Abstract
Regulatory T cells play an important role in tumor escape from host antitumor immunity. Increased frequencies of CD4+CD25+ regulatory T cells have been documented in the tumor sites, malignant effusions, and peripheral blood of patients with ovarian carcinoma. However, the mechanism involved remains unclear. In the present study, we collected high‐purity human CD4+CD25−CD45RA+ naïve T cells by microbead cell separation. These cells did not express FOXP3 by single‐cell analysis, and few cells expressed FOXP3 when they were activated with anti‐CD3/CD28 dual signal. However, more cells expressed FOXP3 when the supernatant of human epithelial ovarian carcinoma cell culture was added, yet not the supernatant of normal human ovarian surface epithelia cell culture. Neutralization assays revealed that neutralizing antibody against transforming growth factor β (TGF‐β), interleukin‐10, and interleukin‐4 did not abrogate elevated FOXP3 expression induced by carcinoma cell culture supernatant, whereas neutralizing leukemia inhibitory factor (LIF) partially abrogated FOXP3 expression, but LIF alone could not increase FOXP3 expression in activated naïve T cells. Further, an in vitro coculture suppression assay showed that these cells could suppress the proliferation of autologous CD4+CD25−CD45RA− T cells. In summary, our findings show that ovarian carcinoma cells are able to induce expression of FOXP3 and exhibit suppressive ability in activated naïve T cells by producing soluble substances, and multiple cytokines involve in the induction of FOXP3 expression. (Cancer Sci 2009)
The existence of tumor‐specific T‐cell immune responses to human malignant tumors has been well documented. Tumor‐specific cytotoxic T lymphocytes have been identified in the tumor‐infiltrating lymphocytes isolated from ovarian carcinoma.( 1 , 2 ) However, in most patients, tumor progression goes on in spite of tumor‐specific immune responses.
Emerging evidence suggests that regulatory T cells play an important role in tumor escape from immunological control. The best‐characterized regulatory T cells are CD4+CD25+ regulatory T cells that specifically express FOXP3. They are anergic and do not proliferate after T‐cell receptor (TCR) stimulation in vitro. They inhibit the proliferation of CD4+ and CD8+ T cells after stimulation via their TCR in a cytokine‐independent yet cell contact‐dependent manner. Regulatory T cells play an important role in the maintenance of peripheral tolerance. They are vital to the peripheral immune regulation mechanism and protect against autoimmunity and transplant rejection. They also suppress antitumor immune response. Experimental tumor models have shown that removal of CD25+ T cells changes the immune response to tumors both in vitro and in vivo.( 3 ) Depletion of CD25+ T cells in mice resulted in a lower incidence or slower growth of B16 melanoma.( 4 ) Attenuation of regulatory T cells by engaging the glucocorticoid‐induced tumor necrosis factor receptor family‐related protein with its natural ligand in combination treatment with existing immune stimulation regimens augmented antitumor immunity and eradicated metastatic 4T1 tumors in mice.( 5 ) Elimination of regulatory T cells in vivo using the recombinant interleukin (IL)‐2 diphtheria toxin conjugate DAB389IL‐2 enhanced the magnitude of vaccine‐mediated, tumor‐specific T‐cell responses in humans.( 6 ) More importantly, studies showed that high regulatory T‐cell frequencies are associated with poor prognosis in tumor patients,( 7 , 8 , 9 , 10 , 11 ) whereas a high CD8+/regulatory T cell ratio predicts favorable prognosis.( 12 , 13 )
Increased frequencies of CD4+CD25+ regulatory T cells have been documented in the tumor sites, malignant effusions, and peripheral blood of patients with ovarian carcinoma( 8 , 14 , 15 ) and several other types of carcinoma.( 9 , 11 , 15 , 16 , 17 , 18 ) However, the mechanism involved in elevating CD4+CD25+ regulatory T cell frequencies remains unclear. Specific recruitment to the tumor could bring increase the number of regulatory T cells in tumor sites, but not in the peripheral blood. Accumulated evidence has shown that regulatory T cells can also be induced in the peripheral condition, and might arise from antigen‐experienced CD4+CD25− T cells in the suppressive cytokine milieu( 19 , 20 , 21 , 22 ) or after interaction with naturally occurring CD4+CD25+ T cells.( 19 ) They may also arise from CD4+CD25− T cells activated by low antigen dose( 23 , 24 ) or by immature dendritic cells.( 25 , 26 ) Studies have shown that tumor cells can convert CD4+CD25− T cells into regulatory T cells by secreting suppressive cytokine( 27 ) or affecting the phenotype of dendritic cells.( 28 ) More recently, it was reported that tumor cells could convert CD4+CD25− naïve T cells into regulatory T cells in the absence of thymus and proliferation.( 29 ) However, most of these studies were conducted using mice. It is not clear whether a similar conversion can occur in humans. Several studies have shown that activated human CD4+CD25− T cells express high levels of FOXP3, and the suppressive capacity in vitro varies.( 30 , 31 , 32 , 33 , 34 ) One recent study showed that a proportion of the regulatory population was generated from rapidly dividing, highly differentiated memory CD4+ T cells in vivo.( 35 ) It is reasonable that the CD4+CD25−CD45RA− memory T cell pool might contain some precursors of regulatory T cells; hence, further isolation of highly purified CD4+CD25−CD45RA+ naïve T cells in humans is needed as the target cells for conversion experiments in vitro.
