Abstract
The E2 protein of hepatitis C virus (HCV) is believed to be a virion surface glycoprotein that is a candidate for inclusion in an antiviral vaccine. A truncated soluble version of E2 has recently been shown to interact with CD81, suggesting that this protein may be a component of the receptor for HCV. When expressed in eukaryotic cells, a significant proportion of E2 forms misfolded aggregates. To analyze the specificity of interaction between E2 and CD81, the aggregated and monomeric forms of a truncated E2 glycoprotein (E2661) were separated by high-pressure liquid chromatography and analyzed for CD81 binding. Nonaggregated forms of E2 preferentially bound CD81 and a number of conformation-dependent monoclonal antibodies (MAbs). Furthermore, intracellular forms of E2661 were found to bind CD81 with greater affinity than the extracellular forms. Intracellular and secreted forms of E2661 were also found to differ in reactivity with MAbs and human sera, consistent with differences in antigenicity. Together, these data indicate that proper folding of E2 is important for its interaction with CD81 and that modifications of glycans can modulate this interaction. Identification of the biologically active forms of E2 will assist in the future design of vaccines to protect against HCV infection.
Enveloped viruses acquire their lipid membranes by budding through host cellular membranes (reviewed in reference 25). The majority of enveloped viruses bud at the plasma membrane. However, several viruses assemble and bud at internal membranes such as those of the endoplasmic reticulum (ER; e.g., rotaviruses), ER-Golgi intermediate compartments (e.g., coronaviruses), or the Golgi complex (e.g., bunyaviruses). This behavior generally reflects the targeting of the viral glycoproteins within subcompartments of the ER or Golgi apparatus. Internally budding viruses may be released from infected cells either by cell lysis or after transport through the cellular secretory pathway to the cell surface.
Hepatitis C virus (HCV), the major cause of non-A, non-B hepatitis, is an enveloped virus classified in the Flaviviridae family (reviewed in references 4 and 28). The genome encodes two putative envelope glycoproteins, E1 (polyprotein residues 192 to 383) and E2 (residues 384 to 746), which are released from the viral polyprotein by signal peptidase cleavages (14, 15, 30). Both glycoproteins are heavily modified by N-linked glycosylation and are believed to be type I integral transmembrane proteins, with C-terminal hydrophobic anchor domains. Expression of the E1 and E2 glycoproteins in mammalian cell lines demonstrates their ER retention with no cell surface glycoprotein expression detectable (9, 10, 27, 32, 34). Immunoelectron microscopic studies localized the glycoproteins to the ER (7, 9). The presence of ER retention signals within the C-terminal regions of both E1 and E2 (5, 6, 13) could explain these observations. Consistent with these data, truncation of E2 at its C terminus leads to its secretion from expressing cells (20, 22, 32, 34). These observations suggest that HCV particle morphogenesis occurs by budding into the ER and subsequent transport of viral particles through the host cell secretory pathway before release into the extracellular space. Modification of flavivirus E and prM protein glycans by trimming and terminal addition suggests that virions do indeed move through an exocytosis pathway similar to that used for host glycoproteins (19, 23). In addition, an analysis of the lectin-binding properties of virions recovered from the sera of chronic hepatitis C patients indicated that the virions contained complex N-linked glycans (31).
When expressed in vitro, the E1 and E2 glycoproteins interact to form noncovalently linked complexes, the size of which is consistent with E1-E2 heterodimers (7, 9). In addition to these noncovalently associated E1-E2 complexes, significant proportions of E1 and E2 are present in disulfide-linked aggregates, which are believed to result from a nonproductive folding pathway (3, 7, 9, 14). Since HCV cannot be propagated efficiently in vitro, it has been difficult to study native E1 and E2 glycoprotein forms as they exist on the virus particle. It is critical when studying the biological activity of the HCV glycoproteins to distinguish between molecules undergoing productive folding and assembly and those following a nonproductive pathway(s) resulting in misfolding and aggregation (8). Recently, a number of conformation-dependent monoclonal antibodies (MAbs H2 and H53) which specifically recognize non-disulfide-bridged E2, have been reported, allowing the study of glycoprotein complexes which may represent native prebudding forms of the HCV glycoprotein complex (6, 7, 22).
The mechanism by which HCV enters target cells is not known; however, the E2 glycoprotein is thought to be responsible for initiating virus attachment to a receptor on potential host cells (29). Indeed, a C-terminal truncated E2 glycoprotein was used to identify CD81 as a putative receptor for HCV (26). CD81 is a broadly expressed protein and is reported to be involved in a variety of biologic responses including cell adhesion, morphology, proliferation, activation, and differentiation of T cells, B cells, and other cell types (17). The E2 glycoprotein has been considered a potential antigen for use in a vaccine against HCV infection. In the chimpanzee model, protection may be achieved by vaccination with recombinant E1-E2, where anti-E2 antibody titers appear to correlate with protection (2). Given the heterogeneity observed when E2 is expressed in tissue culture cells (3, 7, 9, 14), it is important to determine the biologically active forms of E2 to enable optimal vaccine design. Previously, we have demonstrated that a secreted, truncated version of the E2 glycoprotein (E2661) is able to bind to CD81 (12). Here, we report antigenic differences in the secreted and cell-associated forms of E2661, which may be glycosylation dependent. Disulfide-bridged aggregates are present in both secreted and cell-associated forms of E2661; however, the monomeric, nonaggregated form preferentially binds CD81 and a number of conformation-sensitive MAbs. Furthermore, intracellular forms of E2661 were found to bind CD81 with greater affinity than the extracellular form.
