Abstract
XAF1 (X chromosome‐linked inhibitor of apoptosis [XIAP]‐associated factor 1) is a novel XIAP modulator that negatively regulates the anti‐apoptotic effects of XIAP and sensitizes cells to other cell death triggers. It has been reported to be downregulated in a variety of human cancer cell lines. However, the role of XAF1 in pancreatic carcinogenesis remains unclear. In the present study, we investigated the prognostic values of XAF1 expression and its regulation in cancer cell growth and apoptosis both in vitro and in vivo. From the immunohistochemistry staining of tissue microarray, 40 of 89 (44.9%) pancreatic specimens showed low levels of XAF1 expression. Statistical analysis suggested the downregulation of XAF1 was significantly correlated with tumor staging (P = 0.047) and those patients with low XAF1 levels had shorter survival times (P = 0.0162). Multivariate analysis indicated that XAF1 expression was an independent prognostic indicator of the survival of patients with pancreatic cancer (P = 0.007). Furthermore, we found that restoration of XAF1 expression mediated by Ad5/F35 virus suppressed cell proliferation and induced cell cycle arrest and apoptosis, accompanied by the activation of caspases 3, 8, and 9 and poly(ADP‐ribose) polymerase as well as increased level of cytochrome c and Bid cleavage. Notably, XAF1 restoration robustly decreased survivin expression rather than XIAP. In addition, in vivo s.c. xenografts from Ad5/F35‐XAF1 treatment, which showed less cellular proliferation and enhanced apoptosis, were significantly smaller than those from control groups. Our findings document that XAF1 is a valuable prognostic marker in pancreatic cancer and could be a potential candidate for cancer gene therapy. (Cancer Sci 2009)
Abbreviations
- IAP
inhibitor of apoptosis protein
- IRS
immunoreactive score
- PARP
poly(ADP‐ribose) polymerase
- PI
propidium iodide
- TRAIL
tumor necrosis factor‐related apoptosis‐inducing ligand
- XAF1
XIAP‐associated factor 1
- XIAP
X chromosome‐linked IAP
Pancreatic cancer is one of the most aggressive malignant tumors.( 1 ) In China, pancreatic cancer is the sixth leading cause of malignancy‐related death, with an overall cumulative 5‐year survival rate of 1–3%.( 2 ) This extremely poor prognosis is due to the high resistance of pancreatic cancer cells to any available treatment strategy.( 3 , 4 ) Because intact apoptosis pathways are crucial for therapy‐induced cytotoxicity, evasion of apoptosis (programmed cell death) is believed to be one major contributor of treatment failure in pancreatic cancer.( 5 , 6 ) Therefore, restoration of the apoptosis mechanism has attracted substantial attention when pursuing curable therapy for pancreatic cancer.
The process of apoptosis is regulated at multiple levels by several regulatory mechanisms, such as the IAP family that bind and inhibit the activity of caspase.( 7 ) In many experimental systems, XIAP has been recognized as the most potent caspase inhibitor in the IAP family.( 8 , 9 ) Overexpression of XIAP has been indicated in a variety of human cancers, including pancreatic cancer.( 10 ) The caspase inhibiting activity of XIAP can be suppressed by its interacting proteins, like the well‐characterized Smac/DIABLO and HtrA2/Omi.( 11 , 12 ) In response to apoptotic signals, these proteins are released from the mitochondria to modify the XIAP function. Another XIAP modulator is XAF1, a nuclear protein that functions as an antagonist of XIAP by rescuing XIAP‐suppressed caspase activity.( 13 ) XAF1 can dramatically sensitize cancer cells to apoptotic triggers such as TRAIL, etoposide treatments,( 14 ) 5‐fluorouracil, H2O2, γ‐irradiation, ultraviolet,( 15 ) and tumor necrosis factor‐α which was independent of its interaction with XIAP.( 16 ) XAF1 is therefore believed to play an important role in the major apoptosis‐related pathways.
XAF1 also serves as a candidate tumor suppressor gene. Loss of XAF1 has been observed in a variety of cancer cell lines and human cancers,( 17 , 18 , 19 , 20 ) which then is associated with malignant tumor development and progression in a variety of cancers. Loss of XAF1 is due, at least in part, to epigenetic alterations such as DNA methylation at the CpG sites within the promoter region.( 20 , 21 , 22 ) Reactivation of XAF1 by DNA methylation inhibitors restores the sensitivity of cancer cells to apoptosis‐inducing agents.( 23 , 24 ) Therefore, enhancement of XAF1 levels provides a promising strategy for pancreatic cancer therapy.
In this study, we investigated XAF1 expression in pancreatic cancer and its regulation in cancer cell apoptosis both in vitro and in vivo. We identified XAF1 as a biomarker for prognosis as well as a target for therapeutic agents.
Materials and Methods
Patients and tissue samples. Tumor samples were obtained from pancreatic cancer patients who underwent surgery between 2002 and 2005 at Rui Jin Hospital (Shanghai Jiaotong University School of Medicine, Shanghai, China). All cases were staged according the guidelines of the International Union Against Cancer (2002). There were 52 males and 37 females with ages ranging from 31 to 79 years (median, 60.4 years). The median follow‐up data was 13.4 months (range 0.3–42 months) and 77 (86.5%) out of 89 patients died of the disease during the follow‐up period. All available clinicopathological data were collected and is shown in Table 1. The study was approved by the ethical review board of Rui Jin Hospital.
Table 1.
Relationship of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) expression with clinicopathologic parameters in pancreatic cancer
| Total | Low | High | χ2 | P | |
|---|---|---|---|---|---|
| Total cases | 89 | 40 | 49 | ||
| Age (years) | |||||
| High (≥65) | 28 | 10 | 18 | 1.406 | NS |
| Low (<65) | 61 | 30 | 31 | ||
| Gender | |||||
| Female | 37 | 15 | 22 | 0.496 | NS |
| Male | 52 | 25 | 27 | ||
| Tumor staging | |||||
| T2 | 10 | 5 | 5 | 6.095 | 0.047 |
| T3 | 62 | 23 | 39 | ||
| T4 | 17 | 12 | 5 | ||
| Lymph node status | |||||
| Positive | 28 | 16 | 12 | 2.457 | NS |
| Negative | 61 | 24 | 37 | ||
| Distant metastasis | |||||
| Positive | 4 | 2 | 2 | 0.000 | NS |
| Negative | 85 | 38 | 47 | ||
| Tumor grading (n = 86) | |||||
| G1/G2 | 63 | 29 | 34 | 0.044 | NS |
| G3/G4 | 23 | 10 | 13 | ||
| Surgical margins (n = 81) | |||||
| Positive | 9 | 7 | 2 | 3.164 | NS |
| Negative | 72 | 29 | 43 | ||
| Size (n = 78) | |||||
| Large (≥4.5) | 40 | 23 | 17 | 0.187 | NS |
| Small (<4.5) | 38 | 20 | 18 | ||
| Vascular invasion (n = 85) | |||||
| Yes | 45 | 20 | 25 | 0.033 | NS |
| No | 40 | 17 | 23 | ||
| Perineural invasion (n = 86) | |||||
| Yes | 36 | 17 | 19 | 0.231 | NS |
| No | 50 | 21 | 29 | ||
NS, not significant.
