Abstract
The insulin‐like growth factor receptor type 1 (IGF1R) is suggested to play important roles in cancer cell growth through cross‐talk with hormone receptors and growth factor receptors. However, its clinical significance in breast cancers in vivo is still unclear. We examined immunohistochemically the expression of IGF1R, phosphorylated‐AKT (pAKT) and phosphorylated‐ERK1/2 (pERK1/2) using tissue microarray slides containing 150 cases of primary breast carcinoma. Their mutual correlation and correlation with the status of hormone receptors epidermal growth factor receptor and human epidermal growth factor receptor type 2 were also investigated. IGF1R overexpression was detected in 71 cases (47%), and was correlated with lower nuclear grade (P = 0.03), positive estrogen receptor (ER) and/or progesterone receptor status (P = 0.002). pERK1/2 expression, detected in 53 cases (35%), was correlated with positive ER (P < 0.0001) and lower nuclear grade (P = 0.014). pAKT expression, detected in 88 cases (59%), was not correlated with nuclear grade, hormone receptors status or other clinical parameters. Of the 71 IGF1R‐overexpressing tumors, pERK1/2 expression was detected in 27 (56%) of 48 ER‐positive cases but in only four (17%) of 23 ER‐negative cases (P = 0.022). In contrast, pAKT expression was constantly (64% or higher) detected irrespective of hormone receptor status in IGF1R‐overexpressing breast cancers. Taken together, these findings suggest that IGF1R overexpression might activate pERK1/2 and pAKT in hormone receptor‐positive breast cancer, but activate only pAKT in hormone receptor‐negative breast cancer. (Cancer Sci 2006; 97: 597–604)
Recent evidence suggests that potent cross‐talk between growth factor receptor‐induced signaling pathways and estrogen receptor (ER) signaling pathways is involved in the acquisition of resistance to tamoxifen therapy by breast cancer cells.( 1 ) Estrogen‐bound ER activates estrogen‐regulated gene transcription through genomic action, but after long‐term tamoxifen treatment, resistance can develop with the activation of tyrosine kinase receptors, such as insulin‐like growth factor receptor type 1 (IGF1R), human epidermal growth factor receptor (HER) type 1 (HER1 or EGFR), and/or HER type 2 (HER2 or c‐erbB2) by ER phosphorylation through non‐genomic action.( 1 , 2 )
The EGFR and HER2 oncoproteins are overexpressed in 15–20% and 27–30% of primary breast cancers, respectively. Their overexpression has been shown to be correlated with high‐grade and hormone receptor‐negative tumors, and with poorer patient prognosis.( 3 , 4 , 5 , 6 ) Whereas IGF1R overexpression is reported to be detectable in 43–50% of primary breast cancers,( 7 , 8 ) its clinical and prognostic significance remains undetermined in spite of clear evidence of the biological importance of IGF1R overexpression in breast cancer cells in vitro.( 9 , 10 )
EGFR/HER2 and IGF1R show bidirectional signal transduction through the MAPK/ERK1/2 pathway and the PI3K/AKT pathway. The MAPK/ERK1/2 pathway plays a critical role in the regulation of cell growth, differentiation and progression. Phosphorylation of ERK1/2 is a common result of growth factor receptor activation or exposure to an oncogenic agent.( 11 , 12 ) The PI3K/AKT pathway also plays a critical role in controlling the balance between apoptosis and cell survival. This balance in response to extracellular and intracellular signaling is vital for maintaining tissue homeostasis. Aberrant control of cell survival signaling can result in tumor progression and resistance to chemopreventive agents used in cancer treatment.( 13 , 14 ) Recent reports have revealed that activation of the PI3K/AKT pathway under the influence of IGF1R plays an important role in maintaining the proliferation of breast cancer cells that are resistant to gefitinib, trastuzumab or chemoradiotherapy in vitro and in vivo.( 15 , 16 , 17 , 18 ) Furthermore, the latest translational research has revealed that immunohistochemical overexpression of IGF1R is associated with response to lapatinib, a small molecule tyrosine kinase inhibitor of EGFR and HER2, in a clinical trial of patients with advanced or metastatic breast cancer overexpressing HER2. Therefore, IGF1R is expected to be a therapeutic predictive biomarker for the effectiveness of lapatinib. 19
In the present study, we examined the clinicopathological implications of IGF1R expression, aiming at downstream signaling kinases, phosphorylated AKT (pAKT) and phosphorylated ERK1/2 (pERK1/2) in 150 cases of breast carcinoma by using tissue microarray (TMA) and immunohistochemistry.
Patients and Methods
Patients
The present study was carried out after approval by the internal review board and after gaining the patients’ consent to use their cancer specimens for research.
One hundred and fifty patients who underwent resection of breast cancer at the National Defense Medical College Hospital (Tokorozama, Japan) between 1995 and 1997 were enrolled in the present study. The median patient age of the study population was 53.8 years (range 30–81 years). The median tumor diameter was 3.4 cm (range 0.7–13.0 cm). The histological type of the primary tumor was invasive ductal carcinoma in 130 cases (87%), ductal carcinoma in situ in 15 cases (10%), and invasive lobular carcinoma in five cases (3%). According to the nuclear grading system of the Japan Breast Cancer Society, 17 cases (11%), 84 cases (56%) and 49 cases (33%) were classified as grade 1, grade 2 and grade 3, respectively. The numbers of cases with and without axillary lymph node metastasis were 52 (35%) and 77 (51%), respectively, and lymph node status was not recorded in 21 cases (14%). ER was positive in 77 cases (51%) and negative in 73 cases (49%), whereas progesterone receptor (PgR) was positive in 87 cases (58%) and negative in 63 cases (42%). The median time of follow up was 64.9 months, ranging from 12 to 122 months. During the whole time of follow up, 39 (26%) of 150 patients relapsed distantly, whereas the other 111 (74%) patients were alive without recurrence (Table 1).