MATERIALS AND METHODS
Materials.
MAbs specific for E2 (6/16, 9/27, 9/86a, 3/11, 1/39, 11/20c, 6/1a, 6/53, 6/41a, and 9/75) have been described elsewhere (12; C. Shotton, C. Maidens, M. Flint, I. M. Jones, L. Loomis-Price, and J. A. McKeating, submitted for publication) and were generated from rats after immunization with either baculovirus-expressed E1-E2 complexes or mammalian cell (human embryonic kidney [HEK])-expressed secreted E2661 glycoprotein. The MAbs were epitope mapped by using overlapping peptides as shown in Table 1.
TABLE 1.
E2-specific MAbs used in this study
| MAb | Epitope |
|---|---|
| 6/16 | aa 384–391 in the HVR |
| 9/27 | Conformation-dependent epitope in the HVR |
| 9/86a | Conformation-dependent epitope in the HVR |
| 3/11 | aa 412–423 |
| 1/39 | aa 432–443 |
| 11/20c | aa 436–447 |
| 6/1a | aa 464–471 |
| 6/41a | aa 480–493 |
| 9/75 | aa 524–531 |
| 6/53 | aa 544–551 |
The E2-specific conformation-dependent MAbs (H53, H35, and H54) were generated as previously reported (7). Anti-CD81 MAb 1.3.3.22 was purchased from Santa Cruz Biotechnology Inc., Santa Cruz, Calif. HCV-positive human sera (gift from P. Simmonds, University of Edinburgh) were obtained from a number of individuals with chronic infection. Phycoerythrin (PE)- and horseradish peroxidase HRP-conjugated antibodies were purchased from Harlan Sera-Labs. RBL and human CD81 (hCD81)-transfected RBL (RBL-hCD81) cells, described previously (12), were propagated in 5% fetal calf serum (FCS)–Dulbecco modified Eagle medium (Life Technologies, Gibco-BRL) containing G418 (400 μg/ml) for the hCD81-transfected line (gift from P. Monk, University of Sheffield). Molt 4 cells were obtained from the Medical Research Council ADP Repository and were propagated in 10% FCS–RPMI medium (Life Technologies, Gibco-BRL). Recombinant human immunodeficiency virus type 1 (HIV-1) glycoprotein gp120 expressed in CHO cells was obtained from the Medical Research Council AIDS Directed Programme (ADP) Repository. Pooled HIV-positive human sera (QC256) were used to visualize GNA (Galanthus nivalis) lectin-captured gp120 as previously described (21). Recombinant glutathione S-transferase (GST) fusion proteins expressing the second extracellular loop (EC2) from both hCD81 and murine CD81 (mCD81) were constructed and expressed as previously described (11, 12).
Expression of recombinant proteins.
Expression of soluble secreted recombinant E2661 by transient expression in HEK (cell line 293) cells has been described previously (12). Briefly, the eukaryotic expression vector encoding E2661 (p14.tE2.661.hiv) was transfected into 293 cells cultured in 100-mm-diameter dishes by the calcium phosphate precipitation method. After 72 h of incubation at 37°C, the medium was removed, clarified by centrifugation, and used as a source of secreted E2661. The cells were removed from the tissue culture dishes by phosphate-buffered saline (PBS)-EDTA treatment, resuspended in PBS, and lysed by a single freeze-thaw. After clarification, this lysate was used as a source of cell-associated E2661.
Western blotting.
After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), E2 protein was detected by Western blotting using rat anti-E2 MAbs 7/16, 3/11, and 6/53. Hybridoma supernatants were used at a 1:10 dilution. Proteins were detected via an anti-rat HRP-conjugated secondary antibody, enhanced chemiluminescence detection reagents (Amersham Life Sciences), and exposure to photographic film.
Flow cytometric analysis of E2-cell binding.
The interaction of E2661 glycoprotein with cells was quantified by using a fluorescence-activated cell sorting (FACS)-based assay. In brief, cells under test were washed twice in PBS–1% FCS–0.05% sodium azide (wash buffer) and resuspended at 2 × 106/ml; 2 × 105 cells were incubated with E2661 (containing a known amount of MAb H53-reactive antigen) at room temperature for 1 h, and unbound antigen was removed by two washes in wash buffer. Cells were incubated with MAb H53 (1.0 μg/ml) for 1 h at room temperature. Finally, cell-bound MAb was visualized with an anti-mouse immunoglobulin G-PE conjugate (Seralabs, Loughborough, United Kingdom) and analyzed by FACS (Becton Dickinson, Oxford, United Kingdom). Median fluorescence intensities (FIs) were determined by using CellQuest software (Becton Dickinson).
GNA lectin capture EIA and CD81-capture EIA.
Briefly, GNA lectin (Boehringer GmbH, Mannheim, Germany) was used to coat Immulon II enzyme immunoassay (EIA) plates (Dynal, Wirral, United Kingdom) at 1 μg/ml overnight at 4°C. After being washed in Tris-buffered saline, the plates were blocked with 4% milk powder (Cadburys, Stafford, United Kingdom), and E2661 or gp120 was allowed to bind for 2 h at room temperature. Bound antigen was visualized with MAbs specific for E2 or pooled HIV-positive human sera (QC256), an antispecies immunoglobulin G-HRP conjugate (Seralabs), and tetramethylbenzidine substrate. Absorbance values were determined at 450 nm (Dynatech, Billinghurst, United Kingdom). Purified E2 protein was used as a calibrant to enable quantification of E2 levels in transient transfection samples.
