Abstract
The sentinel node (SN) concept has been found to be feasible in gastric cancer. However, the lymphatic network of gastric cancer may be more complex, and it may be difficult to visualize all the SN distributed in unexpected areas by conventional modalities. In this study, we evaluate the feasibility and efficacy of CT lymphography for the detection of SN in gastric cancer. A total 24 patients with early gastric cancer were enrolled in the study. Three modalities (CT lymphography, dye and radioisotope [RI] methods) were used for the detection of SN. The images of CT lymphography were obtained at 10 min after injection of contrast agents. The SN were successfully identified by CT lymphography in 83.3% of patients; detection rates by the dye and RI methods were 95% and 100%, respectively. Most patients, in whom SN were successfully detected by CT lymphography, had positive results at 5 min after injection of the contrast material. The SN stations detected by CT lymphography were consistent with or included those detected by dye and/or RI methods. In conclusion, CT lymphography for the detection of SN in gastric cancer is feasible and has several advantages. However, based on this initial experience, CT lymphography had a relatively low detection rate compared with conventional methods, and further efforts will be necessary to improve the detection rate and widen the clinical application of CT lymphography for the detection of SN in gastric cancer. (Cancer Sci 2010; 101: 2586–2590)
Sentinel node (SN) biopsy has become a standard surgical procedure for patients with early stage breast cancer.( 1 , 2 ) We have reported that the SN concept can be successfully applied in patients with early gastric carcinoma.( 3 , 4 , 5 ) Similar results have been reported by other authors.( 6 , 7 , 8 , 9 , 10 ) Since 2000, we have offered individualized surgery to patients with T1 gastric cancer based on the results of a SN biopsy, in which we used a combination of the dye and radioisotope (RI) methods.( 4 ) Although the dye and RI methods are commonly used as SN biopsy tracers in some malignancies,( 11 ) they have several advantages and disadvantages with respect to each other in terms of their kinetics in the lymphatic system, convenience, cost and associated side‐effects.( 12 ) Several authors reported that dye‐guided and RI‐guided techniques are complementary, and the combination of these methods has been used to improve SN biopsy results in melanoma, breast and gastric cancer.( 6 , 11 , 13 , 14 ) However, the lymphatic network of gastric cancer may be more complex than in melanoma and breast cancer, and may limit the ability of conventional modalities to visualize all the SN distributed in unexpected areas.
The currently used multidetector‐row computed tomography (MDCT) scanners allow thinner collimation and faster scanning, which markedly improves the scanning resolution.( 15 ) Recently, there have been several reports on the successful identification of enhanced SN and lymphatics visualized by MDCT (CT lymphography) using a nonionic contrast medium in breast cancer and superficial esophageal cancer.( 16 , 17 , 18 ) Thus, we speculated that CT lymphography may be of use for SN mapping and biopsy by enabling direct visualization of lymphatic drainage pathways from primary tumor sites in gastric cancer.
In this study, we evaluated the feasibility, accuracy and efficacy of CT lymphography for the detection of SN in early gastric cancer patients in comparison with conventional SN detecting systems (i.e. dye and RI methods).
Materials and Methods
Patients. Between February 2008 and July 2010, a total of 24 consecutive patients with early gastric cancer were enrolled in this study; SN were detected in these patients using all three modalities (i.e. CT lymphography, dye method and RI method). Gastric cancer was confirmed in all patients by preoperative endoscopic biopsy. Their preoperative stage was diagnosed as T1N0M0 gastric cancer by imaging studies, CT and abdominal ultrasonography. The clinical and pathological findings were described (1, 3), and the lymph node station number was classified according to the second English edition of the Japanese Classification of Gastric Carcinoma (JCGC), which was edited by the Japanese Gastric Cancer Association.( 19 ) Partial or subtotal gastrectomy with lymphadenectomy for lymph node stations where SN were located after detection by any of the modalities was performed. The SN biopsy and CT lymphography procedures reported in this study were reviewed and approved by the Institutional Review Board at the National Defense Medical College. Written informed consent was obtained from every patient before they underwent any study procedures to identify SN.
Table 1.