Table 1.
Clinicopathological features in 150 breast cancers
| Clinical parameter | No. cases |
|---|---|
| Total | 150 (100%) |
| Age (years) | |
| Range | 30–81 (53.8 median) |
| <50 | 57 (38%) |
| ≥50 | 93 (62%) |
| Size of tumor (cm) | |
| Range | 0.7–13.0 (3.4 median) |
| <2.0 | 42 (28%) |
| ≥2.0 | 108 (72%) |
| Histological type | |
| Invasive ductal carcinoma | 130 (87%) |
| Ductal carcinoma in situ | 15 (10%) |
| Invasive lobular carcinoma | 5 (3%) |
| Nuclear grade | |
| Grade 1 | 17 (11%) |
| Grade 2 | 84 (56%) |
| Grade 3 | 49 (33%) |
| Lymph node status | |
| Positive | 52 (35%) |
| Negative | 77 (51%) |
| Unknown | 21 (14%) |
| Estrogen receptor status | |
| Positive | 77 (51%) |
| Negative | 73 (49%) |
| Progesterone receptor status | |
| Positive | 87 (58%) |
| Negative | 63 (42%) |
| Follow‐up duration (months) | 11–122 (64.9 median) |
| Event | |
| No relapse | 111 (74%) |
| Distant relapse | 39 (26%) |
Tissue microarray construction
We reviewed all of the hematoxylin and eosin‐stained sections of archived pathological specimens of primary breast cancer. The histological diagnosis, including histological type and nuclear grade, was confirmed in all cases. For the 150 available cases of breast cancer, the most representative area of each tumor was punched out to construct TMA comprising single tissue cores (diameter 2.0 mm) from the 150 original blocks. One TMA block contained a maximum of 66 tissue cores, and three TMA sets were prepared for the present study. Sections 4 m in thickness were cut from the blocks.
Immunohistochemistry
In brief, the 4 m‐thick sections were deparaffinized in xylene, and dehydrated in a graded ethanol series. Antigen retrieval was carried out by incubation of the tissue sections in a microwave oven in 10 mM sodium citrate (pH 6.0) with 0.1% Tween40 at 120°C for 45 min.
In the present study, we used a rabbit polyclonal anti‐IGF1R antibody (ready to use; NeoMarkers, Fremont, CA, USA), a rabbit polyclonal anti‐pERK1/2 antibody (sc‐7383, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a rabbit polyclonal anti‐pAKT antibody (Ser473, 1:100; Cell Signaling Technology, Danvers, MA, USA). After antigen retrieval, the tissue sections were incubated in 0.3% hydrogen peroxide in methanol for 30 min, reacted with the primary antibody for 1–3 h, incubated with dextran polymer reagent conjugated with peroxidase and secondary antibody (envision; Dakocytomation, Glostrup, Denmark) for 1 h, and subsequently reacted with 3,3‐diaminobenzidine tetrahydrochloride‐hydrogen peroxide as the chromogen.
IGF1R staining of the cells was assessed according to both the intensity and the proportion of membrane staining (Fig. 1A–D) and was scored as follows: 0, membrane staining in less than 10% of constituent cells or no membrane staining; 1+, incomplete membrane staining that did not include the entire circumference of the membrane in 10% or more of the carcinoma cells; 2+, weak or moderate complete membrane staining along the entire circumference of the cell membrane in 10% or more of the carcinoma cells; and 3+, strong complete membrane staining along the entire circumference of the cell membrane in 10% or more of the carcinoma cells. Cases that were scored 2+ and 3+ were considered to have IGF1R overexpression.
Figure 1.
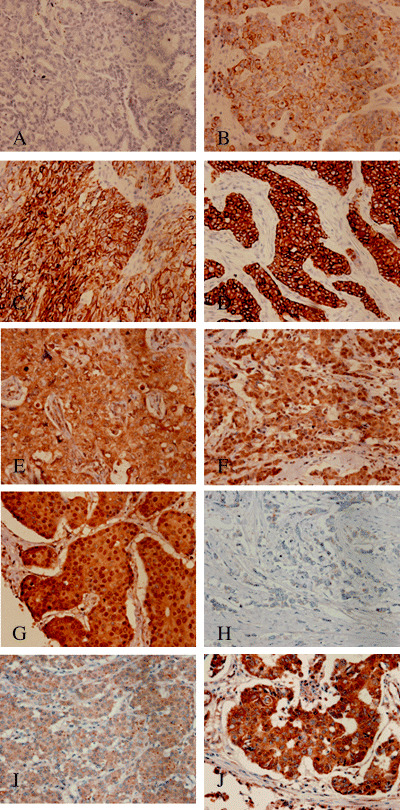
Immunohistochemical staining of insulin‐like growth factor receptor type 1 (IGF1R) protein in primary breast cancer scored according to area and intensity of membrane and cytoplasmic staining. (A) 0, (B) 1+, (C) 2+, (D) 3+ (×200). pERK1/2 staining was evaluated according to the intensity and proportion of nuclear staining. (E) 0, (F) 1+, (G) 2+ (×200). pAKT staining was evaluated according to the intensity and proportion of cytoplasmic staining. (H) 0, (I) 1+, (J) 2+ (×200).