GST-hCD81 and GST-mCD81 fusion proteins expressing the EC2 loop were used to coat Immulon II EIA plates (Dynal) at 0.5 μg/ml and 37°C for 4 h. The ability of E2 protein to specifically bind hCD81 was assessed as described above for the GNA lectin capture EIA, using MAb H53 to detect CD81-captured E2.
HPLC separation of E2661.
Gel filtration chromatography was performed with a TSK G4000PWXL (7.8 by 300 mm) column. The solvent used was PBS at a flow rate of 300 μl/min. Column temperature was maintained at 30°C, and absorbance was monitored at 214 and 280 nm. Samples were injected in a 600-μl volume of PBS, and 600-μl fractions were collected at 2-min intervals, starting 10 min after injection of the sample. Fractions 12 to 19 from the high-pressure liquid chromatography (HPLC) analysis were found to contain E2 by Western blotting and EIA. Each fraction was serially diluted and assayed by GNA lectin capture EIA using either MAb 3/11 (specific for a linear epitope) or MAb H53 (specific for a conformation-dependent epitope) for detection. In addition, samples were quantified for the ability to bind a recombinant form of CD81. At the dilutions used, the optical density obtained was shown to be proportional to the amount of protein present by titration against a standard E2 preparation. From this, the total amount of 3/11-, H53-, and CD81-reactive material present in all fractions was determined. The reactivity present in individual fractions was determined.
RESULTS
The majority of secreted E2661 is unable to bind CD81.
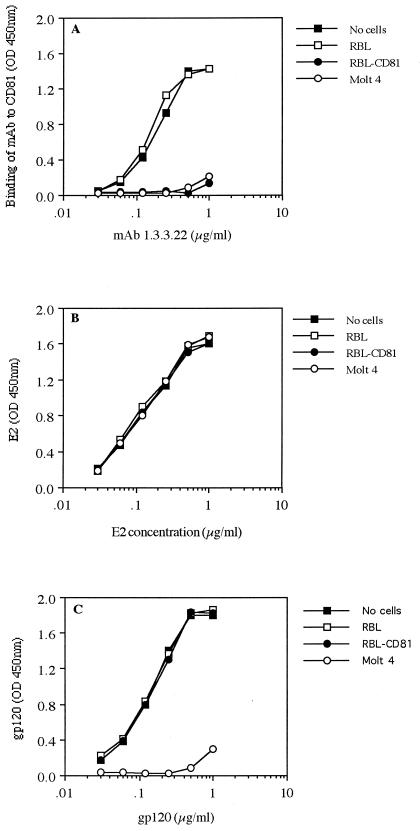
Truncation of the HCV E2 glycoprotein at its C terminus results in secretion of a soluble protein that is able to bind a putative cellular receptor, CD81 (12, 26). To determine the proportion of secreted E2661 able to bind CD81, a soluble antigen preparation was incubated with the rat granulocyte cell line RBL, which does not express hCD81, and the stably transfected clone, RBL-hCD81. In addition, the CD4-positive T-lymphoblastoid cell line Molt 4, which naturally expresses hCD81, was used to attempt to deplete soluble E2661 from the preparation. E2661 glycoprotein was incubated with the various cell types for 2 h at 37°C, the cells were removed by centrifugation, and the residual non-cell-bound E2661 was assayed in a quantitative GNA lectin capture EIA. As a control for this experiment, the ability of CD81-expressing cells to deplete an anti-CD81 MAb (1.3.3.22) from solution was tested. Activity of the cell-free MAb was detected by recognition of a recombinant fusion protein containing the EC2 loop of hCD81 (GST-hCD81) in EIA. Both RBL-CD81 and Molt 4 cells were able to deplete the anti-CD81 MAb from solution, whereas the parental RBL cells were not (Fig. 1A). Similarly, a recombinant form of HIV-1 gp120 could be depleted from solution by preadsorption with Molt 4 cells expressing the principal receptor for HIV, CD4, an interaction mediated via gp120. Incubation of gp120 with either of the RBL cell lines had no effect on antigen levels detected in the EIA (Fig. 1C). In contrast, incubation of E2661 with CD81-expressing cells had minimal effects on the subsequent EIA quantification of total E2 present in the extracellular fluid, using the detection MAb 3/11 (Fig. 1B). It is worth noting that FACS analysis of cells after incubation with E2661 demonstrated minimal but significant levels of cell-bound antigen as detected with MAb H53, such that the percentages of cells staining and median FI signals were as follows: RBL, 0.3% and median FI 3.6; RBL-CD81, 5% and median FI 10.5; and Molt 4, 8.7% and median FI 12.4. Similar results were obtained upon incubation of cell-associated E2661 with CD81-expressing cells (data not shown). These data suggest that the E2-CD81 interaction may be a low-affinity interaction with a high off rate. Alternatively, the majority of E2661 antigen, whether cell associated or extracellular, may not be in the appropriate conformation to bind CD81. Furthermore, since E2 naturally exists in association with E1, the formation of oligomeric glycoprotein structures may affect processing and subsequent receptor interaction.
FIG. 1.