Clinicopathological characteristics of 24 patients
| Age (mean ± SD) (years) | 65.5 ± 11.0 |
| Sex, n (%) | |
| Male | 17 (70.8) |
| Female | 7 (29.2) |
| BMI (mean ± SD) (kg/m2) | 22.5 ± 3.6 |
| Tumor depth, n (%) | |
| Mucosa | 16 (66.7) |
| Submucosa | 6 (25.0) |
| Muscularis propria | 2 (8.3) |
| Tumor size (mean ± SD) (mm) | 31.6 ± 15.8 |
| Tumor location, n (%) | |
| Upper | 4 (16.7) |
| Middle | 14 (58.3) |
| Lower | 6 (25.0) |
| Tumor circumference, n (%) | |
| Less | 7 (29.2) |
| Gre | 6 (25.0) |
| Ant | 5 (20.8) |
| Post | 6 (25.0) |
| Macroscopic types, n (%) | |
| Elevated | 5 (20.8) |
| Depressed | 19 (79.2) |
| Histology, n (%) | |
| Diffuse | 8 (33.3) |
| Intestinal | 16 (66.7) |
| Nodal status, n (%) | |
| pN0 | 22 (91.7) |
| pN1 | 2 (8.3) |
| Lymphatic invasion, n (%) | |
| Yes | 3 (12.5) |
| No | 21 (87.5) |
| Vascular invasion, n (%) | |
| Yes | 4 (16.7) |
| No | 20 (83.3) |
| Prior endoscopic treatment, n (%) | |
| Yes | 2 (8.3) |
| No | 22 (91.7) |
| Surgical procedure, n (%) | |
| Open surgery | 9 (37.5) |
| Laparoscopic surgery | 15 (62.5) |
| Number of dissected LN (mean ± SD) | 19.5 ± 9.0 |
Ant, anterior wall; BMI, body mass index; gre, greater curvature; less, lesser curvature; LN, lymph node; post, posterior wall.
Table 3.
Comparison of CT lymphography, dye and RI methods for the detection of SN
| Case number | Tumor location | CT lymphography | Dye method | RI method | LN metastasis | |
|---|---|---|---|---|---|---|
| 1 | L | Post | #3 (4) | #3 (1) | No | |
| #4d (1) | ||||||
| #6 (1) | #6 (1) | #6 | ||||
| 2 | M | Less | #3 (1) | #3 (1) | #3 (1) | No |
| #4d (1) | ||||||
| 3 | M | Ant | #3 (2) | #3 (3) | #3 (4) | No |
| 4 | U | Post | #1 (1) | #1 (2) | #1 (2) | No |
| 5 | L | Gre | #4d (7) | #4d (3) | No | |
| #6 (1) | #6 (2) | #6 (3) | ||||
| #14v (1) | #14v (1) | |||||
| 6 | M | Post | #3 (2) | #3 (1) | No | |
| ND | #4d (4) | #4d (5) | ||||
| 7 | M | Less | #3 (3) | #3 (7) | #3 (1) | No |
| 8 | M | Post | ND | #3 (5) | #3 (4) | No |
| #4d (3) | #4d (1) | |||||
| 9 | M | Less | #3 (1) | #3 (5) | #3 (6) | No |
| 10 | L | Ant | #6 (2) | #6 (1) | #6 (2) | No |
| 11 | U | Less | #2 (1) | No | ||
| #3 (1) | #3 (3) | #3 (1) | ||||
| 12 | L | Gre | #3 (4) | #3 (4) | No | |
| #4d (3) | #4d (7) | #4d (4) | ||||
| #5 (1) | #5 (1) | |||||
| 13 | L | Post | #3 (1) | |||
| ND | ||||||
| #6 (1) | #6 (1) | |||||
| 14 | U | Gre | ND | #3 (1) | #3 (1) | #3 (1) |
| 15 | M | Ant | #3 (2) | #3 (2) | No | |
| #4sa (1) | #4sa (2) | |||||
| #4sb (1) | #4sb (5) | #4sb (2) | ||||
| #4d (1) | #4d (2) | #4d (2) | ||||
| 16 | L | Ant | #3 (1) | #3 (4) | #3 (4) | No |
| #6 (2) | ||||||
| 17 | U | Less | #1 (1) | #1 (1) | No | |
| #3 (1) | #3 (4) | #3 (3) | ||||
| 18 | M | Post | ND | #3 (1) | #3 (1) | No |
| #4d (1) | #4d (3) | |||||
| 19 | M | Gre | #3 (2) | #3 (2) | No | |
| #4sb (1) | #4sb (1) | #4sb (4) | ||||
| #4d (1) | #4d (2) | #4d (7) | ||||
| 20 | M | Less | #1 (1) | #1 (1) | No | |
| #3 (1) | #3 (8) | #3 (6) | ||||
| #7 (1) | ||||||
| 21 | M | Gre | #4d (1) | #4d (6) | No | |
| #6 (1) | #6 (4) | #4d (4) | ||||