The pERK1/2 staining of the cancer cells was evaluated according to the intensity and proportion of nuclear staining and scored on a three‐point scale as follows (Fig. 1E–G): 0, nuclear staining in less than 10% of constituent cells or no nuclear staining; 1+, weak nuclear staining in 10% or more of the constituent cells; and 2+, strong nuclear staining in 10% or more of the constituent cells. Cases with a score of 2+ were considered to be pERK1/2‐positive.
The pAKT staining of the cancer cells was evaluated according to the intensity and the proportion of cytoplasmic staining and scored on a three‐point scale as follows (Fig. 1H–J): 0, cytoplasmic staining in less than 10% of the constituent cells or no staining; 1+, weak cytoplasmic staining in 10% or more of the constituent cells; and 2+, strong cytoplasmic staining in 10% or more of the constituent cells. Cases with a score 2+ were considered to be pAKT‐positive.
Expression of EGFR, HER2, ER and PgR
The expression of EGFR, HER2, ER and PgR had already been examined immunohistochemically in the 150 tumors.( 20 ) Antibodies or kits used for immunohistochemistry were a PharmDx EGFR Kit for EGFR, a Herceptest for HER2, a monoclonal anti‐ER antibody (clone ID5) and a monoclonal anti‐PgR antibody (clone PgR636) purchased from Dakocytomation. The method used for immunohistochemistry has been described previously.( 20 )
The expression of EGFR and HER2 was scored as 2+ and 3+ if the entire circumference of the cell membrane was weakly or moderately stained, or strongly stained, respectively, in 10% or more of the constituent carcinoma cells. A score of 1+ was given if incomplete membrane staining was observed in 10% or more of the carcinoma cells, and a score of 0 was given if there was membrane staining in less than 10% of the constituent cells or if there was no membrane staining. Cases with a score of 2+ or 3+ were judged as showing overexpression. Hormone receptor status was classified into three groups: 0, when no cancer cell nuclei were stained; 1+, when less than 10% of cancer cell nuclei were stained; and 2+, when 10% or more of cancer cell nuclei were stained. In the present study, a score of 2+ was regarded as ER‐positive or PgR‐positive, and score of 0 or 1+ was regarded as ER‐negative or PgR‐negative.
Statistical analysis
We used the χ2‐test or Fisher's exact test to reveal the correlation of the expression of each protein with histological grade and other clinical parameters. A Cox proportional hazards model was used to test the significance of hormone receptors, IGF1R and signaling kinases as predictors of disease‐free survival times. All statistical analyses were carried out using Statview 5.0 software (SAS Institute, Cary, NC, USA).
Results
Relationship of IGF1R, EGFR and HER2 with clinicopathogical parameters and hormone receptor status in breast cancer
Table 2 shows the relationship between overexpression of IGF1R, EGFR and HER2, and clinicopathological parameters and hormone receptor status in breast cancer. We have already shown the inverse correlation of EGFR and HER2 with hormone receptor status and the correlation of EGFR with higher tumor grade in our previous report.( 20 ) In this study, we re‐evaluated ER and PgR status according to the criteria described above and classified them into four groups by combining ER and PgR.
Table 2.
Correlation between insulin‐like growth factor receptor type 1 (IGF1R), epidermal growth factor receptor and human epidermal growth factor receptor type 2 overexpression with clinicopathological parameters and hormone receptor status in breast cancer
| Parameter | Total | Cases | |||||
|---|---|---|---|---|---|---|---|
| IGF1R | EGFR | HER2 | |||||
| n | % | n | % | n | % | ||
| Total | 150 | 71 | 47 | 12 | 8 | 23 | 15 |
| Hormone receptor status | |||||||
| ER+ | 77 | 48 | 62* | 0 | 0 † | 2 | 3 ‡ |
| ER− | 73 | 23 | 32 | 12 | 16 | 21 | 29 |
| PgR+ | 87 | 54 | 62 § | 1 | 1 ¶ | 6 | 7** |
| PgR− | 63 | 17 | 27 | 11 | 17 | 17 | 27 |
| Hormone receptor status combined | |||||||
| ER+/PgR+ | 63 | 42 | 67 †† | 0 | 0 ‡‡ | 2 | 3 §§ |
| ER+/PgR− | 14 | 6 | 43 | 0 | 0 | 0 | 0 |
| ER−/PgR+ | 24 | 12 | 50 | 1 | 4 | 4 | 17 |
| ER−/PgR− | 49 | 11 | 22 | 11 | 22 | 17 | 35 |
| Histological grade | |||||||
| Invasive ductal carcinoma | 130 | 58 | 45 | 11 | 8 | 18 | 14 |
| Ductal carcinoma in situ | 15 | 8 | 53 | 1 | 7 | 5 | 33 |
| Invasive lobular carcinoma | 5 | 3 | 60 | 0 | 0 | 0 | 0 |
| Nuclear grade | |||||||
| Grade 1 | 17 | 12 | 71 ¶¶ | 0 | 0*** | 2 | 12 |
| Grade 2 | 84 | 42 | 50 | 2 | 2 | 10 | 12 |
| Grade 3 | 49 | 17 | 35 | 10 | 20 | 11 | 22 |
| Age (years) | |||||||
| <50 | 57 | 21 | 37 | 6 | 11 | 9 | 16 |
| ≥50 | 93 | 50 | 54 | 6 | 6 | 14 | 15 |
| Tumor size (cm) | |||||||
| <2.0 | 42 | 15 | 36 | 3 | 7 | 8 | 19 |
| ≥2.0 | 108 | 56 | 52 | 9 | 8 | 15 | 14 |
| Lymph node status | |||||||
| Positive | 52 | 23 | 44 | 6 | 12 | 7 | 13 |
| Negative | 77 | 33 | 43 | 4 | 5 | 14 | 18 |
| Unknown | 21 | 15 | 71 | 2 | 10 | 2 | 10 |
P = 0.0005,
P < 0.0001 and
P < 0.0001 between estrogen receptor (ER)‐positive and ER‐negative cases;
P < 0.0001,
P < 0.0001 and
P = 0.0007 between progesterone receptor (PgR)‐positive and PgR‐negative cases;
P = 0.002,
P < 0.0001 and
P = 0.003 between ER‐ and/or PgR‐positive cases and double‐negative ER and PgR cases;
P = 0.03,
P = 0.026 between the grade 1, 2 and 3 cases.