The majority of E2661 is unable to bind CD81. To assess the binding of E2661 to CD81, the abilities of various cell types to deplete soluble protein were tested. Cells (5 × 106) were incubated with the protein(s) for 2 h at 37°C and removed by centrifugation, and non-cell-bound protein was assayed by EIA. (A) Depletion of anti-CD81 MAb 1.3.3.22 by CD81-expressing cells. Residual, non-cell-bound MAb was assayed by recognition of GST-hCD81 and compared to results for an untreated preparation of MAb. (B) Incubation of soluble recombinant E2661 with CD81-expressing cells. Cell-free residual E2661 was quantified by GNA lectin capture EIA, using the E2-specific MAb 3/11 for visualization. (C) Abilities of CD4-expressing cells to deplete recombinant HIV-1 gp120 from solution. Cell-free gp120 was detected by EIA with pooled HIV-positive sera. The data presented are the means of duplicate samples from a single experiment; comparable data were obtained in two additional experiments. OD, optical density.
Analysis of cell-associated and secreted forms of E2661.
The HCV glycoproteins have a tendency to form disulfide-linked aggregates, which are believed to result from a nonproductive folding pathway (8). The formation of such aggregates may explain the low CD81-binding activity that we observed. To determine the extent of disulfide-linked aggregation within the truncated protein preparation, E2661 was transiently expressed in HEK cells. The cell-associated and secreted forms of E2661 were analyzed by SDS-PAGE under reducing and nonreducing conditions, followed by immunoblotting (Fig. 2A). When analyzed under nonreducing conditions, disulfide-linked aggregates were observed in both secreted and cell-associated preparations. Due to the large size of the aggregates, however, a complete transfer to nitrocellulose probably does not occur. Thus, the level of aggregated E2661 observed by immunoblotting may be an underrepresentation of that present in the preparations. Comparison of cell-associated and secreted E2661 under reducing conditions showed that the secreted form migrated more slowly during SDS-PAGE (Fig. 2A). This observation is consistent with the acquisition of complex sugars during transit through the host cell secretory pathway and has been observed previously (22). When analyzed under reducing conditions, the secreted E2661 protein was apparent as a single broad band, representing nonaggregated, monomeric E2661. The molecular weight of this form of E2661, given the extensive N-linked glycosylation of E2, was consistent with it being monomeric. A proportion of the nonreduced E2661 comigrated with this nonaggregated band, indicating that some monomeric E2661 was present in the secreted preparation. A smear was observed within the cell-associated preparation, presumably representing different glycoforms of E2661. Deglycosylation using peptide N-glycosidase F caused both cell-associated and secreted E2661 to comigrate by SDS-PAGE (Fig. 2B), indicating that the migrational differences between the cell-associated and secreted forms were due to differences in glycosylation.
FIG. 2.
Analysis of intracellular and secreted forms of E2661. Transient expression of E2661 in HEK (293) cells was used to obtain E2661 protein. (A) E2661 consists of monomers and disulfide-linked aggregates. Cell-associated (Cell) and secreted (Sup) forms of E2661 were analyzed by SDS-PAGE under reducing and nonreducing conditions, followed by immunoblotting with anti-E2 MAbs. Monomeric and aggregated forms of E2661 (as assigned by predicted molecular weight) are indicated; molecular masses in kilodaltons are given on the left. (B) Presence of different glycoforms in the cellular antigen preparation. Cell-associated and secreted E2661 was untreated (No IP) or immunoprecipitated with anti-E2 MAbs and then untreated (−) or treated with peptide N-glycosidase F (F). Proteins were then analyzed by reducing SDS-PAGE and Western blotting for E2. Deglycosylated E2661 is indicated (*).
Only monomeric, nonaggregated E2 can bind CD81.
To determine if both monomeric and aggregated E2661 were able to bind CD81, a preparation of secreted E2661 was separated by HPLC. Fractions were analysed by nonreducing SDS-PAGE and immunoblotting (Fig. 3A). Fractions 17 and 18 contained only monomeric E2661, while fraction 14 consisted of mainly aggregates. Fractions 15 and 16 were found to contain a mixture of both monomeric and disulfide-bridged E2661. These data indicate that HPLC can separate the monomeric and aggregated forms of E2661. The fractions were assayed for the ability to bind recombinant GST-hCD81 and MAbs H53 and 3/11 (Fig. 3B). Recognition by MAb 3/11, specific for a linear epitope, correlated with the total amount of E2661 detected by immunoblotting. Consistent with its ability to bind nonaggregated E2661, MAb H53 was able to recognize E2661 in fractions 17 and 18. When assayed for binding to GST-hCD81 in EIA, the monomeric E2661 present in fractions 17 and 18 bound well, while the aggregates in fraction 14 bound poorly. These observations are consistent with the model that disulfide-bridged E2 aggregates are the product of a nonproductive folding pathway. They further suggest that recognition by MAb H53 correlates with an E2 conformation capable of binding CD81.
FIG. 3.
HPLC separation of aggregated and nonaggregated forms of E2. (A) A preparation of secreted E2661 (lane 0) was applied to an HPLC column. Fractions were collected, separated by nonreducing SDS-PAGE, and visualized by immunoblotting with anti-E2 MAbs. Sizes are indicated in kilodaltons on the left. (B) Fractions were evaluated for reactivity with MAbs 3/11, H53, and GST-CD81 by EIA, and reactivity is shown as a percentage of the total. The data presented are from a single HPLC profile, and the EIA data (B) represent the mean E2 reactivity determined from duplicate samples. Comparable data were obtained in a second experiment.