| 22 | M | Less | #3 (1) | #3 (4) | #3 (4) | No |
| 23 | L | Gre | #3 (1) | No | ||
| #6 (1) | #6 (1) | |||||
| #8a (1) | ||||||
| 24 | M | Ant | #3 (1) | #3 (1) | No | |
| #4d (1) | #4d (3) | #4d (3) | ||||
Number of SN is described in parentheses. #1, right paracardial LN; #3, LN along the lesser curvature; #4sa, LN along the short gastric vessels; #4sb, LN along the left gastroepiploic vessels; #4d, LN along the right gastroepiploic vessels; #5, suprapyloric LN; #6, infrapyloric LN; #7, LN along the left gastric artery; #8a, LN along the common hepatic artery; #14v, LN along the superior mesenteric vein. # indicates lymph node station number. sa, sb, d, v were defined by Japanese Classification of Gastric Carcinoma( 19 ).
Identification of SN by CT lymphography. Approximately 1 week before surgery, CT lymphography was performed as previously described.( 16 , 17 ) Briefly, the CT apparatus used in this study was a 64 detector–row CT (Aquilion 64, Toshiba Medical, Tokyo, Japan). After taking a blowing agent, precontrast CT images from the lower thorax to the upper abdomen were initially obtained while patients were in the supine position with their arms extended cranially. Then, with patients remaining on the CT table, the endoscope was inserted and a total of 2 mL of nonionic contrast medium (Optiray, Covidien, Tokyo, Japan) was injected, using a 25‐gauge sclerotherapy needle, into the submucosal layer in four different areas (with 0.5 mL at each area) that closely surrounded the primary tumors. The time from the initial to the last contrast material injection was 5 min. Post‐contrast images were obtained 1, 3, 5 and 10 min after completion of the contrast material injections. The scanning parameters included 120 kVp, 0.5 s tube rotation time, 27 mm/rotation helical pitch, 55 mm table speed, 0.5 s gantry rotation time and 5‐mm‐thick reconstructed sections. The CT images were reconstructed with a section width of 1.25 mm and a section interval of 0.5 mm. Multi‐planar reconstruction, maximum intensity projection, and 3‐D images were reviewed on a stand‐alone workstation (Zio workstation, AMIN, Inc., Tokyo. Japan). A lymph node was defined as a SN if visual inspection or the CT value (the increase of Hounsfield units [HU] after submucosal injection) confirmed it to be enhanced or if it was confirmed to connect to an enhanced lymph vessel (Fig. 1). The CT value of SN was measured and expressed in HU.
Figure 1.
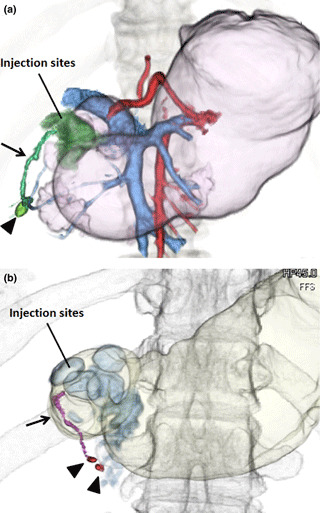
Representative 3‐D computed tomography (CT) lymphograms for early gastric cancer. (a) A tumor was located on the lower side and posterior wall of the stomach (case number 1). The CT lymphography successfully detected an enhanced lymph node (arrowhead) and lymphatic flow toward the infrapyloric lymph node (arrow). Pictures of the portal and artery phases have been merged. (b) A tumor was located on the lower side and anterior wall of the stomach (case number 10). The CT lymphography successfully detected two enhanced lymph nodes (arrowheads) and lymphatics toward the infrapyloric lymph nodes (arrow).