In the present study, we collected high‐purity CD4+CD25−CD45RA+ naïve T cells by microbead cell separation. These cells did not contain any regulatory T cells as no FOXP3 was expressed by single‐cell analysis. Only a few of these CD4+CD25−CD45RA+ T cells expressed FOXP3 when they were activated with anti‐CD3/CD28 dual‐signal for 3 days. However, more cells expressed FOXP3 when the supernatant of human epithelial ovarian carcinoma cell culture was added, yet not with the addition of supernatant of normal human ovarian surface epithelia cell (OSE). Neutralization assays revealed that multiple cytokines could be involved in the induction of FOXP3, because none of the transforming growth factor β (TGF‐β), IL‐4, IL‐10, or leukemia inhibitory factor (LIF) antibodies could completely abrogate the induction of FOXP3. Further, a coculture suppression assay in vitro showed that these cells could suppress the proliferation of autologous CD4+CD25−CD45RA− T cells. Our study explores a novel mechanism of elevated regulatory T cells in the microenvironment of ovarian carcinoma.
Methods
Cell culture and supernatant collection. SKOV3 and CaoV3 ovarian epithelial carcinoma cell lines (ATCC, NA, USA) were cultured in RPMI‐1640 culture medium (Gibco, NY, USA) supplemented with 10% heat‐inactivated fetal bovine serum (FBS; Invitrogen, CA, USA) and 100 U/mL penicillin plus 100 μg/mL streptomycin (Gibco). Cells were washed twice with PBS when they grew to 80% confluence and were then kept in serum‐free culture medium for an additional 48 h. Supernatant was collected and debris was removed by centrifugation and then filtration through a 0.22‐μm filter.
Primary culture of human ovarian carcinoma.
Human ovarian carcinoma tissue was obtained with informed consent from five patients during surgery, whose pathological diagnosis and staging are shown in Table 1. The tissue was digested into single cells by 0.25% trypsin (Sigma, St Louis, USA), and cultured in Maccoy 5A (Gibco) with 15% FBS and 100 U/mL penicillin plus 100 μg/mL streptomycin. They were passaged with 0.25% trypsin when the cells became confluent. When cells grew to approximately 80% confluence, the medium was replaced with RPMI‐1640 without FBS for an additional 48 h. The supernatants were collected as described above.
Table 1.
Clinical information from five patients with epithelial ovarian carcinoma for ovarian carcinoma cell primary culture
| Patient | Age (years) | Pathological diagnosis | FIGO staging |
|---|---|---|---|
| P1 | 54 | Serous adenocarcinoma | IIa |
| P2 | 67 | Mucinous adenocarcinoma | IIIc |
| P3 | 37 | Endometrioid adenocarcinoma | IIb |
| P4 | 61 | Serous adenocarcinoma | IIIb |
| P5 | 46 | Serous adenocarcinoma | IIIc |
OSE culture.
Primary culture of OSE was established from a normal‐looking ovary of a 37‐year‐old patient undergoing surgery for lieomyoma by gently scraping the ovarian surface with the blunt side of a scalpel with informed consent. The OSE fragments were cultured in 199–MCDB 105 (1 : 1) (Gibco) with 15% FBS and 100 U/mL penicillin plus 100 μg/mL streptomycin. The cells were left undisturbed for at least 4 days, and kept growing to confluence for approximately 8–15 days before being routinely passaged with 0.25% trypsin. Positive expression of both vimentin and keratin was used to identify OSE and exclude stromal cells by immunocytochemistry (data not shown). When cells grew to approximately 80% confluence, the medium was replaced with RPMI‐1640 without FBS for an additional 48 h. Supernatant was collected and debris was removed by centrifugation and then filtration through a 0.22‐μm filter.
Isolation of human cells. Human peripheral blood was obtained from normal healthy donors by the Blood Center of Zhejiang Province of China with informed consent. Peripheral blood mononuclear cells (PBMC) were prepared by centrifugation over Ficoll‐Hypaque gradients. Monocytes and macrophages were depleted through plastic adherence in a cell culture flask for 45 min at 37°C. T cells were isolated over the autoMACS Separator (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ T cells were collected by negative selection with a human CD4+ T cell isolation kit II (Miltenyi Biotec) according to the manufacturer’s instructions. These CD4+ cells were then sorted by Depletes procedure with human CD25 microbeads (Miltenyi Biotec) for CD4+CD25− T cells and CD4+CD25+ T cells. CD4+CD25− T cells were then further isolated for CD4+CD25−CD45RA+ T cells and CD4+CD25−CD45RA− T cells by CD45RA microbead (Miltenyi Biotec) using the POSSEL procedure. For high purity, approximately 1 × 107 CD4+CD25−CD45RA+ T cells were isolated from 3–4 × 108 PBMC in general. The purity of all isolated populations was routinely controlled by flow cytometry with phycoerythrin (PE)‐conjugated anti‐CD25 (Miltenyi Biotec), FITC‐conjugated anti‐CD4 mAb (Caltag, CA, USA), allophycocyanin (APC)‐conjugated anti‐CD25 (eBioscience, CA, USA), and APC‐conjugated anti‐CD45RA mAb (eBioscience). The post‐sort purity for CD4+ T cells, CD4+CD25− T cells, and CD4+CD25−CD45RA+ T cells was >96% (Fig. 1A).