Antigenic characterization of secreted and cell-associated E2661.
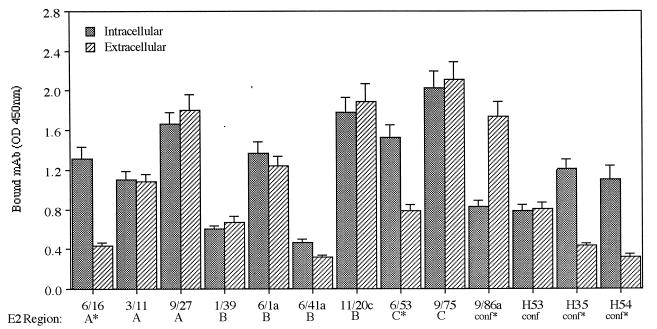
To investigate differences in the conformations of secreted and cell-associated E2661, recognition of these proteins by a panel of MAbs was investigated by EIA (Fig. 4 and 5). A number of MAbs recognizing both conformational and linear epitopes were selected. The linear epitopes reside within the arbitrarily defined regions of E2, A to C (12), based on mapping studies and are detailed further in Table 1 and Materials and Methods. MAbs 6/16, 6/53, H35, and H54 all bound the cell-associated antigen better than the secreted protein, whereas MAb 9/86a, specific for a conformational determinant within the hypervariable region (HVR), bound with greater relative affinity to the secreted form of E2661. It is interesting that MAbs H35, H54, 6/16, and 6/53 were raised against cell-associated antigen, whereas MAb 9/86a was raised against a secreted form of E2661. All of the remaining MAbs showed equivalent recognition of cell-associated and secreted E2661 antigen (Fig. 4). The epitopes recognized by MAbs 6/16 amino acids [aa 384 to 391; ETHVTGGS within the HVR) and 6/53 (aa 544 to 551; PPLGNWFG) do not encode predicted N-linked glycosylation sites; however, the 6/53 epitope is flanked by two predicted sites (NNTRPPLGNWFGCTWMNST). MAbs H35 and H54, showing differences in recognition between the antigens, are both specific for conformation-dependent epitopes.
FIG. 4.
Antigenic characterization of intracellular and secreted forms of E2661 based on recognition by a panel of anti-E2 MAbs in GNA lectin capture EIA. The epitopes recognized by these MAbs fall into three arbitrarily defined regions of E2 (A to C) or are conformational in nature. An asterisk indicates that the MAb was raised against a cell-associated E2 antigen. The data represent the mean ± standard error of triplicate measurements within a single experiment; comparable data were obtained in two additional experiments. OD, optical density.
FIG. 5.
Titration of the recognition of intracellular (●) and secreted (○) E2661 by MAbs H35 (A), H54 (B), 9/86a (C), and H53 (D) in a GNA lectin capture EIA. The data presented are the mean value of two samples from a single experiment; comparable data were obtained in two additional experiments. OD, optical density.
In addition, we compared the abilities of human sera from a number of chronically infected individuals to recognize either intracellular or secreted E2661 (Fig. 6). The majority of sera were previously shown not to react with denatured E2661, suggesting that most of the antibody response was specific for conformation-dependent epitopes (data not shown). Of the 58 sera tested, 21 could discriminate between the two forms of antigen, showing increased reactivity with the intracellular form of E2 (Wilcoxon U = 52, P < 0.0001). The remaining sera reacted equivalently with both forms of antigen.
FIG. 6.
Human serum reactivity with intracellular and secreted forms of E2661. Serum samples from 58 individuals chronically infected with HCV were tested for the ability to recognize cell-associated and secreted E2661 by GNA lectin capture EIA. Reciprocal serum endpoint titers are shown.
Comparative ability of intracellular and secreted forms of E2661 to bind CD81.
Since MAb H53 is able to distinguish between aggregated and nonaggregated forms of E2 (Fig. 3B) and reacts equivalently with intracellular and secreted E2 it is an ideal probe to assess the amount of nonaggregated material in different E2 preparations. The ratio of H53-reactive to total E2661 in several preparations varied between 0.8 and 2.4 (data not shown). Equal amounts of aggregated material were generally found within the intracellular and secreted forms of E2661 (data not shown). We compared the abilities of equivalent amounts of H53-reactive intracellular and secreted E2661 to bind RBL-CD81 cells by FACS. Both glycoproteins failed to bind to the parental RBL cells (mean FI of 7.6), whereas mean FIs were 286 and 96.0 for intracellular E2661 and secreted E2661, respectively (Fig. 7A). To further quantify this interaction, we established an EIA to measure CD81-E2 interactions, based on a GST-hCD81 fusion protein capture (12). E2661 binding in this EIA is dependent on the concentration of GST-hCD81 protein present and is specific for the hCD81 sequence, showing no interaction with GST-fusion protein expressing the murine EC2 sequence (GST-mCD81) (Fig. 7B). Equivalent amounts of H53-reactive intracellular and secreted E2661 were quantified for the ability to bind hCD81 in the EIA (Fig. 7C). The intracellular form was found to saturate hCD81 at lower levels of antigen compared to the secreted form, consistent with it having a greater relative affinity.
FIG. 7.