Identification of SN by conventional (RI and dye) methods. For the identification of SN by the RI method, on the day before surgery 3 mL of 99mTc–labeled tin colloid solution (4 mCi), prepared by mixing 99mTc–pertechnate and stannous chloride (II) (Nihon Medi–Physics, Tokyo, Japan) at a ratio of 1:2, was injected endoscopically into the submucosa around the tumor (with 0.5 mL at each area) with a 25‐gauge sclerotherapy needle, in the same manner as for CT lymphography. Radioactivity of the lymph nodes was detected by a hand‐held gamma probe (Navigator System, USCC, Norwalk, CT, USA), with a lead plate covering radioactivity around the primary tumor during surgery. The radioactivity of the retrieved lymph nodes was determined on the back table. Hot nodes were defined as lymph nodes with a radioactivity of 10 counts per 10 s or higher. The SN were defined as hot nodes; SN stations were defined as lymph node stations where hot nodes were distributed.
For the identification of SN using the dye method, 4 mL of 1.25% indocyanine green solution (ICG) was injected around the tumor (with 1 mL at each area) into the submucosa endoscopically with a 25‐gauge sclerotherapy needle immediately after laparotomy or after insertion of laparoscopy. Five minutes after injection of the ICG solution, green‐dyed lymphatics and/or green‐dyed lymph nodes (green nodes) were identified. The SN were defined as green nodes; SN stations were defined as lymph node stations where green lymphatics or green nodes were distributed.
Statistical analyses. Data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using either the Mann–Whitney U test or the Chi‐squared test. A P‐value <0.05 was considered statistically significant. All analyses were performed using the StatView version 5.0 software program (SAS Institute Inc., Cary, NC, USA).
Results
Contrast agent injection was successfully performed in all patients, without acute or late adverse effects; this was easily confirmed by CT as positive contrast medium at the injection site with no leakage into the gastric lumen (Fig. 1). Clinicopathological characteristics of the 24 patients are presented in Table 1. The average age of the patients was 65.5 ± 11.0 years (range, 41–87). Two patients had prior endoscopic treatment that identified positive cancer cells in the submucosal vertical margin, and 15 patients underwent laparoscopic surgery as surgical procedures. Two patients had lymph node metastasis in their SN. Out of the 24 patients, SN of 20 (83.3%) were successfully detected by CT lymphography, while those of the remaining four patients (16.7%) were not detected throughout the investigation periods. Results of CT lymphography at each time point are presented in Table 2. Three patients (case numbers 1, 3 and 4) were not examined by CT lymphography at 1 min after contrast material injection because of practical problems. Most patients in whom SN were successfully detected by CT lymphography had positive results at 5 min after injection of contrast material; the exception to this was case number 1, in which a positive result was not detected until 10 min. In the group of patients in whom SN was successfully detected, it took an average of 2.5 ± 2.2 min to detect the first SN, and it took an average 4.5 ± 3.5 min to have the greatest CT value of SN.
Table 2.