Figure 1.
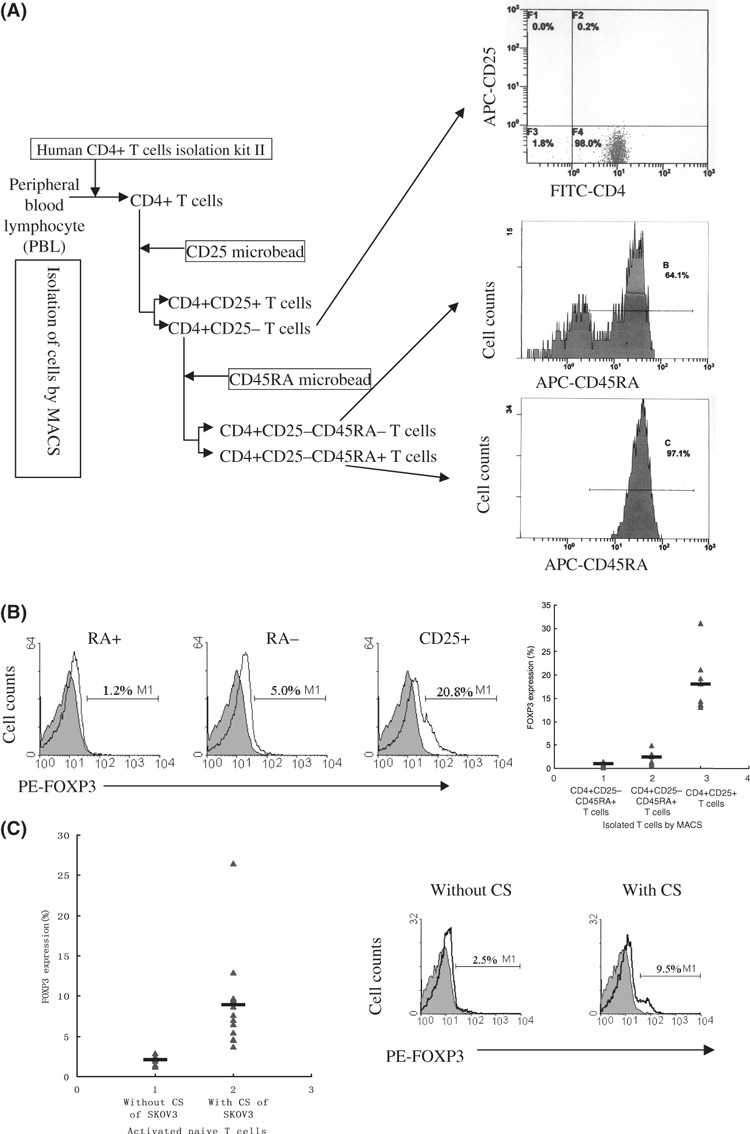
Supernatant of SKOV3 cell culture increased the FOXP3 expression in activated naïve T cells. (A) CD4+CD25−CD45RA+ naïve T cells were isolated by MACS (left), and the purity of isolated populations was controlled by flow cytometry (right). (B) FOXP3 expression in post‐sorted CD4+CD25−CD45RA+, CD4+CD25−CD45RA−, and CD4+CD25+ T cells (left) by flow cytometry. The average percentage and range of FOXP3 expression in post‐sorted CD4+CD25−CD45RA+, CD4+CD25−CD45RA−, and CD4+CD25+ T cells from at least eight independent experiments (right). (C) FOXP3 expression in activated naïve T cells treated with and without supernatant of SKOV3 cell culture (right) on day 3 by flow cytometry. The average percentage and range of FOXP3 expression in activated naïve T cells treated with and without culture supernatant (CS) of SKOV3 on day 3 from at least eight independent experiments (left).