Tissue microarray construction and immunohistochemistry. Formalin‐fixed paraffin‐embedded samples were reviewed for tissue array construction by a pathologist. At least two core tissue biopsies were taken from morphologically representative regions of each pancreatic tumor. Samples of 89 primary tumor cases and 21 normal tissue samples were arranged in rows and columns to construct a tissue microarray. For staining, sections (5 μm) were transferred to glass slides using an adhesive slide system (PSA‐CS 4; Instrumedics, Hackensack, NJ, USA) to support cohesion of the array elements.
Slides were de‐waxed in xylene, and rehydrated in ethanol with descending concentration. Anti‐XAF1 antibody (1 : 500; Abcam, Cambridge, UK) or XIAP (1 : 100; BD Biosciences, San Jose, CA, USA) was added after blocking of endogenous peroxidase and proteins, and each section was incubated with HRP‐labeled antigoat IgG antibody. The immunostained specimens were assessed semiquantitatively by two independent observers without prior knowledge of the clinicopathologic characteristics. XAF1 or XIAP expression was scored using two measures: staining intensity was graded as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong); the percentage of positive cells was scored as 0 (negative), 1 (<10%), 2 (11–50%), 3 (51–80%), or 4 (>80%). The two scores were multiplied as the IRS and two categories of expression levels were set up: 1, low expression (meaning 0–2); and 2, “high expression” (meaning 3–12).
Construction of expression plasmids. XAF1 full‐length cDNA was cloned into a pDC316 carrier plasmid (Benyuanzhengyang, Beijing, China) to generate pDC316‐XAF1. The plasmid pDC316‐XAF1 and the skeleton plasmid pBHG‐fiber5/35 (Benyuanzhengyang) were co‐transfected into HEK293 cells using Polyfect (Qiagen, Hilden, Germany). The co‐transfection yielded the recombinant Ad5/F35‐XAF1 plasmid. Successful recombination was confirmed by observation of cytotoxicity as well as by PCR. The control vector Ad5/F35‐Null was obtained by the same method.
Cell culture and gene transduction. Human pancreatic cancer cell lines BxPC‐3, SW1990, and PANC‐1 were maintained in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10% FBS and 1% penicillin–streptomycin under standard culture conditions (37°C, 95% humidified air and 5% CO2). For gene transduction, cells were incubated with adenoviral aliquots at a desired MOI for 4 h before addition of culture medium. Ad5/F35‐Null virus was used as a non‐specific control for gene transfer.
Cell viability analysis. Cells were seeded at 1 × 104 per well into 96‐well plates. Cell viability was determined by MTT assay (Sigma, St. Louis, MO, USA) according to the standard protocol. The ratio of absorbance at 570 nm wavelength of treated cells relative to that of untransfected cells was calculated and expressed as a proliferation index. Each treatment was repeated at least three times and the proliferation values were expressed as mean ± SEM.
Cell cycle analysis by FACS. Cell cycle distribution was determined by staining DNA with PI. Briefly, cells were incubated for 48 h after treatment then harvested. The collected cells were fixed in 70% ethanol and cell pellets were suspended with PI with simultaneous RNase treatment at 37°C for 30 min. The percentage of cells in the different phases of the cell cycle was measured with a FACSCalibur flow cytometer (BD Biosciences).
Cell apoptosis analysis by FACS. Early‐stage apoptosis was assessed using an Annexin V–FITC Apoptosis Detection Kit (BD Pharmingen, San Diego, CA, USA) according to the manufacturer’s protocols. Briefly, cells were washed with cold PBS, resuspended in binding buffer, and 5 μL FITC‐conjugated Annexin V was added. After incubation at room temperature for 15 min, an additional 5 μL PI was added, and the cells were analyzed by flow cytometry. Each treatment was triplicated and the results were expressed as mean ± SEM.
Western blot analysis. Whole‐cell lysates were obtained using lysis buffer and soluble cell lysates were collected after centrifugation at 13 400g for 30 min. Protein samples (20 μg) were subjected to SDS gel electrophoresis, transferred to PVDF membranes (Bio‐Rad, Hercules, CA, USA), and the blot was probed with primary antibodies at the indicated dilutions: XAF1 (1 : 1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), caspase 3 (1 : 1000; Cell Signaling Technology, Beverly, MA, USA), PARP (1 : 1000; Cell Signaling Technology), caspase 8 (1 : 1000; Cell Signaling Technology), caspase 9 (1 : 1000, Cell Signaling Technology), Bid (1 : 1000; R&D Systems, Minneapolis, MN, USA), cytochrome c (1 : 1000; Sigma), survivin (1 : 1000; Cell Signaling Technology), XIAP (1 : 1000; BD Pharmingen), β‐actin (1 : 5000; Sigma). Following incubation with an HRP‐conjugated secondary antibody, the antibody‐bound proteins were detected by the ECL system (Amersham Pharmacia Biotech, Uppsala, Sweden).
In vivo xenograft experiment. Athymic nude mice, 6–8 weeks old, were obtained from the Shanghai Experimental Animals Centre of the Chinese Academy of Sciences (Shanghai, China). All animal studies were carried out under approved guidelines of the Animal Care and Use Committee of Shanghai Jiaotong University. SW1990 cells were s.c. injected into the right rear flank of five athymic nude mice. As the tumor grew larger, it was divided into small even pieces and replanted into the right rear flank of 25 athymic nude mice. When the tumors were palpable, animals were randomized to five groups: the untreated group (n = 5), injection of PBS into tumors every other day; the vector group (n = 5), injection of Ad5/F35‐Null into tumors every other day; the XAF1 group (n = 5), injection of Ad5/F35‐XAF1 tumors every other day; the gemcitabine group (n = 5), injection of 50 mg/kg gemcitabine (Eli Lilly, Indianapolis, IN, USA) i.p. twice a week; and the combination group (n = 5), injection of Ad5/F35‐XAF1 into tumors every other day plus 50 mg/kg gemcitabine i.p. twice a week. Tumor size was observed and recorded twice a week. Mice were killed on the day of the last treatment. Tumors were weighed and measured, and a portion of each was placed in either 4% paraformaldehyde or was snap‐frozen in liquid nitrogen. Tumor volume was calculated as 0.5 × (length) × (width)2.