IGF1R overexpression was detected in 71 (47%) of the 150 cases. In non‐cancerous mammary gland tissue, IGF1R was also expressed with a score of 1+ or 2+ localized in both luminal and myoepithelial cells. IGF1R was overexpressed in 48 (62%) of 77 ER‐positive cases, 23 (32%) of 73 ER‐negative cases, 54 (62%) of 87 PgR‐positive cases, and 17 (27%) of 63 PgR‐negative cases. When the two hormone receptors were combined, IGF1R was overexpressed in 42 (67%) of 63 ER‐positive/PgR‐positive cases, six (43%) of 14 ER‐positive/PgR‐negative cases, 12 (50%) of 24 ER‐negative/PgR‐positive cases, and 11 (22%) of 49 ER‐negative/PgR‐negative cases. ER‐positive/PgR‐positive cells and IGF1R‐positive cells were almost identical in ER‐positive/PgR‐positive and IGF1R‐positive tumors. There was a significant correlation of IGF1R overexpression with ER or PgR positivity, compared with ER or PgR negativity (P = 0.0005, P < 0.0001, respectively). The frequency of IGF1R overexpression with positivity for ER and PgR was significantly higher than that of IGF1R overexpression with double negativity for ER and/or PgR (P = 0.002).
IGF1R was overexpressed in 12 (71%) of 17 cases with grade 1, 42 (50%) of 84 cases with grade 2, and 17 (35%) of 49 cases with grade 3. IGF1R overexpression was correlated with lower nuclear grade (P = 0.03), but was not significantly correlated with age, tumor size, histological type or axillary lymph node status.
EGFR was overexpressed in 0 (0%) of 63 ER‐positive/PgR‐positive cases, 0 (0%) of 14 ER‐positive/PgR‐negative cases, one (4%) of 24 ER‐negative/PgR‐positive cases, and 11 (22%) of 49 ER‐negative/PgR‐negative cases. Frequency of EGFR overexpression in cases showing double negativity for ER and PgR was significantly higher than in cases that were positive for ER and/or PgR (P < 0.0001).
HER2 was overexpressed in 2 (3%) of 63 ER+/PgR + cases, 0 (0%) of 14 ER+/PgR – cases, 4 (17%) of 24 ER –/PgR + cases, and 17 (35%) of 49 ER –/PgR – cases. The frequency of HER2 overexpression in cases that were double negative for ER and PgR was also significantly higher than in cases that were positive for ER and/or PgR (P = 0.003).
In the group with ER‐positive and/or PgR‐positive breast cancers, the incidence of IGF1R overexpression was strikingly higher than that of EGFR or HER2 overexpression. In contrast, in the group of double negativity of ER and PgR, the incidence of IGF1R overexpression was almost equal to that of EGFR and was rather lower than that of HER2 overexpression (Table 2).
Relationship of pERK1/2 and pAKT with clinical parameters and hormone receptor status in breast cancer
As shown in Table 3, pERK1/2 was detected as 2+ in 53 (35%) of the 150 cases, including 41 (53%) of 77 ER‐positive cases, 12 (16%) of 73 ER‐negative cases, 40 (46%) of 87 PgR‐positive cases, and 13 (21%) of 63 PgR‐negative cases. When ER and PgR were combined, pERK1/2 was expressed in 35 (56%) of 63 ER‐positive/PgR‐positive cases, six (43%) of 14 ER‐positive/PgR‐negative cases, five (21%) of 24 ER‐negative/PgR‐positive cases, and seven (14%) of 49 ER‐negative/PgR‐negative cases. There was a significant correlation of pERK1/2 with ER or PgR positivity, compared with ER or PgR negativity (P < 0.0001, P = 0.002, respectively). pERK1/2 expression was significantly higher in cases that were positive for ER and/or PgR than in cases that were double negative for both ER and PgR (P = 0.0001).
Table 3.
