecture Cohort Study in Japan to the Cancer Prevention Study II in the USA. Cancer Sci 2005; 96: 120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sobue T, Yamaguchi N, Suzuki T et al. Lung cancer incidence rate for male ex‐smokers according to age at cessation of smoking. Jpn J Cancer Res 1993; 84: 601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wakai K, Seki N, Tamakoshi A et al. Decrease in risk of lung cancer death in males after smoking cessation by age at quitting: findings from the JACC Study. Jpn J Cancer Res 2001; 92: 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Watanabe S, Tsugane S, Sobue T, Konishi M, Baba S. Study design and organization of the JPHC Study. J Epidemiol 2001; 11 (Suppl): S3–7. [DOI] [PubMed] [Google Scholar]
- 16. Ohno Y, Tamakoshi A. Japan Collaborative Cohort Study for Evaluation of Cancer Risk Sponsored by Monbusho (JACC Study). J Epidemiol 2001; 11: 144–50. [DOI] [PubMed] [Google Scholar]
- 17. Tamakoshi A, Yoshimura T, Inaba Y et al. Profile of the JACC Study. J Epidemiol 2005; 15 (Suppl 1): S4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsugane S, Sobue T. Baseline survey of JPHC Study–design and participation rate. J Epidemiol 2001; 11 (Suppl): S24–9. [DOI] [PubMed] [Google Scholar]
- 19. Rothman KJ. Analysis of crude data. In: Rothman KJ, ed. Modern Epidemiology, 1st edn. Boston: Little, Brown, 1986; 153–76. [Google Scholar]
- 20. Breslow NE, Day NE. Fitting models to grouped data. In: Heseltine E, ed. Statistical Methods in Cancer Research, Vol. 2. Lyon: IARC, 1987; 120–76. [Google Scholar]
- 21. SAS Institute Inc. SAS/STAT User's Guide, Version 8. Cary, NC: SAS Institute, 1999. [Google Scholar]
- 22. Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ 1994; 309: 901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ajiki W, Tsukuma H, Oshima A. Trends in cancer incidence and survival in Osaka. In: Tajima K, Kuroishi T, Oshima A, eds. Cancer Mortality and Morbidity Statistics: Japan and the World 2004. Tokyo: Japan Scientific Societies Press, 2004; 137–63. [Google Scholar]
- 24. Marugame T, Kamo K, Sobue T et al. Trends in smoking by birth cohorts born between 1900 and 1977 in Japan. Prev Med 2006; 42: 120–7. [DOI] [PubMed] [Google Scholar]
- 25. World Cancer Research Fund, American Institute for Cancer Research. Cancers, nutrition and food: lung. In: Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington: American Institute for Cancer Research, 1997; 130–47. [Google Scholar]