Histologic analysis of growth and apoptosis. The tumor tissues were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned at 4 μm thicknesses. Growth in tumor sections was evaluated by Proliferating Cell Nuclear Antigen (PCNA), and apoptosis was assessed by TUNEL assay using an in situ cell death detection kit (Roche, Basel, Switzerland) according to the manufacturer’s recommendations. The tumor sections were also stained with H&E.
Statistical analysis. Statistical analyses were conducted using SPSS 13.0 software. The χ2‐test was used to evaluate the correlation between XAF1 or XIAP expression and clinicopathologic characters. Survival curves were drawn according to the Kaplan–Meier method and differences between the curves were analyzed by applying the log–rank test. A multivariate regression analysis (Cox) was used to analyze the independent prognostic factors. One‐way ANOVA was used to compare values of test and control samples. P‐values less than 0.05 were considered to be statistically significant.
Results
XAF1 is less expressed in pancreatic cancers than in normal tissues. Immunohistochemical analyses were carried out in 89 pancreatic tumors and 21 normal pancreatic tissues. XAF1 staining was observed in normal and malignant epithelium and acinar cells. Similar to the nucleus and cytoplasm localization of XAF1 previously reported,( 18 , 25 ) we showed a strong XAF1 signal in the cytoplasm and/or the nuclei. In total, XAF1 expression was low in 40 (44.9%) of 89 pancreatic cancers and 2 (9.5%) of 21 normal pancreatic tissues (Fig. 1). The mean score of XAF1 expression was significantly higher in normal (IRS = 8.381) compared with cancer (IRS = 3.938; P < 0.01).
Figure 1.
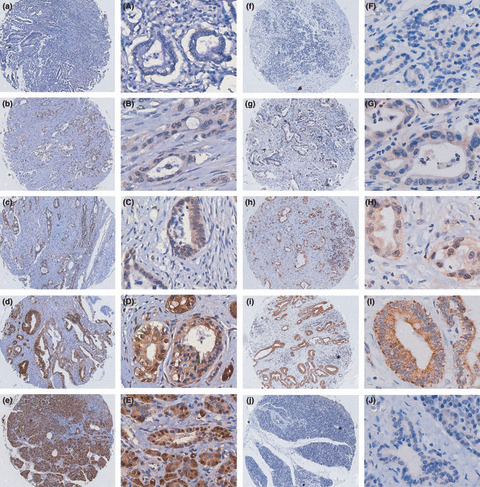
Immunohistochemical evaluation of X chromosome‐linked inhibitor of apoptosis protein (XIAP) expression and XIAP‐associated factor 1 (XAF1) in pancreatic cancers and normal pancreas on tissue microarrays. Invasive pancreatic cancers are shown with negative (a,A), weak (b,B), moderate (c,C), and strong (d,D) XAF1 staining. (e,E) Normal pancreatic tissue showing strong staining of epithelial and acinar cells. (f–i and F–I) Pancreatic cancers showing weak, moderate, and strong XIAP expression. (j,J) Normal pancreatic tissue showing negative XIAP expression. Magnification, (a–j) ×100; (A–J) ×400.
XIAP was diffusely stained in the cytoplasm of malignant epithelium cells. In contrast, minimal to absent labeling was seen in non‐neoplastic pancreatic ductules. XIAP expression was high in 64 (71.9%) of 89 pancreatic cancers and 4 (19.0%) of 21 normal pancreatic tissues (Fig. 1). The mean score of XIAP expression was significantly higher in cancer (IRS = 3.19) compared with normal (IRS = 1.05; P < 0.01).
Lack of XAF1 correlates with poor prognosis of pancreatic cancer. We then examined the correlations between XAF1 or XIAP expression and clinicopathologic variables. Tumor staging (P = 0.047 by χ2‐test) was significantly associated with low expression of XAF1 (Table 1). However, a non‐significant correlation was observed between XIAP expression and clinicopathologic factors (Table 2). No correlations were observed between XAF1and XIAP expression (P > 0.05).
Table 2.
Relationship of X chromosome‐linked inhibitor of apoptosis protein (XIAP) expression with clinicopathologic parameters in pancreatic adenocarcinomas
| Total | Negative | Positive | χ2 | P | |
|---|---|---|---|---|---|
| Total cases | 89 | 25 | 64 | NA | |
| Age (years) | |||||
| High (≥65) | 28 | 4 | 24 | 3.854 | NS |
| Low (<65) | 61 | 21 | 40 | NA | |
| Gender | |||||
| Female | 37 | 11 | 26 | 0.084 | NS |
| Male | 52 | 14 | 38 | NA | |
| Tumor extent | |||||
| T2 | 10 | 3 | 7 | 3.978 | NS |
| T3 | 62 | 14 | 48 | NA | |
| T4 | 17 | 8 | 9 | NA | |
| Lymph node status | |||||
| Positive | 28 | 7 | 21 | 0.660 | NS |
| Negative | 61 | 18 | 43 | NA | |
| Distant metastasis | |||||
| Positive | 4 | 2 | 2 | 0.000 | NS |
| Negative | 85 | 23 | 62 | NA | |
| Tumor grading (n = 86) | |||||
| G1/G2 | 63 | 19 | 44 | 0.713 | NS |
| G3/G4 | 23 | 6 | 17 | NA | |
| Surgical margins (n = 81) | |||||
| Positive | 9 | 1 | 8 | 0.861 | NS |
| Negative | 72 | 23 | 49 | NA | |
| Size (n = 78) | |||||
| Large (≥4.5) | 40 | 12 | 28 | 0.395 | NS |
| Small (<4.5) | 38 | 9 | 29 | NA | |
| Vascular invasion (n = 85) | |||||
| Yes | 45 | 15 | 30 | 1.907 | NS |
| No | 40 | 8 | 32 | NA | |
| Perineural invasion (n = 86) | |||||
| Yes | 36 | 10 | 26 | 0.001 | NS |
| No | 50 | 14 | 36 | NA | |
NS, not significant; NA, not applicable.