Correlation of pERK1/2 and pAKT with clinicopathological parameters and hormone receptor status in breast cancer
| Parameter | Total | Cases | |||
|---|---|---|---|---|---|
| pERK1/2 | pAKT | ||||
| n | % | n | % | ||
| Total | 150 | 53 | 35 | 88 | 59 |
| Hormone receptor status | |||||
| ER+ | 77 | 41 | 53* | 45 | 58 |
| ER− | 73 | 12 | 16 | 43 | 59 |
| PgR+ | 87 | 40 | 46 † | 53 | 61 |
| PgR− | 63 | 13 | 21 | 35 | 56 |
| Hormone receptor status combined | |||||
| ER+/PgR+ | 63 | 35 | 56 ‡ | 38 | 60 |
| ER+/PgR− | 14 | 6 | 43 | 7 | 50 |
| ER−/PgR+ | 24 | 5 | 21 | 15 | 63 |
| ER−/PgR− | 49 | 7 | 14 | 28 | 57 |
| Histological type | |||||
| Invasive ductal carcinoma | 130 | 42 | 32 § | 75 | 58 |
| Ductal carcinoma in situ6 | 15 | 6 | 40 | 10 | 67 |
| Invasive lobular carcinoma | 5 | 4 | 80 | 2 | 40 |
| Nuclear grade | |||||
| Grade 1 | 17 | 10 | 59 ¶ | 11 | 65 |
| Grade 2 | 84 | 32 | 38 | 44 | 52 |
| Grade 3 | 49 | 11 | 22 | 33 | 67 |
| Age (years) | |||||
| <50 | 57 | 16 | 28 | 31 | 54 |
| 50 | 93 | 37 | 40 | 57 | 61 |
| Tumor size (cm) | |||||
| <2.0 | 42 | 12 | 29 | 17 | 40 |
| 2.0 | 108 | 41 | 38 | 71 | 66 |
| Lymph node status | |||||
| Positive | 52 | 18 | 35 | 28 | 54 |
| Negative | 77 | 29 | 38 | 48 | 62 |
| Unknown | 21 | 6 | 29 | 12 | 57 |
P < 0.0001 between estrogen receptor (ER)‐positive and ER‐negative cases;
P = 0.002 between progesterone receptor (PgR)‐positive and PgR‐negative cases;
P = 0.0001 between ER‐ and/or PgR‐positive cases and double‐negative ER and PgR cases;
P = 0.03 between the cases with invasive ductal carcinoma and invasive lobular carcinoma;
P = 0.014 between grade 1, 2 and 3 cases.
pERK1/2 was positive in 42 (32%) of 130 cases with invasive ductal carcinoma, in six (40%) of 15 cases with ductal carcinoma in situ, and in four (80%) of five cases with invasive lobular carcinoma. There was a significant difference in correlation of pERK1/2 between invasive ductal carcinoma and invasive lobular carcinoma (P = 0.03).
With regard to nuclear grade, pERK1/2 was positive in 10 (59%) of 17 grade 1 cases, 32 (38%) of 84 grade 2 cases, and 11 (22%) of 49 grade 3 cases. There was a significant correlation of pERK1/2 with lower nuclear grade (P = 0.014). There was no correlation of pERK1/2 with age, tumor size or axillary lymph node status.
pAKT was detected as 2+ in 88 cases (59%) (Table 3). There was no significant correlation of pAKT overexpression with hormone receptor status, histological type, nuclear grade or other clinical parameters. pERK1/2‐positive cancer cells and pAKT‐positive cancer cells were almost identical in pERK1/2‐positive and pAKT‐positive tumors.
Correlation of pERK1/2 and pAKT with hormone receptor status in breast cancers overexpressing IGF1R
In Table 4, pERK1/2 positivity rates are presented for the four subsets of hormone receptors status in the 71 IGF1R‐overexpressing breast cancers. pERK1/2 expression was detected in 24 (57%) of 42 ER‐positive/PgR‐positive cases, three (50%) of six ER‐positive/PgR‐negative cases, two (17%) of 12 ER‐negative/PgR‐positive cases, and two (18%) of 11 ER‐negative/PgR‐negative cases. There was a significant difference of pERK1/2 expression rates between ER‐positive and ER‐negative cases in breast cancers overexpressing IGF1R (P = 0.022).
Table 4.
Correlation of hormone receptor status with pERK1/2 and with pAKT in breast cancer with overexpression of insulin‐like growth factor receptor type 1 (IGF1R)
| Hormone receptor status | Total | Cases | |||
|---|---|---|---|---|---|
| pERK1/2 | pAKT | ||||
| n | % | n | % | ||
| Total | 71 | 33 | 46 | 48 | 68 |
| ER+/PgR+ | 42 | 24 | 57* | 28 | 67 |
| ER+/PgR− | 6 | 3 | 50 | 4 | 67 |
| ER−/PgR+ | 12 | 2 | 17 | 9 | 75 |
| ER−/PgR− | 11 | 2 | 18 | 7 | 64 |
P = 0.022, between the cases with estrogen receptor (ER) positivity and ER negativity irrespective of progesterone receptor (PgR) status.
In the 71 IGF1R‐overexpressing tumors, pAKT was detected in 28 (67%) of the 42 ER‐positive/PgR‐positive cases, four (67%) of the six ER‐positive/PgR‐negative cases, nine (75%) of the 12 ER‐negative/PgR‐positive cases, and seven (64%) of the 11 ER‐negative/PgR‐negative cases. Thus, pAKT was detected frequently regardless of hormone receptor status in IGF1R‐overexpressing breast cancers.
Significance of hormone receptors, IGF1R and signaling kinases as predictors of disease‐free survival
In Table 5, proportional hazards analysis revealed that the risk of relapse of ER‐negative and PgR‐negative cases was approximately twice as high as for those that were ER‐positive and/or PgR‐positive (95% confidential interval 1.12–4.0, P = 0.02). Cases with IGF1R overexpression tended to have better prognosis than cases without IGF1R overexpression in hormone receptor‐positive breast cancers (P = 0.09). However, IGF1R status was not correlated with prognosis in 49 ER‐negative and PgR‐negative cases. In pERK1/2 and pAKT status, the groups with pERK1/2 or pAKT expression were not correlated with prognosis regardless of ER/PgR status and IGF1R status.