T‐cell culture. All T‐cell cultures were conducted in RPMI‐1640 medium supplemented with penicillin G (100 U/mL), streptomycin (100 μg/mL), l‐glutamine (2 mM) (Gibco), and 10% heat‐inactivated FBS at 37°C in a humidified atmosphere containing 5% CO2. The MACS sorted cells were activated in vitro with 2 μg/mL plate‐bound anti‐CD3 (eBioscience) and 2 μg/mL soluble anti‐CD28 (eBioscience) in a 24‐well culture plate (Corning, NJ, USA) at 1 000 000 cells/mL in the presence or absence of human recombinant (hr) IL‐2 (2 ng/mL) (Cytolab) for 3 days. The culture plate was coated overnight at 4°C with 2 μg/mL anti‐CD3. In neutralization experiments, 10 μg/mL of neutralizing anti‐TGFβ (R&D Systems, MN, USA), anti‐IL‐10 (Biosource), anti‐IL‐4 (PEPROTECH, NJ, USA), anti‐LIF (R&D Systems) antibody or a combination of neutralizing anti‐TGFβ and anti‐IL‐10 antibody was used. In induction experiments, hrLIF (R&D Systems) or IL‐6 (R&D Systems) at 10 ng/mL was used.
Flow cytometry. Flow cytometry was carried out on an EPICS XL (Beckman Coulter, CA, USA). Data were analyzed with the XL SYSTEM II, CA, USA. For staining of FOXP3, the cells were fixed and permeabilized using the eBioscience Fixation/Permeabilization kit (eBioscience) according to the manufacturer’s protocol. The FOXP3 was stained with PCH101 PE and isotype control (eBioscience). Cell apoptosis was detected using the rh Annexin V‐FITC Kit (Bendermedsystems, CA, USA) according to the manufacturer’s protocol.
T‐cell suppression assay in vitro. A coculture suppression assay in vitro was determined using a BrdU proliferation ELISA kit (Roche, IN, USA) according to the manufacturer’s instructions. First, highly purified CD4+CD25−CD45RA+ T cells were cultured in RPMI‐1640 with 50% supernatant of SKOV3 cell culture for 3 days, then these cells were collected and isolated for CD4+CD25+ T cells (Ti) by CD25 microbead using the POSSEL procedure. Previously isolated autologous CD4+CD25−CD45RA− T cells (1 × 105/well) were cocultured with SKOV3‐induced CD4+CD25+ T cells at different ratios (1 : 1, 0.5 : 1, 0.25 : 1, 0 : 1) for 72 h in 96‐well flat‐bottom plates (tissue culture grade, clear bottom) at 1 × 105/100 μL cell density, and the induced CD4+CD25+ T cells also were cultured solely in parallel. Cells were activated with 2 μg/mL soluble anti‐CD3 and 2 μg/mL anti‐CD28 in the presence of 2 ng/mL hrIL‐2. During the final 20 h, 10 μL/100 μL BrdU labeling solution was added to the culture. The 450‐nm absorbance of each well was measured using contrast absorbance at 630 nm on an ELISA plate reader. The proliferation index (PI) was calculated using the equation
where A(T cells + Ti) is the absorbance of autologous CD4+CD25−CD45RA− T cells cocultured with SKOV3‐induced CD4+CD25+ T cells, ATi is the absorbance of SKOV3‐induced CD4+CD25+ T cells cultured alone, AT cells is the absorbance of autologous CD4+CD25−CD45RA− T cells cultured without induced CD4+CD25+ T cells, and Amedium is the absorbance of culture medium.
Results
Supernatant of ovarian carcinoma cell culture increased the FOXP3 expression in activated naïve T cells. The forkhead box transcription factor FoxP3 is specifically expressed in regulatory T cells. Several recent reports have further shown that expression of FoxP3 is sufficient to confer suppressive activity on naïve T cells.( 36 , 37 , 38 , 39 , 40 )
To investigate whether the supernatant of ovarian carcinoma cell culture increases the FOXP3 expression in activated naïve T cells, highly purified CD4+ CD25−CD45RA+ T cells were collected and cultured in RPMI‐1640 with 50% supernatant of SKOV3 cell culture. The FOXP3 expression of activated T cells was determined by single‐cell analysis using flow cytometry after 3 days. FOXP3 expression was observed in control CD4+CD25+ T cells as well as in activated naïve T cells cultured with supernatant of SKOV3 cell culture. In contrast, Foxp3 expression was absent in unstimulated CD4+CD25−CD45RA+ T cells, and few cells expressed FOXP3 in activated CD4+CD25−CD45RA+ naïve T cells without SKOV3 cell culture supernatant (Fig. 1B,C).
To further confirm that the supernatant of ovarian carcinoma cell culture could increase FOXP3 expression in activated naïve T cells, the cell culture supernatant from another ovarian carcinoma cell strain, CAOV3, was collected. We also established several primary cultures of human ovarian carcinoma (PCOC) cells (PCOC1, PCOC2, PCOC3, PCOC4, PCOC5) and collected the cell culture supernatant. As observed in the culture with supernatant of SKOV3 cell culture, the supernatant of CAOV3 and PCOC cell cultures also increased FOXP3 expression in activated naïve T cells (Fig. 2).
Figure 2.