The Kaplan–Meier analysis revealed that patients with high levels of XAF1 expression had longer overall survival time, whereas patients with low XAF1 expression had worse survival (P = 0.0162 by the log–rank test; Fig. 2a). In addition, patients with low levels of XIAP expression had longer overall survival time (P = 0.0213 by the log–rank test; Fig. 2b). Therefore, we divided subjects into three groups for survival analysis: group 1, XIAP low; group 2, XIAP high and XAF1 high; and group 3, XIAP high and XAF1 low. Survival analysis showed that patients with high XIAP expression and low XAF1 expression had the most unfavorable outcomes (P = 0.0033 by the log–rank test; Fig. 2c).
Figure 2.
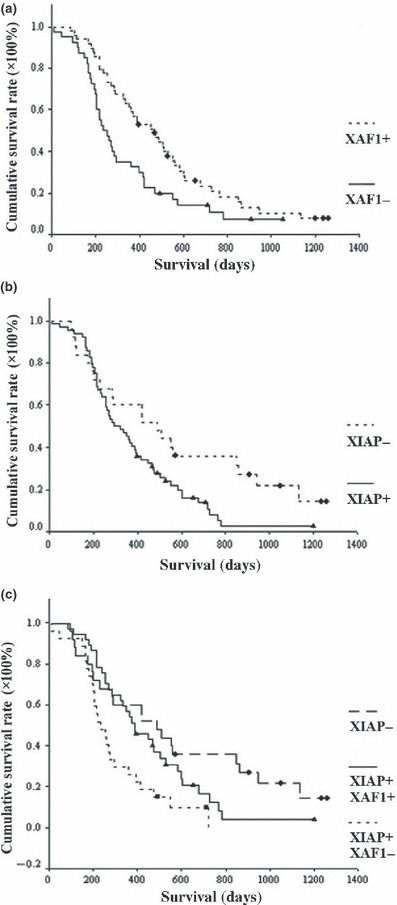
Association of X chromosome‐linked inhibitor of apoptosis protein (XIAP) and XIAP‐associated factor 1 (XAF1) expression with clinical outcomes for patients with pancreatic cancer. (a) Disease‐specific survival for patients with pancreatic cancer. Patients with low XAF1 expression had more unfavorable outcomes than patients with high XAF1 expression (P = 0.016). (b) Patients with high XIAP expression had more unfavorable outcomes than patients with low XIAP expression (P = 0.021). (c) Patients with high XIAP expression and low XAF1 expression had the most unfavorable outcomes (P = 0.003).
Multivariate analysis using the Cox proportional hazard model indicated that, apart from lymph node metastasis (CI = 0.290, P < 0.001), distant metastasis (CI = 0.083, P = 0.001), and tumor grading (CI = 0.009, P = 0.002), low expression of XAF1 (CI = 0.483, P = 0.007) significantly correlated with poor tumor‐specific survival and was an independent prognostic factor for pancreatic cancer (Table 3).
Table 3.
Cox proportional hazards model analysis of prognostic factors in patients with pancreatic cancer (n = 89)
| Variables | Univariate analysis Hazard ratio (95% CI) | P | Multivariate analysis Hazard ratio (95% CI) | P |
|---|---|---|---|---|
| Age (years) | 0.990 (0.962–1.019) | 0.500 | NA | NA |
| Gender | 0.794 (0.426–1.479) | 0.467 | NA | NA |
| Tumor staging | 1.036 (0.226–4.755) | 0.933 | NA | NA |
| Lymph node status | 0.335 (0.173–0.646) | 0.001 | 0.290 (0.160–0.524) | 0.000 |
| Distant metastasis | 0.070 (0.013–0.376) | 0.002 | 0.083 (0.018–0.385) | 0.001 |
| Tumor grading | 0.003 (0.000–0.097) | 0.001 | 0.009 (0.000–0.205) | 0.002 |
| Surgical margins | 0.958 (0.383–2.397) | 0.927 | NA | NA |
| Size (cm) | 0.946 (0.741–1.208) | 0.658 | NA | NA |
| Vascular invasion | 0.999 (0.484–2.063) | 0.998 | NA | NA |
| Perineural invasion | 1.681 (0.923–3.060) | 0.089 | NA | NA |
| XAF1 expression | 0.494 (0.272–0.900) | 0.021 | 0.483 (0.284–0.821) | 0.007 |
| XIAP expression | 1.310 (0.660–2.601) | 0.440 | NA | NA |
NA, not applicable; XAF1, X chromosome‐linked inhibitor of apoptosis protein (XIAP)‐associated factor 1.
XAF1 overexpression inhibits cell growth in pancreatic cancer cell lines. As XAF1 expression was lower in pancreatic cancer cells, which are characterized by a greater incidence of proliferation than normal tissues, we hypothesized that XAF1 might regulate pancreatic cancer cell survival. Therefore, we overexpressed XAF1 in BxPC‐3 and SW1990 cells using the Ad5/F35 virus (Fig. 3a). Compared with the untreated cells, there was a 54.63 ± 5.79% and 29.52 ± 2.52% decrease in cell viability in BxPC‐3 cells and SW1990 cells, respectively (Fig. 3b). To identify the mechanisms of growth inhibition in pancreatic cancer cells, we used flow cytometry and found an increase of G2/M phase population at 48 h after Ad5/F35‐XAF1 infection (Fig. 3c), which partially explained the XAF1‐induced growth inhibition.
Figure 3.