Table 5.
Significance of hormone receptors, insulin‐like growth factor receptor type 1 (IGF1R) and signaling kinases as predictors of disease‐free survival
| Cases | Hazard rate | 95% confidence interval | P‐value |
|---|---|---|---|
| ER− and PgR− vs ER+ and/or PgR+ | 2.12 | 1.12–4.00 | 0.02 |
| ER+ and/or PgR+ (n = 101) | |||
| IGF1R− vs IGF1R+ | 1.9 | 0.85–4.83 | 0.09 |
| pERK1/2− vs pERK1/2+ | 1.12 | 0.45–2.78 | 0.81 |
| pAKT− vs pAKT+ | 1.25 | 0.51–3.07 | 0.63 |
| ER− and PgR− cases (n = 49) | |||
| IGF1R− vs IGF1R+ | 1.22 | 0.41–3.69 | 0.71 |
| pERK1/2− vs pERK1/2+ | 0.57 | 0.17–2.0 | 0.38 |
| pAKT− vs pAKT+ | 0.76 | 0.28–1.84 | 0.49 |
| Breast cancer cases with IGF1R overexpression (n = 71) | |||
| pERK1/2− vs pERK1/2+ | 1.2 | 0.35–4.09 | 0.78 |
| pAKT− vs pAKT+ | 0.64 | 0.17–2.38 | 0.5 |
The total number of cases was 150. Proportional hazards analysis revealed that estrogen receptor (ER)‐negative and progesterone receptor (PgR)‐negative cases were approximately twice as likely to relapse (95% confidential interval 1.12–4.00, P = 0.02) than ER‐positive and/or PgR‐positive cases, and that cases with IGF1R overexpression tended to have a better prognosis than cases without IGF1R overexpression (P = 0.09). Other groups did not show a significant correlation of hormone receptors, IGF1R and signal kinases with disease‐free survival.
Discussion
IGF1R overexpression was comprehensively detected in breast cancers showing all subsets of hormone receptor status, but was more frequent in cases that were positive for ER and/or PgR than in cases that were double negative for both ER and PgR. These results indicate that IGF1R‐overexpressing breast cancers might frequently develop through cellular activation induced by hormone receptors. It has been shown that estrogens induce insulin‐like growth factor type 1 (IGF‐1) in ER‐positive breast cancer cells, and IGF‐1 stimulates their growth through IGF1R. The reason for the association between IGF1R overexpression and ER positivity can be, at least in part, explained by the presence of this loop.( 21 , 22 ) However, more than 20% of ER/PgR double‐negative breast cancers also overexpressed IGF1R.
With regard to downstream signaling kinases, the incidence of pAKT expression did not differ significantly among all the subsets of ER/PgR status in breast cancer. In contrast, the incidence of pERK1/2 expression was highest in ER/PgR double‐positive cancers, intermediate in ER‐positive/PgR‐negative and ER‐negative/PgR‐positive cancers, and lowest in ER/PgR double‐negative cancers.
Several papers have described the relationship between pAKT or pERK and clinicopatholgical characteristics of breast cancer. Kirkegaard et al. reported that immunohistochemical expression of pAKT (Ser473) predicted decreased overall survival, but was not significantly associated with disease‐free survival.( 23 ) Cineas et al. showed that a high level of pAKT measured by chemiluminesence‐linked immunosorbent assay was a predictor of decreased disease‐free survival.( 24 ) However, we couldn't show a significant correlation between immunohistochemical expression of pAKT and shorter disease‐free survival.
In contrast, Svensson et al. reported that ERK1/2 phosphorylation in breast cancer correlated with better survival and a less‐aggressive phenotype.( 25 ) We also showed that pERK1/2 was frequently expressed in breast cancers that were hormone receptor‐positive with lower nuclear grade, but that pERK1/2 didn't significantly correlate with better prognosis. A part of our results was compatible with the report by Svensson et al.
The manifold effects of the ER pathway can be partly explained by the newly discovered intense cross‐talk of ER with the growth factor receptor‐regulated system. Many recent studies have emphasized the importance of this kind of cross‐talk in breast cancer etiology and progression. The latest reports suggest that cross‐talk between ER and growth factor receptor pathways, such as the EGFR family and IGF1R, contributes to tamoxifen resistance, which is intimately associated with the dynamic equilibrium of multiple signaling pathways.( 26 , 27 , 28 , 29 )
The difference in distribution of pERK1/2‐expressing tumors and pAKT‐expressing tumors among the subsets of hormone receptor status observed in the present study appears to be informative in terms of breast cancer development. Most human mammary cancers originate in luminal mammary epithelial cells lining the mammary ducts and alveoli. These cancers are histopathologically diverse and are classified on the basis of their growth requirements as hormone‐dependent or hormone‐independent tumors.( 30 ) During the process in which breast precancerous cells deviate from a hormone‐dependent status, which represents a less aggressive phenotype, the MAPK signaling pathway modulated by phosphorylation of ERK1/2 might lose its dominant effect on cellular proliferation through complex remodeling of the intracellular signaling network. Instead, the PI3K/AKT signaling pathway might come to play a relatively potent role in breast cancer cells that have lost their expression of ER and/or PgR. In fact, we found that most cases of hormone receptor‐negative breast cancer had a low level of pERK1/2 expression but a high level of pAKT expression, accompanied by overexpression of EGFR and/or HER2. These findings suggest that the growth of hormone receptor‐negative breast cancers might be frequently regulated by activation of EGFR and/or HER2 growth factor receptors, and that phosphorylation of AKT becomes dominant in these cancers through the change in balance between pAKT and pERK1/2.