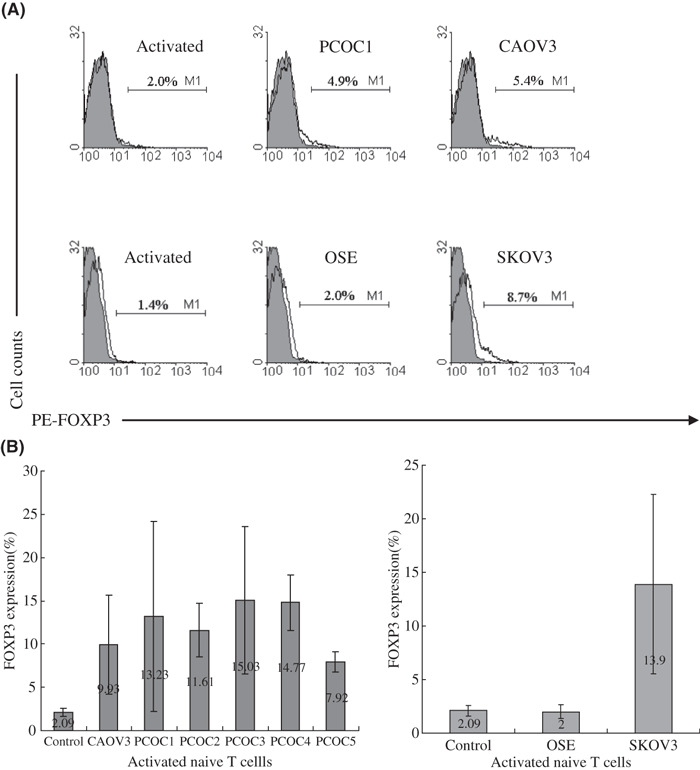
Supernatants from CAOV3 and ovarian carcinoma cell culture revealed the same function as that from SKOV3, but supernatant from ovarian surface epithelia cells (OSE)did not. (A) FOXP3 expression in activated naïve T cells treated with culture supernatant (CS) of PCOC1, CAOV3, or OSE on day 3 by flow cytometry. One representative staining is shown for at least three independent experiments. (B) The average percentage and range of FOXP3 expression in activated naïve T cells treated with CS of five PCOC, CAOV3, or OSE on day 3 from at least three independent experiments.
Supernatant of OSE cell culture did not increase FOXP3 expression in activated naïve T cells. Most human epithelial ovarian carcinomas arise from ovarian surface epithelial cells. To investigate whether the supernatant of OSE cell culture also induces FOXP3 expression in activated naïve T cells, we established a primary culture of OSE and collected the cell culture supernatant. Interestingly, the supernatant of OSE cell culture did not increase the FOXP3 expression in activated naïve T cells (Fig. 2), implying that human epithelial ovarian carcinoma cells acquire the capacity to induce regulatory T cells during tumor progression.
Neutralization of TGF‐β and IL‐10 did not influence the effect of SKOV3 cell culture supernatant to activated naïve T cells. Numerous studies have shown that regulatory T cells can be induced from naïve peripheral T cells with the suppressive cytokine milieu. Several cytokines, including TGF‐β, IL‐10, IL‐4, and IL‐13, are involved in the peripheral expansion of regulatory T cells. More recently, Victoria and colleagues observed the tumor conversion of naïve CD4+CD25− T cells into CD4+CD25+Foxp3+ regulatory T cells through the production of high levels of TGF‐β in mice, and neutralization of TGF‐β abrogated this conversion both in vitro and in vivo.( 27 ) To investigate whether TGF‐β in SKOV3 cell culture supernatant was required for the induction of FOXP3 expression, a neutralizing antibody against TGF‐β was used. However, neutralization of TGF‐β did not affect FOXP3 expression (Fig. 3).
Figure 3.
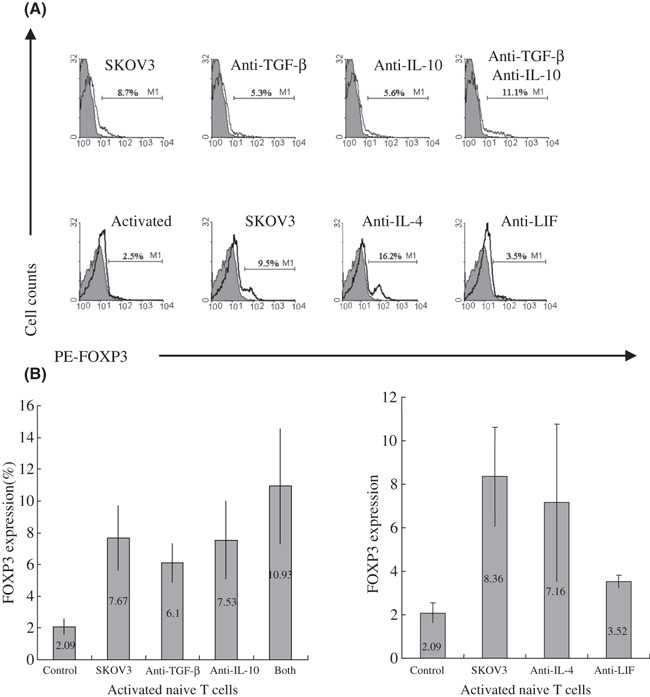
Neutralization of transforming growth factor β (TGF‐β), interleukin (IL)‐10, or IL‐4 did not abrogate elevated FOXP3 expression induced by SKOV3 cell culture supernatant, but neutralization of leukemia inhibitory factor (LIF) partially counteracted the effect of supernatant on naïve T cells. (A) FOXP3 expression in activated naïve T cells treated with culture supernatant (CS) of SKOV3 and neutralizing Ab against TGF‐β, IL‐10 (top), IL‐4, and LIF (bottom) on day 3 by flow cytometry. One representative staining is shown for at least three independent experiments. (B) The average percentage and range of FOXP3 expression in activated naïve T cells treated with CS of SKOV3 and neutralizing Ab against TGF‐β, IL‐10 (left), IL‐4, and LIF (right) on day 3 from at least three independent experiments.