Time course of computed tomography lymphography for the detection of SN after injection of contrast agent
| Case number | Time course | Time to the first appearance of SN (min) | Time to greatest nodal enhancement (min) and HU at that time | |||
|---|---|---|---|---|---|---|
| 1 min | 3 min | 5 min | 10 min | |||
| 1 | NE | − | − | + | 10 | 10 (46.3 HU) |
| 2 | − | + | + | + | 3 | 10 (88.0 HU) |
| 3 | NE | + | + | − | 3 | 3 (77.2 HU) |
| 4 | NE | − | + | + | 5 | 10 (85.6HU) |
| 5 | + | + | + | + | 1 | 1 (126.1HU) |
| 6 | − | − | − | − | – | – |
| 7 | + | + | + | + | 1 | 1 (92.0HU) |
| 8 | − | − | − | − | – | – |
| 9 | + | + | + | + | 1 | 3 (133.5HU) |
| 10 | − | + | + | + | 3 | 3 (273.4HU) |
| 11 | − | + | + | + | 3 | 3 (100.0HU) |
| 12 | + | + | + | + | 1 | 5 (86.2HU) |
| 13 | + | + | + | + | 1 | 1 (102.8HU) |
| 14 | − | − | − | − | – | – |
| 15 | + | + | + | + | 1 | 3 (723.0HU) |
| 16 | + | + | + | + | 1 | 3 (78.2HU) |
| 17 | + | + | + | + | 1 | 1 (101.3HU)) |
| 18 | − | − | − | − | – | – |
| 19 | + | + | + | + | 1 | 5 (69.6HU)) |
| 20 | − | + | + | + | 3 | 3 (361.2HU) |
| 21 | − | + | + | + | 1 | 5 (199.7HU) |
| 22 | + | + | + | + | 1 | 10 (210.5HU) |
| 23 | − | − | + | + | 3 | 10 (157.9HU) |
| 24 | + | + | + | + | 1 | 1 (140.0HU) |
| Detection rate | 11/21 (52.4%) | 17/24 (70.8%) | 19/24 (79.2%) | 19/24 (79.2%) | ||
HU, Hounsfield units; NE, not examined; SN, sentinel node.
Next, we compared the detection rates and the station where SN were distributed, using CT lymphography and the conventional SN detection methods (Table 3). The detection rates of SN by CT lymphography, dye method and RI method were 83.3%, 95.8% and 100%, respectively. The average number of SN in 24 patients using CT lymphography, dye method and RI method was 1.3 ± 0.9 (range, 0–3), 5.0 ± 3.4 (range, 0–12) and 4.3 ± 3.0 (range, 1–13), respectively. The average number of stations where SN were located was 1.1 ± 0.7 (range, 0–3), 1.7 ± 1.0 (range, 0–4) and 1.8 ± 0.8 (range, 1–3), respectively. The SN stations detected using CT lymphography were consistent with or included those by the dye and/or RI methods. Of note, one patient (case number 13) had a conflicting result regarding the SN station using CT lymphography and the RI method, and he had a lymph node metastasis at station #6, which was only identified by CT lymphography.
Discussion
In this study, we have demonstrated for the first time the results of CT lymphography for the detection of SN in gastric cancer patients. The SN detection rate using CT lymphography was 83.3% (20 of 24 patients), which was inferior to conventional SN detection methods, such as the dye and RI methods.
The reason for unsuccessful detection of SN by CT lymphography remains unclear because of the small number of patients. In the patients in whom SN were not detected by CT lymphography, the injection site with positive contrast medium could be visualized by CT. Our department has investigated more than 200 patients with SN navigation surgery since 2000 with satisfactory results.( 3 , 4 , 5 , 20 ) Thus, we believe that the relatively higher false–negative rate of CT lymphography may not be caused just by an injection failure. In this study, we set the investigation time points at 1, 3, 5 and 10 min after injection of the contrast material, based on previous reports using animal and esophageal cancer patients.( 18 ) Suga et al. ( 18 ) reported that CT lymphography enabled direct visualization of the enhanced lymphatic vessels and enhanced lymph nodes within 3 min after injection of contrast agent in dogs, and time to greatest nodal enhancement was obtained at an average of 1.8 min (range, 1–3 min). Like this animal study, Hayashi et al. ( 16 ) demonstrated that enhanced lymphatic vessels and enhanced lymph nodes were observed within 5 min after contrast agent injection, peaking at 1 min after injection, in patients with superficial esophageal cancer; this was faster than our current results in gastric cancer. Our current data showed that 5 and 10 min after injection of contrast agent had higher detection rates than 1 and 3 min after injection, and it took an average 4.5 ± 3.5 min to have the greatest CT value of SN. Thus, we can conclude that 5 and 10 min after injection of contrast agent were best for detecting SN node in gastric cancer.