Comparative abilities of intracellular and secreted forms of nonaggregated E2661 to bind CD81. (A) Equivalent amounts of H53-reactive intracellular and secreted E2661 were assessed for the ability to bind CD81-expressing cells. RBL-hCD81 cells were incubated with secreted (bold line) or cell-associated (thin line) E2661. Binding was detected with anti-E2 MAb H53 and an anti-mouse PE conjugate and analyzed by FACS. Cells incubated with no E2661 are indicated by the filled graph. (B) An EIA based on capture of E2661 by a recombinant fusion protein consisting of GST and the EC2 loop of CD81 was established. Binding of E2661 to hCD81 (●) or mCD81 (○) sequence is shown. (C) Binding of intracellular and secreted forms of E2 to GST-hCD81. Equivalent amounts of MAb H53-reactive secreted (○) or cell-associated (●) E2661 were tested for the ability to bind GST-hCD81 in EIA. The data represent the mean of duplicate (B) or triplicate (C) measurements within a single experiment; error bars represent the standard error of triplicate samples. Comparable data were obtained in two additional experiments. OD, optical density.
DISCUSSION
The E2 glycoprotein has been suggested to be a key antigen for the development of a vaccine against HCV. Indeed, in a chimpanzee model, protection from challenge could be induced by vaccination with recombinant E1E2 proteins, and this correlated with the titer of anti-E2 antibodies elicited (2). A truncated, secreted version of the E2 protein has previously been shown to bind the putative HCV receptor CD81 (12, 26). Here, we describe a functional analysis of the cell-associated and secreted forms of this truncated E2. Several of these findings may have implications for the design of a successful E2-based vaccines. First, antigenic differences between the cell-associated and secreted form of E2661 were detected (Fig. 4 to 6). Second, we found that the majority of E2661 was unable to bind CD81 (Fig. 1). Third, disulfide-bridged, aggregated E2661 did not bind CD81 (Fig. 3). These observations suggest that the source of antigen for an E2-based subunit vaccine(s) should be chosen carefully, ideally to optimize the levels of E2 in a suitable conformation, capable of interacting with CD81. The immunogenicity of intracellular and secreted forms of E2 antigen and their ability to elicit protective antibody responses are not known. Recently, we have shown that immunization of rats with a secreted form of E2661 induces a polyclonal antibody response able to recognize both intracellular and secreted forms of E2, with preferential recognition of secreted E2, as demonstrated by MAb 9/86a (Fig. 4 and 5) (Shotton et al., submitted).
It was interesting that some human sera bound with greater affinity to intracellular antigen compared to the secreted form (Fig. 6), suggesting that some individuals have an antibody response to specific epitopes which are differentially exposed on intracellular and secreted forms of E2. The ability of sera to discriminate between the antigen preparations did not correlate with the clinical status of the patient, the genotype of the infecting HCV strain, the endpoint titer of the serum for E2, or the ability of the sera to recognize native versus SDS- or DTT-denatured antigens (data not shown). Furthermore, the 21 sera showing preferable reactivity with intracellular E2661 did not show any differences in the ability to inhibit E2661 binding to CD81 (J. A. McKeating, unpublished data), a surrogate marker for neutralizing antibodies (D. Y. Chien, P. Arcangel, G. Kuo, P. Pileri, S. Coates, M. Baumeister, M. Houghton, and S. Abrignani, Design of a quantitative hCD81-HCV envelope binding assay to evaluate envelope binding and antibody titres in vaccinated animals, presented at the 6th International Symposium on Hepatitis C and Related Viruses, Washington, D.C., 6 to 9 June, 1999), suggesting that such antibody responses had no protective advantage for the individual.
The secreted monomeric and aggregated forms of E2661 could be separated by HPLC (Fig. 3). We found that monomeric E2661 was able to bind CD81, while the aggregated E2661 was not. This observation is consistent with previous data suggesting that aggregation results from a nonproductive folding pathway (3, 7). We have found that divergent truncated E2 glycoproteins cloned from different sources can vary both in the ability to bind CD81 and the ability to form disulfide-linked aggregates (McKeating, unpublished data). Clearly, care has to be taken in comparing the affinities of different antigens for CD81 to consider both the extent of E2 aggregation and whether intracellular or secreted antigens are being studied. Unfortunately, MAb H53 is restricted in recognizing the strain H (genotype 1a) E2 sequence and will not be a universal tool for quantifying the levels of nonaggregated native E2 gp present in a preparation. However, a number of conformation-dependent human MAbs have recently been found; these MAbs show broad patterns of cross-reactivity and may prove useful in this regard (K. G. Hadlock, R. Lanford, S. Perkins, J. Rowe, Q. Yang, S. Levy, S. Abrignani, and S. K. H. Foung, submitted for publication).
If CD81 is the primary receptor for HCV, our observations imply that the aggregated form of E2 may not be incorporated into HCV virions. Even though the formation of aggregates is observed when E2 is expressed in several cell lines and with several different expression systems, such disulfide-bridging may be an artifact of relatively high-level expression within tissue culture cells. The level of HCV replication, and therefore E2 expression, may be so low within infected cells in vivo that the nonproductive folding pathway is not followed. This may present a significant barrier to the development of a tissue culture system capable of supporting efficient HCV replication. Alternatively, the disulfide-bridging event may occur in vivo, and the aggregates so formed may actually play a role in the HCV life-cycle (A. Choukhi, A. Pillez, H. Drobecq, C. Sergheraert, C. Wychowski, and J. Dubuisson, submitted for publication). It is known that accumulation of misfolded proteins within the ER activates an intracellular signalling pathway known as the ER stress response (24). Indeed, Liberman and colleagues (18) recently reported that intracellular ER-retained E2 glycoprotein could activate the promoters of the grp78 (BiP) and grp94 chaperones. Since overexpression of grp78 has been reported to decrease the sensitivity of cells to cytotoxic T-cell killing, this activity may be important in HCV persistence and pathogenesis. Further work will be necessary to define the possible role(s) for aggregated E2 in HCV persistence and pathogenesis.