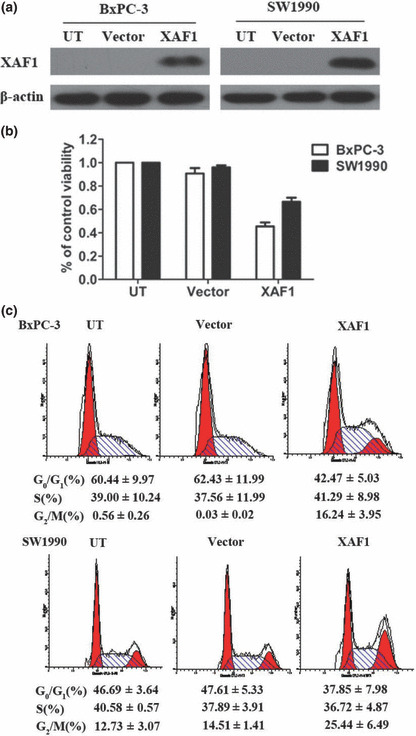
Effect of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) overexpression on cell growth and cell cycle distribution of pancreatic cancer cell lines. (a) Western blot analysis showing expression of XAF1 in BxPC‐3 and SW1990 cells after they had been infected with an adenovirus containing XAF1 construct (XAF1). Untreated (UT) cells and cells infected with adenovirus alone (vector) served as the control. (b) Viability of BxPC‐3 and SW1990 cells evaluated by MTT assay in response to XAF1 overexpression. Columns, mean (n = 3); bars, SE. P < 0.05, significantly different compared with control. (c) Cell cycle distribution of BxPC‐3 and SW1990 cells infected with Ad5/F35‐XAF1 or vector. All assays were done three times, and in triplicate wells.
XAF1 overexpression induces apoptosis in pancreatic cancer cell lines. To examine whether the decrease in cell viability was accompanied by apoptosis, we used flow cytometry with Annexin V–PI staining. As shown in Figure 4, treatment of BxPC‐3 cells with Ad5/F35‐XAF1 enhanced apoptosis (19.90 ± 3.09%) at a greater level than the vector control (6.72 ± 0.76%, P < 0.01) or untreated group (7.22 ± 1.53%, P < 0.01). Similar results were obtained in SW1990 cells. Ad5/F35‐XAF1 treatment yielded 34.44 ± 6.37% of early apoptotic cells compared with the control (10.11 ± 3.05%, P < 0.01) or untreated group (9.41 ± 4.14%, P < 0.01). Induction of apoptosis in Ad5/F35‐XAF1‐infected cultures was also confirmed by TUNEL staining (data not shown).
Figure 4.
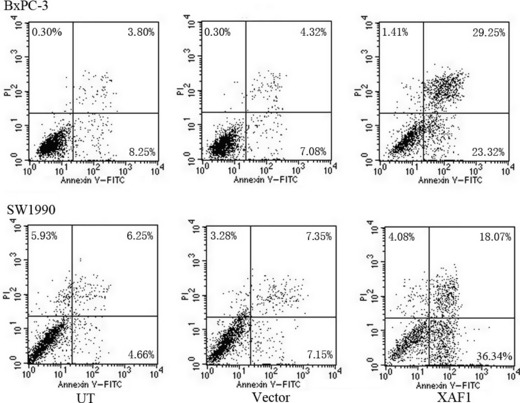
Analysis of apoptosis using flow cytometry in BxPC‐3 and SW1990 pancreatic cancer cells after treatment with an adenovirus containing X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) construct, Ad5/F35‐XAF1. Enhanced apoptosis was indicated by a shift to cells that were FITC+/propidium iodide (PI)−.
Next, we explored the potential molecular mechanisms involved. Overexpression of XAF1 activated caspase 3, 8, and 9, and PARP as evidenced by the increasing protein level of cleaved caspase (Fig. 5a). We then wondered if XAF1‐induced apoptosis was dependent on caspase activation. In SW1990 cells, the apoptotic rate was significantly abrogated in the presence of z‐VAD‐fmk (Fig. 5b), indicating that XAF1‐induced apoptosis occurs in a caspase‐dependent manner.
Figure 5.
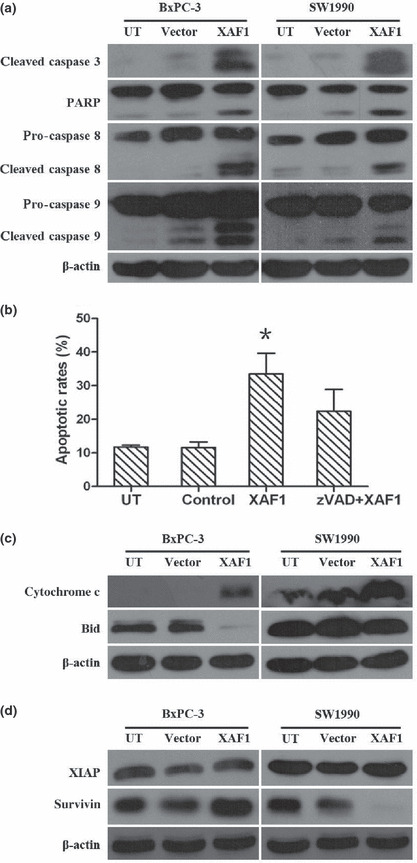
Effect of X chromosome‐linked inhibitor of apoptosis protein (XIAP)‐associated factor 1 (XAF1) overexpression on proteins involved in cell apoptosis, by Western blot analysis. (a) Caspase activation and poly(ADP‐ribose) polymerase (PARP) cleavage in pancreatic cancer cells following Ad5/F35‐XAF1 (XAF1) infection. (b) XAF1‐mediated cell apoptosis dependent on caspase activation. SW1990 cells were incubated with media (UT, untreated), vector (adenovirus alone), Ad5/F35‐XAF1 (XAF1), or a combination of Z‐VAD‐fmk (a cell‐permeable pancaspase inhibitor) and Ad5/F35‐XAF1 (zVAD+XAF1). Flow cytometric analysis was done 48 h later. Columns, mean (n = 3); bars, SE. *P < 0.05, significantly different compared with control. (c) Expression levels of proteins involved in intrinsic pathway in response to XAF1 overexpression in pancreatic cancer cells. (d) Change in expression of IAP family proteins, XIAP and survivin, in pancreatic cancer cells following Ad5/F35‐XAF1 treatment.
Furthermore, we investigated whether XAF1 had an effect on the mitochondrial pathway during apoptosis. As shown in Figure 5(c), an increase in cytochrome c level was observed in both BxPC‐3 and SW1990 cells following Ad5/F35‐XAF1 infection. We also assessed Bid cleavage as a possible link between caspase activation and mitochondrial injury, and overexpression of XAF1 enhanced Bid cleavage (Fig. 5c). Together, these show that XAF1 overexpression triggers caspase activation and mitochondrial perturbations in pancreatic cancer cells.
XAF1 reverses the anti‐caspase activity of XIAP, a physiological inhibitor of apoptosis, so we further investigated the function of XAF1 by examining its relationship with other IAPs. Interestingly, we found that overexpressed XAF1 downregulated the protein expression of survivin rather than XIAP (Fig. 5d).