Considering the present results, it is possible that in IGF1R‐overexpressing breast cancers, the levels of expression of pERK1/2 and pAKT might alter in accordance with the changes in ER and/or PgR expression during cancer progression.
Some in vitro experimental studies have suggested that the IGF1R signaling pathway and its function may differ between hormone‐dependent and hormone‐independent breast cancer cells. Bartucci et al. have reported that in the ER‐positive cell line MCF‐7, IGF1R transmits various signals, such as those for growth, survival, migration and adhesion, whereas in ER‐negative MDA‐MB‐231 cells, the growth‐related function of IGF1R is attenuated, although it is still able to control non‐mitogenic processes, such as migration. 31 One of the most significant differences in IGF1R signaling between ER‐negative and ER‐positive cells was impaired long‐term stimulation of the PI3K/AKT pathway. Although sustained AKT activity could be important for the survival of breast cancer cells, a proper equilibrium between AKT and other pathways such as ERK1/2 is also critical in determining the biological behavior of breast cancer. There is additional evidence that hyperactivation of AKT can downregulate the MAPK/ERK1/2 pathway in ER‐negative breast cancer.( 31 , 32 )
A study by Surmacz has also suggested a requirement for the IGF1R pathway by the ER‐negative MDA‐MB‐435 cell line.( 30 ) The growth of these cell lines is not enhanced by IGF‐I. Interestingly, despite the lack of a mitogenic response to IGF‐I, the metastatic potential of these ER‐negative breast cancer cell lines can be effectively inhibited by IGF1R antagonists.( 33 , 34 ) These experimental findings in vitro are compatible with the present data for IGF1R‐overexpressing breast cancer.
In retrospective clinical studies, a highly significant correlation between IGF1R overexpression and better patient prognosis has been reported,( 35 ) whereas patients with ER‐negative but IGF1R‐positive tumors, which are reported to occur infrequently, tend to show shorter disease‐free survival.( 36 )
In conclusion, we have found that breast cancers showing double positivity for hormone receptors have the highest level of IGF1R overexpression (67%), and that high levels of both pERK1/2 and pAKT expression are also detected frequently. However, in hormone receptor‐negative breast cancers, IGF1R was overexpressed in 22% of cases, and the expression of pERK1/2 was low, although the level of pAKT expression was relatively higher than that of pERK1/2. From these findings it is evident that the role of IGF1R in hormone receptor double‐negative breast cancer differs from that in hormone receptor‐positive breast cancer. The balance between pERK1/2 and pAKT expression might be influenced by differential overexpression of IGF1R in the constituent cancer cells of a tumor.
This is the first report to have demonstrated a critical role for IGF1R expression in vivo in association with hormone receptors in breast cancer.
References
- 1. Johnston SR. Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin Cancer Res 2005; 11 (2 Part 2): S889–99. [PubMed] [Google Scholar]
- 2. Geisler J, Lonning PE. Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res 2005; 11: 2809–21. [DOI] [PubMed] [Google Scholar]
- 3. Hayes DF, Thor AD. c‐erbB‐2 in breast cancer: development of a clinically useful marker. Semin Oncol 2002; 29: 231–45. [DOI] [PubMed] [Google Scholar]
- 4. Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer 1998; 34: 791–808. [DOI] [PubMed] [Google Scholar]
- 5. Walker RA, Dearing SJ. Expression of epidermal growth factor receptor mRNA and protein in primary breast carcinomas. Breast Cancer Res Treat 1999; 53: 167–76. [DOI] [PubMed] [Google Scholar]
- 6. Tsutsui S, Ohno S, Murakami S et al. Prognostic value of the combination of epidermal growth factor receptor and c‐erbB‐2 in breast cancer. Surgery 2003; 133: 219–21. [DOI] [PubMed] [Google Scholar]
- 7. Shimizu C, Hasegawa T, Tani Y et al. Expression of insulin‐like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol 2004; 35: 1537–42. [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim YH, Yee D. Insulin‐like growth factor‐I and breast cancer therapy. Clin Cancer Res 2005; 11 (2 Part 2): S944–50. [PubMed] [Google Scholar]
- 9. Van Den Berg HW, Claffie D, Boylan M et al. Expression of receptors for epidermal growth factor and insulin‐like growth factor I by ZR‐75–1 human breast cancer cell variants is inversely related: the effect of steroid hormones on insulin‐like growth factor I receptor expression. Br J Cancer 1996; 73: 477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu H, Rohan T. Role of the insulin‐like growth factor family in cancer development and progression. J Natl Cancer Inst 2000; 92: 1472–89. [DOI] [PubMed] [Google Scholar]
- 11. Furstenberger G, Senn HJ. Insulin‐like growth factors and cancer. Lancet Oncol 2002; 3: 298–302. [DOI] [PubMed] [Google Scholar]
- 12. Furstenberger G, Morant R, Senn HJ. Insulin‐like growth factors and breast cancer. Onkologie 2003; 26: 290–4. [DOI] [PubMed] [Google Scholar]