The cell-associated form of E2661 was found to bind CD81 with a greater affinity than the secreted form (Fig. 7). One possible reason for this observation could be that the expressing cell contains a glycoform of E2661 with a particularly high affinity for CD81. This glycoform may be modified during transit through the secretory pathway and therefore be absent from the secreted preparations of E2661. However, when cell-associated E2661 was bound to CD81-expressing cells and subsequently analyzed by SDS-PAGE and immunoblotting, a heterogeneous population of E2661 molecules was observed (data not shown). A similar result was observed when secreted E2661 was bound to CD81-expressing cells and analyzed by immunoblotting. These data suggest that multiple glycoforms of E2661 are capable of binding CD81. An alternative explanation of the higher affinity of the cell-associated protein for CD81 is that the addition of complex sugars and glycan trimming may sterically reduce the availability of the CD81 binding site on E2661. It is possible that virion-bound E2 does not undergo the glycan modifications observed during E2661 processing and secretion. Clearly, the various strategies of expressing E2 at the plasma membrane (11, 16) with the intention of studying HCV glycoprotein-mediated cell fusion may be compromised by the reduced affinity of cell surface-expressed E2 for CD81. One must consider that association of E2 with E1, and subsequent heterodimer formation (7, 9), may affect E2 glycosylation. In a study on the oligomerization of Semliki Forest virus (SFV) membrane proteins, Barth and colleagues (1) found that of the two N-linked glycans on the SFV E2 protein (when in E2-E1 heterodimers), one was retained in an untrimmed, endo-β-N-acetylglucosaminidase H-sensitive form. When the SFV E2 protein was expressed in the absence of SFV E1, however, both glycans were trimmed. Clearly, it will be important to assess the glycosylation status of virion bound E1 and E2 proteins to enable the selection of a recombinant antigen which optimally mimics the native form present within the virus.
ACKNOWLEDGMENTS
We are indebted to Yasmin Chaudhry, Louise Wilson, Barbara Konig, and André Pillez for excellent technical assistance and to Shoshana Levy for providing recombinant CD81.
M.F. was supported by The Wellcome Trust. J.D. was supported by grant 9736 from the Association pour la Recherche sur le Cancer. J.A.M. was supported by The Lister Institute for Preventive Medicine.
Footnotes
Publication no. 10 from the Edward Jenner Institute for Vaccine Research.
REFERENCES
- 1.Barth, B.-U., J. M. Wahlberg, and H. Garroff. The oligomerization reaction of the Semliki forest virus membrane protein subunits. J. Cell Biol. 128:283–291. [DOI] [PMC free article] [PubMed]
- 2.Choo Q-L, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kuo C, Kansopon J, McFarland J, Tabrizi A, Ching K, Moss B, Cummins L B, Houghton M, Muchmore E. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choukhi A, Ung S, Wychowski C, Dubuisson J. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J Virol. 1998;72:3851–3858. doi: 10.1128/jvi.72.5.3851-3858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke B. Molecular virology of hepatitis C virus. J Gen Virol. 1997;78:2397–2410. doi: 10.1099/0022-1317-78-10-2397. [DOI] [PubMed] [Google Scholar]
- 5.Cocquerel L, Duvet S, Meunier J-C, Pillez A, Cacan R, Wychowski C, Dubuisson J. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J Virol. 1999;73:2641–2649. doi: 10.1128/jvi.73.4.2641-2649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocquerel L, Meunier J-C, Pillez A, Wychowski C, Dubuisson J. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J Virol. 1998;72:2183–2191. doi: 10.1128/jvi.72.3.2183-2191.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deleersnyder V, Pillez A, Wychowski C, Blight K, Xu J, Hahn Y S, Rice C M, Dubuisson J. Formation of native hepatitis C virus glycoprotein complexes. J Virol. 1997;71:697–704. doi: 10.1128/jvi.71.1.697-704.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubuisson J. Folding, assembly and subcellular localization of HCV glycoproteins. Curr Top Microbiol Immunol. 1999;242:135–148. doi: 10.1007/978-3-642-59605-6_7. [DOI] [PubMed] [Google Scholar]
- 9.Dubuisson J, Hsu H H, Cheung R C, Greenberg H B, Russell D G, Rice C M. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J Virol. 1994;68:6147–6160. doi: 10.1128/jvi.68.10.6147-6160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvet S, Cocquerel L, Pillez A, Cacan R, Verbert A, Moradpour D, Wychowski C, Dubuisson J. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J Biol Chem. 1998;273:32088–32095. doi: 10.1074/jbc.273.48.32088. [DOI] [PubMed] [Google Scholar]