The molecular weight of nonionic contrast medium used in this study was the same as that of ICG, that is, 777.1 vs 775 Dalton, respectively. In CT lymphography, we injected half the amount of contrast agent used for an ICG injection, and this volume was reported to not cause pathological damage( 18 ) and did not interfere in the intraoperative SN detection methods using dye and RI in the current study. Therefore, in order to improve the sensitivity of SN detection by CT lymphography in human gastric cancer, further examinations are necessary regarding the optimal investigation period and the optimal amount of injection of contrast agent.
In gastrointestinal cancers, the lymphatic network may be more complex than in breast cancer, and it may be difficult to visualize all the SN distributed in unexpected areas by the vital dye method. Kitagawa et al. ( 21 ) demonstrated a relatively high incidence of direct distribution of radioactive tracer in the second tier lymph node in gastric cancer. This may be the major mechanism of so‐called skip metastasis in gastric cancer.( 22 ) In this regard, lymphoscintigraphy, as well as CT lymphography, may be useful for the preoperative detection of areas where SN are located.( 23 , 24 ) However, lymphoscintigraphy is inferior in various respects, such as the lack of detailed anatomical information, limited spatial resolution of images and the difficulty of SN detection adjacent to injection sites because of the thine‐through phenomenon.( 25 ) Thus, we can conclude that preoperative CT lymphography with the assistance of intraoperative conventional methods is a promising strategy for the detection of SN in gastric cancer, although the current, relatively low detection rate with CT lymphography alone needs to be improved.
The potential advantages of CT lymphography for SN detection are summarized as follows: (i) prior knowledge of SN locations using CT lymphography may be useful for intraoperative survey, especially if they are found in unexpected areas; (ii) surgeons can preoperatively predict the accurate anatomical location of primary areas and which stations are where the SN are distributed; and (iii) CT apparatus is available in all hospitals and no specific training or license is required for handling RI.
In conclusion, CT lymphography for the detection of SN in gastric cancer is feasible. However, our initial experiences with CT lymphography indicated a relatively low detection rate, and further effort is necessary to improve the detection rate and thus widen the clinical application of CT lymphography for the detection of SN in gastric cancer.
Abbreviations
- Ant
anterior wall
- BMI
body mass index
- CT
computed tomography
- Gre
greater curvature
- HU
Hounsfield units
- Less
lesser curvature
- LN
lymph node
- ND
not detected
- NE
not examined
- Post
posterior wall
- RI
radioisotope
- SN
sentinel node
Acknowledgments
The authors greatly thank Shigeru Kosuda and Yoshio Hoshito in the Department of Radiology, National Defense Medical College Hospital, for valuable contributions to this study.
References
- 1. Veronesi U, Paganelli G, Viale G et al. Sentinel‐lymph‐node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol 2006; 7: 983–90. [DOI] [PubMed] [Google Scholar]
- 2. Motomura K, Egawa C, Komoike Y et al. Sentinel node biopsy for breast cancer: technical aspects and controversies. Breast Cancer 2007; 14: 25–30. [DOI] [PubMed] [Google Scholar]
- 3. Ichikura T, Sugasawa H, Sakamoto N, Yaguchi Y, Tsujimoto H, Ono S. Limited gastrectomy with dissection of sentinel node stations for early gastric cancer with negative sentinel node biopsy. Ann Surg 2009; 249: 942–7. [DOI] [PubMed] [Google Scholar]
- 4. Ichikura T, Chochi K, Sugasawa H et al. Individualized surgery for early gastric cancer guided by sentinel node biopsy. Surgery 2006; 139: 501–7. [DOI] [PubMed] [Google Scholar]