We have found that ovarian carcinoma cells are able to synthesize and secrete IL‐10.( 41 ) It was reported that the IL‐10 serum level was elevated in ovarian cancer patients compared with that in patients with benign ovarian tumors.( 42 ) To further explore the mechanism by which SKOV3 cell culture supernatant induces FOXP3 expression, neutralizing antibody against IL‐10 was used alone or in combination with anti‐TGF‐β. Similarly, neutralization of IL‐10 also did not affect FOXP3 expression. Furthermore, when anti‐IL‐10 was combined with anti‐TGF‐β, the expression of FOXP3 even slightly increased (Fig. 3).
Neutralization of LIF partially counteracted the effect of SKOV3 cell culture supernatant to activated naïve T cells. Other cytokines could be involved in the induction of FOXP3 by supernatant of ovarian carcinoma cell culture. We have found that more than 20 types of cytokine were increased, yet approximately 20 types of cytokine were decreased in ovarian carcinoma cell culture supernatants compared with OSE cell supernatants in for 79 types of cytokine detected by protein microarray screening.( 43 ) To further elucidate the cytokines involved, neutralizing antibodies against IL‐4 and LIF were selected for neutralization assays, as the IL‐4 levels in supernatants of SKOV3, CAOV3, and PCOC1 were increased more than two‐fold, and LIF levels were increased more than two‐fold in the cell culture supernatants of SKOV3 and the PCOC1 and 1.8‐fold in CAOV3 supernatant.( 43 ) In addition, IL‐4 was able to induce the development of CD25+CD4+ T cells with regulatory capacity in an Ag‐specific manner from peripheral naïve CD4+ T cells,( 19 ) and LIF was associated with immune tolerance.( 44 , 45 ) Unexpectedly, neutralization of IL‐4 with 10 μg/mL anti‐IL‐4 also did not affect FOXP3 expression. Surprisingly, neutralizing LIF markedly decreased the FOXP3 expression, though it did not completely abrogate the effect of SKOV3 cell culture supernatant on activated naïve T cells (Fig. 3).
LIF alone did not increase FOXP3 expression in activated naïve T cells. There is no evidence to date that LIF is able to directly induce FOXP3 expression in activated naïve T cells or to induce CD25+CD4+ T cells to produce regulatory capacity from peripheral naïve CD4+ T cells. To clarify whether LIF could induce FOXP3 expression in activated naïve T cells, 10 ng/mL LIF was added to the T cell culture medium. As shown in Figure 4, LIF alone could not increase FOXP3 expression in activated naïve T cells.
Figure 4.
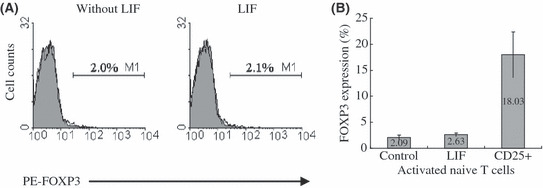
Leukemia inhibitory factor (LIF) alone did not increase the level of FOXP3 expression in activated naïve T cells. (A) FOXP3 expression in activated naïve T cells treated with and without exogenous LIF on day 3 by flow cytometry. One representative staining is shown for four independent experiments. (B) The average percentage and range of FOXP3 expression in activated naïve T cells treated with and without exogenous LIF on day 3 from four independent experiments.
IL‐6 did not inhibit FOXP3 expression in activated naïve T cells induced by SKOV3 cell culture supernatant. Our study had revealed that all neutralizing antibodies specific to the four cytokines mentioned above could not completely abrogated the effect of SKOV3 cell culture supernatant on activated naïve T cells. Hence, another approach to inhibit FOXP3 expression induced by ovarian carcinoma cell culture supernatant should be considered. In mice, it has been shown that IL‐6 completely inhibits the generation of Foxp3+ regulatory T cells induced by TGF‐β. Instead, IL‐6 and TGF‐β together induce the differentiation of T(H)17 cells from naïve T cells, that is a subset of IL‐17‐producing T (T[H]17) cells distinct from T(H)1 or T(H)2 cells, which has been described and shown to have a crucial role in the induction of autoimmune tissue injury.( 46 ) To investigate whether IL‐6 could inhibit the expression of FOXP3 of human activated naïve T cells induced by SKOV3 cell culture supernatant, 10 ng/mL IL‐6 was added to the culture medium. Unexpectedly, IL‐6 also could not abrogate the effect of SKOV3 cell culture supernatant on activated naïve T cells (Fig. 5).