- 13. Schmitz KJ, Grabellus F, Callies R et al. High expression of focal adhesion kinase (p125FAK) in node‐negative breast cancer is related to overexpression of HER‐2/neu and activated Akt kinase but does not predict outcome. Breast Cancer Res 2005; 7: R194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmitz KJ, Otterbach F, Callies R et al. Prognostic relevance of activated Akt kinase in node‐negative breast cancer: a clinicopathological study of 99 cases. Mod Pathol 2004; 17: 15–21. [DOI] [PubMed] [Google Scholar]
- 15. Jones HE, Goddard L, Gee JM et al. Insulin‐like growth factor‐I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer 2004; 11: 793–814. [DOI] [PubMed] [Google Scholar]
- 16. Albanell J, Baselga J. Unraveling resistance to trastuzumab (Herceptin): insulin‐like growth factor‐I receptor, a new suspect. J Natl Cancer Inst 2001; 93: 1830–2. [DOI] [PubMed] [Google Scholar]
- 17. Werner H, Roberts CT Jr. The IGFI receptor gene: a molecular target for disrupted transcription factors. Genes Chromosomes Cancer 2003; 36: 113–20. [DOI] [PubMed] [Google Scholar]
- 18. Cui X, Zhang P, Deng W et al. Insulin‐like growth factor‐I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3‐kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol 2003; 17: 575–88. [DOI] [PubMed] [Google Scholar]
- 19. Gomez HL, Doval DC, Chavez MA et al. Biomarker results from a phase II randomized study of lapatinib as first‐line treatment for patients with ErbB2 FISH‐amplified advanced or metastatic breast cancer. San Antonio Breast Cancer Symp 2005; 94 (Suppl. 1): S63. [Google Scholar]
- 20. Tsuda H, Morita D, Kimura M et al. Correlation of KIT and EGFR overexpression with invasive ductal breast carcinoma of the solid‐tubular subtype, nuclear grade 3, and mesenchymal or myoepithelial differentiation. Cancer Sci 2005; 96: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Surmacz E, Bartucci M. Role of estrogen receptor alpha in modulating IGF‐I receptor signaling and function in breast cancer. J Exp Clin Cancer Res 2004; 23: 385–94. [PubMed] [Google Scholar]
- 22. Gee JM, Robertson JF, Gutteridge E et al. Epidermal growth factor receptor/HER2/insulin‐like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr Relat Cancer 2005; 12 (Suppl. 1): S99–111. [DOI] [PubMed] [Google Scholar]
- 23. Kirkegaard T, Witton CJ, McGlynn LM et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol 2005; 207: 139–46. [DOI] [PubMed] [Google Scholar]
- 24. Cicenas J, Urban P, Vuaroqueaux V et al. Increased level of phosphorylated akt measured by chemiluminescence‐linked immunosorbent assay is a predictor of poor prognosis in primary breast cancer overexpressing ErbB‐2. Breast Cancer Res 2005; 7: R394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Svensson S, Jirstrom K, Ryden L et al. ERK phosphorylation is linked to VEGFR2 expression and Ets‐2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene 2005; 24: 4370–9. [DOI] [PubMed] [Google Scholar]
- 26. Yue W, Wang JP, Li Y et al. Tamoxifen versus aromatase inhibitors for breast cancer prevention. Clin Cancer Res 2005; 11 (2 Part 2): S925–30. [PubMed] [Google Scholar]
- 27. Thiantanawat A, Long BJ, Brodie AM. Signaling pathways of apoptosis activated by aromatase inhibitors and antiestrogens. Cancer Res 2003; 63: 8037–50. [PubMed] [Google Scholar]
- 28. Schiff R, Massarweh SA, Shou J et al. Cross‐talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 2004; 10 (1 Part 2): S331–6. [DOI] [PubMed] [Google Scholar]
- 29. Schiff R, Massarweh S, Shou J, Osborne CK. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res 2003; 9 (1 Part 2): S447–54. [PubMed] [Google Scholar]
- 30. Surmacz E. Function of the IGF‐I receptor in breast cancer. J Mammary Gland Biol Neoplasia 2000; 5: 95–105. [DOI] [PubMed] [Google Scholar]
- 31. Bartucci M, Morelli C, Mauro L et al. Differential insulin‐like growth factor I receptor signaling and function in estrogen receptor (ER) ‐positive MCF‐7 and ER‐negative MDA‐MB‐231 breast cancer cells. Cancer Res 2001; 61: 6747–54. [PubMed] [Google Scholar]
- 32. Moelling K, Schad K, Bosse M et al. Regulation of Raf‐Akt Cross‐talk. J Biol Chem 2002; 277: 31 099–106. [DOI] [PubMed] [Google Scholar]
- 33. Dunn SE, Ehrlich M, Sharp NJ et al. A dominant negative mutant of the insulin‐like growth factor‐I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res 1998; 58: 3353–61. [PubMed] [Google Scholar]
- 34. Doerr ME, Jones JI. The roles of integrins and extracellular matrix proteins in the insulin‐like growth factor I‐stimulated chemotaxis of human breast cancer cells. J Biol Chem 1996; 271: 2443–7. [DOI] [PubMed] [Google Scholar]
- 35. Peyrat JP, Bonneterre J, Laurent JC et al. Presence and characterization of insulin‐like growth factor 1 receptors in human benign breast disease. Eur J Cancer Clin Oncol 1988; 24: 1425–31. [DOI] [PubMed] [Google Scholar]
- 36. Railo MJ, Von Smitten K, Pekonen F. The prognostic value of insulin‐like growth factor‐I in breast cancer patients. Results of a follow‐up study on 126 patients. Eur J Cancer 1994; 30A: 307–11. [DOI] [PubMed] [Google Scholar]