XAF1 overexpression can reduce tumor growth in xenograft‐bearing nude mice. Nude mice, bearing s.c. xenografts of SW1990 cell line, were treated with direct injection of Ad5/F35‐XAF1 and/or gemcitabine as well as vehicle control. At 10 days after initiating therapy, mice treated with Ad5/F35‐XAF1 or gemcitabine showed a significant reduction of tumor growth, whereas combined treatment did not further reduce tumor growth (Fig. 6a). Furthermore, there was a significant reduction of tumor weight in Ad5/F35‐XAF1 and gemcitabine, and gemcitabine alone, treatment groups (Fig. 6b). Decreased tumour volume matched the decreased PCNA expression in the tumor cell. In addition, we observed an increased apoptosis rate in the Ad5/F35‐XAF1 treated group and gemcitabine treated groups compared to the untreated control group. A representative example is shown in Figure 6(c).
Figure 6.
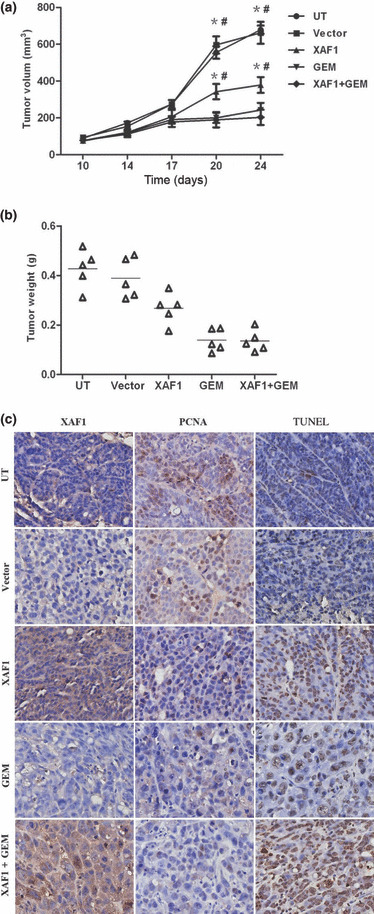
Inhibition of tumor growth in SW1990 pancreatic tumor xenograft study. (a) Antitumor effect of adenovirus containing X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 construct, Ad5/F35‐XAF1 (XAF1), in nude mice implanted s.c. with SW1990 pancreatic cancer cells. *P < 0.05 versus UT (untreated); #P < 0.05 versus Vector (adenovirus alone). GEM, gemcitabine. (b) Scatter plot of tumor weight. Black bars, median weight for the tumors in each group (n = 5); triangle, tumor weight of a xenograft. (c) Immunohistochemical analysis of tumor sections from each treatment group, with antibodies against XAF1, the proliferation marker Proliferating Cell Nuclear Antigen (PCNA) and the apoptotic marker TUNEL.
Discussion
The identification of molecules that can promote tumor progression and compromise patient survival is essential to develop adequate therapeutic modalities against pancreatic cancer. An increased number of molecular markers have been shown to correlate with tumor development and progression in pancreatic cancer.( 26 , 27 , 28 ) The present study indicated an overall lower expression of XAF1 and higher expression of XIAP in pancreatic cancer. XAF1 was originally identified based on its ability to bind to XIAP and was shown to be underexpressed in several cancer cell lines and primary carcinomas,( 17 , 18 , 20 ) which correlated with relatively high levels of XIAP expression in these cells.( 17 , 20 ) However, we did not find any correlation between XAF1 and XIAP expression, suggesting that XAF1 might not directly modulate XIAP expression and function in other mechanisms independently of XIAP. Further studies are necessary to clarify this point. In addition, we found that XAF1 or XIAP expression was an independent prognostic factor in pancreatic cancer, and patients with high XIAP expression and low XAF1 expression had the most unfavorable outcomes. This observation is consistent with a previous study, which reported that XIAP/XAF1 status acted as an independent prognostic factor in gastric cancer.( 29 ) Moreover, XAF1 generated a larger hazard ratio than the other prognostic indicators, including tumor grading, lymph node involvement, and distant metastasis. These findings suggest XAF1 might widely serve as a prognostic indicator in patients with pancreatic cancer.
Additionally, we found restoration of XAF1 expression in pancreatic cancer cells with absent or low levels of endogenous XAF1 could reverse malignancy. Both BxPC‐3 and SW1990 cell lines showed inhibited cell growth, arrested cell cycle, and increased apoptosis after overexpression of XAF1. Furthermore, restoration of XAF1 reduced SW1990 tumor xenografts in athymic nude mice without any remarkable toxicity. Collectively, these findings indicated a powerful antineoplastic benefit of XAF1 overexpression in pancreatic cancer both in vitro and in vivo.
In exploration of the potential molecular mechanisms underlying the pro‐apoptotic effect of XAF1, we found activation of both the extrinsic and intrinsic pathways. The XAF1‐mediated cell death could be prevented by caspase inhibitor Z‐VAD‐fmk, suggesting a predominant caspase‐dependent mechanism was involved. However, pancreatic cancer cells belong to so‐called type II cells regarding death receptor mediated apoptosis.( 30 , 31 , 32 ) In this type of cell, a mitochondrial amplification loop is required for efficient induction of apoptosis. Consistently, we found XAF1 could significantly induce cytochrome c expression. It has also been reported recently that an increased production of cytochrome c in XAF1‐inducible cells was inhibited by Bcl‐2 overexpression.( 16 ) The role of XAF1 in the mitochondrial pathway independent of its caspase‐activating function is additionally supported by our findings that the Bid cleavage was observed in XAF1‐expressing BxPC‐3 cells. Bid, as one of the major players in the crosstalk between extrinsic and intrinsic apoptotic signaling, has to be cleaved by caspase 8 to activate mitochondria.( 33 , 34 ) Similar changes were not observed in SW1990 cells, suggesting that differences are likely to exist between different neoplastic cell types in the molecular mechanisms of XAF1. Therefore, we concluded that both the extrinsic and intrinsic pathways participate in the XAF1‐mediated apoptosis.