Figure 5.
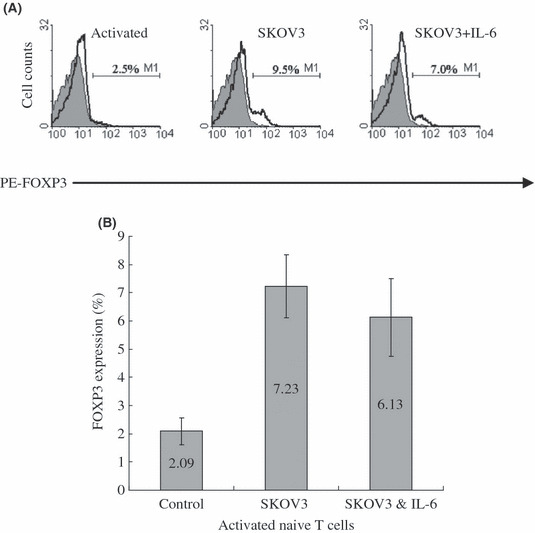
Interleukin (IL)‐6 did not inhibit FOXP3 expression in activated naïve T cells induced by SKOV3 culture supernatant. (A) FOXP3 expression in activated naïve T cells treated with culture supernatant (CS) of SKOV3+/− IL‐6 on day 3 by flow cytometry. One representative staining is shown for four independent experiments. (B) The average percentage and range of FOXP3 expression activated in naïve T cells treated with CS of SKOV3+/− IL‐6 on day 3 from four independent experiments.
Isolated CD4+CD25+ T cells from naïve T cells cultured with SKOV3 cell culture supernatant suppress CD4+CD25− T‐cell proliferation. To examine the suppressive ability of induced FOXP3+ T cells from activated naïve T cells cultured with SKOV3 cell culture supernatant, we isolated a CD25+ population using the autoMACS Separator with CD25 microbeads to enrich the FOXP3+ population. A coculture suppression assay in vitro was determined using a BrdU proliferation ELISA kit as described in the Methods. At the end of a 3‐day culture, previously isolated autologous CD4+CD25−CD45RA− T cells were cocultured with various induced CD25+ T‐cell populations for another 3 days, then we measured the 450‐nm absorbance of each well using a contrast absorbance 630 nm on an ELISA plate reader and calculated the PI with the equation mentioned in the Methods. As shown in Figure 6, the induced CD25+ T cells showed a potent suppressive ability of CD4+ CD25−CD45RA− T‐cells proliferation in a dose‐dependent manner. These data demonstrate that CD4+CD25+ T cells with suppressive ability can arise from CD4+CD25−CD45RA+ naïve T cells cultured with SKOV3 cell culture supernatant, which should be associated with the expression of FOXP3.
Figure 6.
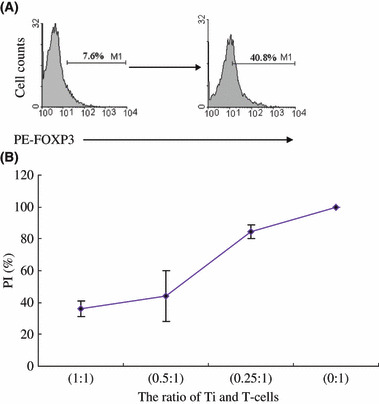
Isolated CD4+CD25+ T cells from naïve T cells cultured with SKOV3 cell culture supernatant suppress CD4+CD25− T cell proliferation. (A) CD4+CD25+ T cells were isolated by MACS from activated naïve T cells cultured with SKOV3 cell culture supernatant, and FOXP3 expression was detected by flow cytometry before (left) and after (right) sorting. (B) Autologous CD4+CD25−CD45RA− T cells were cocultured with induced CD25+ T cell populations at diffierent ratios (1 : 1, 0.5 : 1, 0.25 : 1, 0 : 1) and the proliferation index (PI) was calculated. The average PI of three independent experiments is shown. Error bars are SD of triplicate samples. Ti, induced CD4+ CD25+ T cells.