- 5. Ichikura T, Morita D, Uchida T et al. Sentinel node concept in gastric carcinoma. World J Surg 2002; 26: 318–22. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi H, Ochiai T, Mori M et al. Sentinel lymph node mapping for gastric cancer using a dual procedure with dye‐ and gamma probe‐guided techniques. J Am Coll Surg 2003; 196: 68–74. [DOI] [PubMed] [Google Scholar]
- 7. Kitagawa Y, Saikawa Y, Takeuchi H et al. Sentinel node navigation in early stage gastric cancer – updated data and current status. Scand J Surg 2006; 95: 256–9. [DOI] [PubMed] [Google Scholar]
- 8. Miwa K, Kinami S, Taniguchi K, Fushida S, Fujimura T, Nonomura A. Mapping sentinel nodes in patients with early‐stage gastric carcinoma. Br J Surg 2003; 90: 178–82. [DOI] [PubMed] [Google Scholar]
- 9. Osaka H, Yashiro M, Sawada T, Katsuragi K, Hirakawa K. Is a lymph node detected by the dye‐guided method a true sentinel node in gastric cancer? Clin Cancer Res 2004; 10: 6912–8. [DOI] [PubMed] [Google Scholar]
- 10. Palaia R, Cremona F, Delrio P, Izzo F, Ruffolo F, Parisi V. Sentinel node biopsy in gastric cancer. J Chemother 1999; 11: 230–1. [DOI] [PubMed] [Google Scholar]
- 11. Lee JH, Ryu KW, Kim CG et al. Sentinel node biopsy using dye and isotope double tracers in early gastric cancer. Ann Surg Oncol 2006; 13: 1168–74. [DOI] [PubMed] [Google Scholar]
- 12. Schlag PM, Bembenek A, Schulze T. Sentinel node biopsy in gastrointestinal‐tract cancer. Eur J Cancer 2004; 40: 2022–32. [DOI] [PubMed] [Google Scholar]
- 13. Van Der Veen H, Hoekstra OS, Paul MA, Cuesta MA, Meijer S. Gamma probe‐guided sentinel node biopsy to select patients with melanoma for lymphadenectomy. Br J Surg 1994; 81: 1769–70. [DOI] [PubMed] [Google Scholar]
- 14. Albertini JJ, Lyman GH, Cox C et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 1996; 276: 1818–22. [PubMed] [Google Scholar]
- 15. Kim HJ, Kim AY, Oh ST et al. Gastric cancer staging at multi‐detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology 2005; 236: 879–85. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi H, Tangoku A, Suga K et al. CT lymphography‐navigated sentinel lymph node biopsy in patients with superficial esophageal cancer. Surgery 2006; 139: 224–35. [DOI] [PubMed] [Google Scholar]
- 17. Minohata J, Takao S, Hirokaga K. Sentinel lymph node biopsy using CT lymphography in breast cancer. Breast cancer 2009. [E pub ahead of publishing]. [DOI] [PubMed] [Google Scholar]
- 18. Suga K, Shimizu K, Kawakami Y et al. Lymphatic drainage from esophagogastric tract: feasibility of endoscopic CT lymphography for direct visualization of pathways. Radiology 2005; 237: 952–60. [DOI] [PubMed] [Google Scholar]
- 19. Japanese Gastric Cancer A . Japanese Classification of Gastric Carcinoma – 2nd English Edition. Gastric Cancer 1998; 1: 10–24. [DOI] [PubMed] [Google Scholar]
- 20. Morita D, Tsuda H, Ichikura T et al. Analysis of sentinel node involvement in gastric cancer. Clin Gastroenterol Hepatol 2007; 5: 1046–52. [DOI] [PubMed] [Google Scholar]
- 21. Kitagawa Y, Fujii H, Mukai M, Kubota T, Otani Y, Kitajima M. Radio‐guided sentinel node detection for gastric cancer. Br J Surg 2002; 89: 604–8. [DOI] [PubMed] [Google Scholar]
- 22. Lee SE, Lee JH, Ryu KW et al. Sentinel node mapping and skip metastases in patients with early gastric cancer. Ann Surg Oncol 2009; 16: 603–8. [DOI] [PubMed] [Google Scholar]
- 23. Khafif A, Schneebaum S, Fliss DM et al. Lymphoscintigraphy for sentinel node mapping using a hybrid single photon emission CT (SPECT)/CT system in oral cavity squamous cell carcinoma. Head Neck 2006; 28: 874–9. [DOI] [PubMed] [Google Scholar]
- 24. Nakahara T, Kitagawa Y, Yakeuchi H et al. Preoperative lymphoscintigraphy for detection of sentinel lymph node in patients with gastric cancer – initial experience. Ann Surg Oncol 2008; 15: 1447–53. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz GF, Giuliano AE, Veronesi U. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer 2002; 94: 2542–51. [DOI] [PubMed] [Google Scholar]