The ability of XAF1 to sensitize pancreatic cancer cells to apoptosis might further be related to their downregulation of the IAP family (c‐IAP1, c‐IAP2, survivin, and XIAP). Solid data have suggested IAP proteins contribute to chemoradiation resistance in solid tumors and downregulation of IAP proteins increases the sensitivity of neoplastic cells to a broad category of therapeutic agents.( 35 , 36 ) Interestingly, we did not detect a reduction of XIAP expression in each cell line (BxPC‐3 and SW1990) following Ad5/F35‐XAF1 infection. This observation is in contradiction with a previous study, which showed that, in first trimester trophoblast cells, XAF1 restoration elicited XIAP degradation and decreased the active form of XIAP.( 25 ) However, we did observe a remarkable decrease of survivin level in the SW1990 cell line, suggesting that XAF1 is likely to mediate cell death in pancreatic cancer cells by acting through different pathways. In accordance with our findings, a recent study found high levels of XAF1 could suppress the expression of survivin.( 37 ) As a member of the IAP family, survivin shows unique features compared to other apoptosis regulators.( 38 ) It is capable of suppressing apoptosis and inducing cytotoxic resistance.( 39 , 40 ) Therefore, decreased survivin level and concomitantly increased caspase 3 activity could be the potential mechanism for XAF1‐mediated apoptosis in pancreatic cancer. Moreover, survivin is also involved in cell cycle regulation in many cell types,( 41 , 42 ) which may contribute to the observed G2/M arrest in XAF1 expressing cells. Therefore, downregulation of survivin by XAF1 could have a dual favorable advantage, inducing apoptotic cell death, as well as cell cycle arrest, in pancreatic cancer.
Furthermore, we observed an anticancer function of Ad5/F35‐XAF1 in a murine xenotransplantation model, including decreased cell proliferation as well as an increased apoptotic rate. We then compared the effect of Ad5/F35‐XAF1 with that of gemcitabine, an anticancer agent emerging recently as the first‐line treatment of locally advanced and metastatic pancreatic cancers.( 43 , 44 ) However, the reduction of tumor growth in the Ad5/F35‐XAF1 treated group was slightly less significant than in the gemcitabine treated group. In addition, the combination of Ad5/F35‐XAF1 and gemcitabine did not show either additive or synergistic effects. As most chemotherapy like gemcitabine affects only fast growing cells, it is very likely that Ad5/F35‐XAF1‐mediated cell cycle arrest is responsible for a failure of synergistic effects of XAF1 and gemcitabine. A recent study showed that restoration of XAF1 enhanced TRAIL‐ and anticancer drug‐induced apoptosis in gastric cancer.( 45 ) However, the effect on pancreatic tumor growth by XAF1, in combination with other drugs, needs to be further explored.
In summary, reduction of XAF1 expression has an important role in tumor growth and/or malignant transformation of pancreatic cancer. Low expression of XAF1 might be a useful marker for adjuvant therapy in patients with high risk of poor outcome. Our data strongly suggest the potential clinical benefits of restoration of the XAF1 signal pathway in pancreatic cancer therapy.
Disclosure Statement
Qi Zhu received a research grant from the National Nature Science Foundation of China (no. 30670941). Shuiping Tu received a research grant from the National Nature Science Foundation of China (no. 30572142). Yaozong Yuan received a research grant from the National Nature Science Foundation of China (no. 30770965).
Supporting information
Fig. 1. Immunohistochemical staining of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) expression in standard tissue sections with pancreatic cancer and normal pancreas. Magnification, ×100.
Fig. 2. Expression of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) in pancreatic cancer. Magnification, ×400.
Fig. 3. Expression of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) in normal pancreas. Magnification, ×400.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
This work was supported by the National Nature Science Foundation of China (no. 30770965).
References
- 1. Lohr M. Is it possible to survive pancreatic cancer? Nat Clin Pract Gastroenterol Hepatol 2006; 3: 236–7. [DOI] [PubMed] [Google Scholar]
- 2. Guo X, Cui Z. Current diagnosis and treatment of pancreatic cancer in China. Pancreas 2005; 31: 13–22. [DOI] [PubMed] [Google Scholar]
- 3. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363: 1049–57. [DOI] [PubMed] [Google Scholar]
- 4. Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: basic and clinical aspects. Gastroenterology 2005; 128: 1606–25. [DOI] [PubMed] [Google Scholar]
- 5. Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology 2004; 4: 567–86. [DOI] [PubMed] [Google Scholar]
- 6. Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006; 25: 4798–811. [DOI] [PubMed] [Google Scholar]
- 7. Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol 2002; 3: 401–10. [DOI] [PubMed] [Google Scholar]
- 8. Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X‐linked IAP is a direct inhibitor of cell‐death proteases. Nature 1997; 388: 300–04. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R. X‐linked inhibitor of apoptosis protein (XIAP) inhibits caspase‐3 and‐7 in distinct modes. J Biol Chem 2001; 276: 27058–63. [DOI] [PubMed] [Google Scholar]
- 10. Karikari CA, Roy I, Tryggestad E et al. Targeting the apoptotic machinery in pancreatic cancers using small‐molecule antagonists of the X‐linked inhibitor of apoptosis protein. Mol Cancer Ther 2007; 6: 957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c‐dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell 2001; 8: 613–21. [DOI] [PubMed] [Google Scholar]
- 13. Liston P, Fong WG, Kelly NL et al. Identification of XAF1 as an antagonist of XIAP anti‐Caspase activity. Nat Cell Biol 2001; 3: 128–33. [DOI] [PubMed] [Google Scholar]
- 14. Leaman DW, Chawla‐Sarkar M, Vyas K et al. Identification of X‐linked inhibitor of apoptosis‐associated factor‐1 as an interferon‐stimulated gene that augments TRAIL Apo2L‐induced apoptosis. J Biol Chem 2002; 277: 28504–11. [DOI] [PubMed] [Google Scholar]