- 11.Flint M, Thomas J M, Maidens C, Shotton C, Levy S, Barclay W, McKeating J A. Functional analysis of cell surface-expressed hepatitis C virus E2 glycoprotein. J Virol. 1999;73:6782–6790. doi: 10.1128/jvi.73.8.6782-6790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flint M, Maidens C, Shotton C, Loomis-Price L, Dubuisson J, Monk P, Levy S, McKeating J A. Characterization of hepatitis C virus E2 glycoprotein interaction with the putative cellular receptor, CD81. J Virol. 1999;73:6235–6244. doi: 10.1128/jvi.73.8.6235-6244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flint M, McKeating J A. The C-terminal region of the hepatitis C virus E1 glycoprotein confers localization within the endoplasmic reticulum. J Gen Virol. 1999;80:1943–1947. doi: 10.1099/0022-1317-80-8-1943. [DOI] [PubMed] [Google Scholar]
- 14.Grakoui A, Wychowski C, Lin C, Feinstone S M, Rice C M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67:1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hijikata M, Kato N, Ootsuyama Y, Nakagawa M, Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci USA. 1991;88:5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagging L M, Meyer K, Owens R J, Ray R. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J Virol. 1998;72:3539–3546. doi: 10.1128/jvi.72.5.3539-3546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy S, Todd S C, Maecker H T. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 18.Liberman A, Fong Y-L, Selby M J, Choo Q-L, Cousens L, Houghton M, Benedict Yen T S. Activation of grp78 and grp94 promoters by hepatitis C virus E2 envelope protein. J Virol. 1999;73:3718–3722. doi: 10.1128/jvi.73.5.3718-3722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason P W. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology. 1989;169:354–364. doi: 10.1016/0042-6822(89)90161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuura Y, Suzuki T, Suzuki R, Sato M, Aizaki H, Saito I, Miyamura T. Processing of E1 and E2 glycoproteins of hepatitis C virus expressed in mammalian and insect cells. Virology. 1994;205:141–150. doi: 10.1006/viro.1994.1629. [DOI] [PubMed] [Google Scholar]
- 21.McKeating J A, Zhang Y J, Arnold C, Frederiksson R, Fenyo E-M, Balfe P. Chimeric viruses expressing primary envelope glycoproteins of HIV-1 show increased sensitivity to neutralization by human sera. Virology. 1996;220:450–460. doi: 10.1006/viro.1996.0332. [DOI] [PubMed] [Google Scholar]
- 22.Michalak J-P, Wychowski C, Choukhi A, Meunier J-C, Ung S, Rice C M, Dubuisson J. Characterization of truncated forms of hepatitis C virus glycoproteins. J Gen Virol. 1997;78:2299–2306. doi: 10.1099/0022-1317-78-9-2299. [DOI] [PubMed] [Google Scholar]
- 23.Nowak T, Farber P M, Wengler G, Wengler G. Analyses of the terminal sequences of West Nile virus structural proteins and of the in vitro translation of these proteins allow the proposal of a complete scheme of the proteolytic cleavages involved in their synthesis. Virology. 1989;169:365–376. doi: 10.1016/0042-6822(89)90162-1. [DOI] [PubMed] [Google Scholar]
- 24.Pahl H L, Baeuerle P A. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 25.Pettersson R F. Protein localization and virus assembly at intracellular membranes. Curr Top Microbiol Immunol. 1991;170:67–106. doi: 10.1007/978-3-642-76389-2_3. [DOI] [PubMed] [Google Scholar]
- 26.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner A J, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 27.Ralston R, Thudium K, Berger K, Kuo C, Gervase B, Hall J, Selby M, Kuo G, Houghton M, Choo Q-L. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia viruses. J Virol. 1993;67:6753–6761. doi: 10.1128/jvi.67.11.6753-6761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 931–959. [Google Scholar]
- 29.Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner A J, Lau J Y N, Choo Q-L, Chien D, Pileri P, Houghton M, Abrignani S. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759–1763. doi: 10.1073/pnas.93.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santolini E, Migliaccio G, Monica N L. Biosynthesis and biochemical properties of the hepatitis C virus core protein. J Virol. 1994;68:3631–3641. doi: 10.1128/jvi.68.6.3631-3641.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato K, Okamoto H, Aihara S, Hoshi Y, Tanaka T, Mishiro S. Demonstration of sugar moiety on the surface of hepatitis C virions recovered from the circulation of infected humans. Virology. 1993;196:354–357. doi: 10.1006/viro.1993.1488. [DOI] [PubMed] [Google Scholar]
- 32.Selby M J, Glazer E, Masiarz F, Houghton M. Complex processing and protein:protein interactions in the E2:NS2 region of HCV. Virology. 1994;204:114–122. doi: 10.1006/viro.1994.1515. [DOI] [PubMed] [Google Scholar]
- 33.Selby M J, Choo Q-L, Berger K, Kuo G, Glazer E, Eckart M, Lee C, Chien D, Kuo C, Houghton M. Expression, identification and subcellular localization of the proteins encoded by the hepatitis C viral genome. J Gen Virol. 1993;74:1103–1113. doi: 10.1099/0022-1317-74-6-1103. [DOI] [PubMed] [Google Scholar]
- 34.Spaete R R, Alexander D A, Rugroden M E, Choo Q-L, Berger K, Crawford K, Kuo C, Leng S, Lee C, Ralston R, Thudium K, Tung J W, Kuo G, Houghton M. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology. 1992;188:819–830. doi: 10.1016/0042-6822(92)90537-y. [DOI] [PubMed] [Google Scholar]