- 15. Chung SK, Lee MG, Ryu BK et al. Frequent alteration of XAF1 in human colorectal cancers: implication for tumor cell resistance to apoptotic stresses. Gastroenterology 2007; 132: 2459–77. [DOI] [PubMed] [Google Scholar]
- 16. Xia Y, Novak R, Lewis J, Duckett CS, Phillips AC. Xaf1 can cooperate with TNFalpha in the induction of apoptosis, independently of interaction with XIAP. Mol Cell Biochem 2006; 286: 67–76. [DOI] [PubMed] [Google Scholar]
- 17. Fong WG, Liston P, Rajcan‐Separovic E, St Jean M, Craig C, Korneluk RG. Expression and genetic analysis of XIAP‐associated factor 1 (XAF1) in cancer cell lines. Genomics 2000; 70: 113–22. [DOI] [PubMed] [Google Scholar]
- 18. Ng KC, Campos EI, Martinka M, Li G. XAF1 expression is significantly reduced in human melanoma. J Invest Dermatol 2004; 123: 1127–34. [DOI] [PubMed] [Google Scholar]
- 19. Lee MG, Huh JS, Chung SK et al. Promoter CpG hypermethylation and downregulation of XAF1 expression in human urogenital malignancies: implication for attenuated p53 response to apoptotic stresses. Oncogene 2006; 25: 5807–22. [DOI] [PubMed] [Google Scholar]
- 20. Byun DS, Cho K, Ryu BK et al. Hypermethylation of XIAP‐associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinomas. Cancer Res 2003; 63: 7068–75. [PubMed] [Google Scholar]
- 21. Zou B, Chim CS, Zeng H et al. Correlation between the single‐site CpG methylation and expression silencing of the XAF1 gene in human gastric and colon cancers. Gastroenterology 2006; 131: 1835–43. [DOI] [PubMed] [Google Scholar]
- 22. Kempkensteffen C, Hinz S, Schrader M et al. Gene expression and promoter methylation of the XIAP‐associated Factor 1 in renal cell carcinomas: correlations with pathology and outcome. Cancer Lett 2007; 254: 227–35. [DOI] [PubMed] [Google Scholar]
- 23. Fang X, Liu Z, Fan Y et al. Switch to full‐length of XAF1 mRNA expression in prostate cancer cells by the DNA methylation inhibitor. Int J Cancer 2006; 118: 2485–9. [DOI] [PubMed] [Google Scholar]
- 24. Reu FJ, Bae SI, Cherkassky L et al. Overcoming resistance to interferon‐induced apoptosis of renal carcinoma and melanoma cells by DNA demethylation. J Clin Oncol 2006; 24: 3771–9. [DOI] [PubMed] [Google Scholar]
- 25. Straszewski‐Chavez SL, Visintin IP, Karassina N et al. XAF1 mediates tumor necrosis factor‐alpha‐induced apoptosis and X‐linked inhibitor of apoptosis cleavage by acting through the mitochondrial pathway. J Biol Chem 2007; 282: 13059–72. [DOI] [PubMed] [Google Scholar]
- 26. Mimeault M, Brand RE, Sasson AA, Batra SK. Recent advances on the molecular mechanisms involved in pancreatic cancer progression and therapies. Pancreas 2005; 31: 301–16. [DOI] [PubMed] [Google Scholar]
- 27. Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006; 20: 1218–49. [DOI] [PubMed] [Google Scholar]
- 28. Jimeno A, Hidalgo M. Molecular biomarkers: their increasing role in the diagnosis, characterization, and therapy guidance in pancreatic cancer. Mol Cancer Ther 2006; 5: 787–96. [DOI] [PubMed] [Google Scholar]
- 29. Shibata T, Noguchi T, Takeno S, Gabbert HE, Ramp U, Kawahara K. Disturbed XIAP and XAF1 expression balance is an independent prognostic factor in gastric adenocarcinomas. Ann Surg Oncol 2008; 15: 3494–502. [DOI] [PubMed] [Google Scholar]
- 30. Igney FH, Krammer PH. Death and anti‐death: tumour resistance to apoptosis. Nat Rev Cancer 2002; 2: 277–88. [DOI] [PubMed] [Google Scholar]
- 31. Trauzold A, Wermann H, Arlt A et al. CD95 and TRAIL receptor‐mediated activation of protein kinase C and NF‐kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene 2001; 20: 4258–69. [DOI] [PubMed] [Google Scholar]
- 32. Hinz S, Trauzold A, Boenicke L et al. Bcl‐XL protects pancreatic adenocarcinoma cells against CD95‐ and TRAIL‐receptor‐mediated apoptosis. Oncogene 2000; 19: 5477–86. [DOI] [PubMed] [Google Scholar]
- 33. Scaffidi C, Fulda S, Srinivasan A et al. Two CD95 (APO‐1/Fas) signaling pathways. EMBO J 1998; 17: 1675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gross A, Yin XM, Wang K et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL‐XL prevents this release but not tumor necrosis factor‐R1/Fas death. J Biol Chem 1999; 274: 1156–63. [DOI] [PubMed] [Google Scholar]
- 35. Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 2005; 5: 876–85. [DOI] [PubMed] [Google Scholar]
- 36. Reed JC. Apoptosis‐targeted therapies for cancer. Cancer Cell 2003; 3: 17–22. [DOI] [PubMed] [Google Scholar]
- 37. Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the X‐linked inhibitor of apoptosis (XIAP)‐XAF1 complex. J Biol Chem 2007; 282: 26202–9. [DOI] [PubMed] [Google Scholar]
- 38. Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 2003; 22: 8581–9. [DOI] [PubMed] [Google Scholar]
- 39. Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003; 3: 46–54. [DOI] [PubMed] [Google Scholar]
- 40. Shin S, Sung BJ, Cho YS et al. An anti‐apoptotic protein human survivin is a direct inhibitor of caspase‐3 and ‐7. Biochemistry 2001; 40: 1117–23. [DOI] [PubMed] [Google Scholar]
- 41. Deveraux QL, Reed JC. IAP family proteins – suppressors of apoptosis. Genes Dev 1999; 13: 239–52. [DOI] [PubMed] [Google Scholar]
- 42. Li F, Ambrosini G, Chu EY et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998; 396: 580–4. [DOI] [PubMed] [Google Scholar]
- 43. Burris HA, Moore MJ, Andersen J et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreatic cancer: a randomized‐trial. J Clin Oncol 1997; 15: 2403–13. [DOI] [PubMed] [Google Scholar]
- 44. Rothenberg ML, Moore MJ, Cripps MC et al. A phase II trial of gemcitabine in patients with 5‐FU‐refractory pancreas cancer. Ann Oncol 1996; 7: 347–53. [DOI] [PubMed] [Google Scholar]
- 45. Tu SP, Liston P, Cui JT et al. Restoration of XAF1 expression induces apoptosis and inhibits tumor growth in gastric cancer. Int J Cancer 2009; 125: 688–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1. Immunohistochemical staining of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) expression in standard tissue sections with pancreatic cancer and normal pancreas. Magnification, ×100.
Fig. 2. Expression of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) in pancreatic cancer. Magnification, ×400.
Fig. 3. Expression of X chromosome‐linked inhibitor of apoptosis protein‐associated factor 1 (XAF1) in normal pancreas. Magnification, ×400.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