Discussion
FOXP3 is the most specific marker yet for regulatory T cells. In mice, expression of FoxP3 is strictly correlated with regulatory activity, ectopic expression of a transgene encoding Foxp3 or a retroviral vector encoding Foxp3 in isolated CD4+CD25− T cells arouses acquisition of suppressor properties,( 36 , 37 , 38 , 39 ) and induced ablation of a loxP‐flanked Foxp3 allele in mature regulatory T cells results in the loss of their suppressive function in vivo and acquisition of the ability to produce IL‐2 and T helper type 1 cytokines.( 39 ) Similar to the mouse system, ectopic expression of FOXP3 in human CD4+CD25− T cells( 47 , 48 ) or human leukemic CD4+ Jurkat‐T cells( 49 ) also results in acquisition of suppressor properties, and genetic defects in FOXP3 cause IPEX, an X‐linked autoimmune/inflammatory syndrome.( 50 , 51 ) However, several studies in vitro have shown that activated T cells in humans do not display suppressive function despite high FOXP3 expression,( 33 , 34 ) even cells that are activated in the presence of TGF‐β,( 52 ) although more similar studies have reported that human T cells with regulatory function are differentiated from both the naïve and memory CD4+ T‐cell pools, which is correlated with FOXP3 expression.( 22 , 30 , 31 , 53 , 54 , 55 ) Differences in culture conditions and activation procedure of T cells could explain the notable inconsistency in the results among different research groups. Remarkably, most cultures without generation of regulatory T cells despite high FOXP3 expression have 5 days or longer anti‐CD3/CD28 dual‐signal stimulation,( 33 , 34 , 52 ) whereas generation cultures of regulatory T cells have irradiated (3000 cGy) allogeneic T cell‐depleted PBMC stimulation or less 3 days anti‐CD3/CD28 dual‐signal stimulation.( 22 , 30 , 31 , 53 , 54 ) In addition, in one study although the authors considered that activated human CD4+CD25− T cells transiently expressed FOXP3 but did not obtain suppressive ability, these T cells from three of nine donors displayed suppressive ability in vitro.( 34 ) We also found that activated CD4+CD25−CD45RA− T cells expressed high levels of FOXP3, and their suppressive capacity in vitro varied. Interestingly, activated CD4+CD25−CD45RA− T cells without suppressive ability despite high FOXP3 expression were correlated with a high cell apoptosis rate by flow cytometry (unpublished data, Zhao XF, Ye F, Chen LL, Lu WG, Xie X, 2008). It was conceivable that 5‐day or longer activated CD4+CD25− T cells without suppressive ability were the result of a high cell death rate that partially resulted from activation‐induced cell death. As a result, we affirmed that FOXP3 was competent as a specific marker in our assays.
It is well known that partial antigen‐touched naïve T cells will develop into memory T cells, and these memory T cells could quickly differentiate into helper or effector T cells upon re‐exposure to their cognate antigen. However, the development of antigen‐touched naïve regulatory T cells remains largely unknown. Neither do we know whether the antigen‐touched naïve regulatory T cells could develop into memory T cells, nor whether the possible memory regulatory T cells express FOXP3 in rest state. As mentioned above, activated CD4+CD25− T cells in vitro express a high level of FOXP3,( 30 , 31 , 32 , 33 , 34 ) and human CD4+CD25hi‐Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo.( 35 ) There should be a regulatory population that is included in memory populations, which does not express FOXP3 in rest state, quickly expresses FOXP3 once exposed to its cognate antigen, and then acquires suppressive ability. Therefore, we further isolated CD4+CD25− T cells for CD4+CD25−CD45RA+ naïve T cells. Interestingly, isolated CD4+CD25−CD45RA+ naïve T cells did not express FOXP3, and few cells expressed FOXP3 when activated with anti‐CD3/CD28 dual‐signal stimulation for 3 days in the absence of suppressive cytokines in vitro. The expression of FOXP3 was obviously increased when the supernatant of ovarian carcinoma cell culture was added into the culture system. However, the supernatant of OSE cell culture did not increase the expression of FOXP3. Therefore, we consider that supernatant of ovarian carcinoma cell culture may induce naïve T cells to differentiate into regulatory T cells at the initial stage when these cells are activated, and thus may inhibit the induction of effective antitumor immunity in ovarian carcinoma patients.
Several mechanisms should be involved in the tumor‐induced expansion of regulatory T cells. Cytokines derived from tumor cells or local tumor‐infiltrating immune cells play a significant role. However, up to the present, most of those conversion phenomena were observed in mice. Valzasina et al. reported that tumor cells could convert CD4+CD25− naïve T cells into regulatory T cells in the absence of thymus and proliferation using an undifferentiated colon carcinoma of BALB/c mice.( 29 ) Liu et al. reported that mouse prostate tumor TRAMP‐C2 cells and mouse renal cell carcinoma RENCA cells could convert CD4+CD25− T cells into regulatory T cells by secreting TGF‐β.( 27 ) Several studies showed that TGF‐β, IL‐10, and IL‐4 were able to induce the development of CD4+CD25+ T cells with regulatory capacity.( 19 , 20 , 21 , 22 , 53 ) The present study indicated that supernatant of ovarian carcinoma cell culture induced the expression of FOXP3 in activated naïve T cells, its effective components probably were the ovarian carcinoma cell‐derived soluble substances including various kinds of cytokines. Thus, we selected TGF‐β, IL‐10, IL‐4, and LIF, which were elevated in cell culture supernatants and tissues of ovarian cancer, for further study. Unexpectedly, none of the neutralizing Abs specific to these cytokines could completely abrogate the induction of FOXP3 by ovarian cancer cells, although LIF neutralizing Ab markedly decreased the expression of FOXP3 induced by supernatant of ovarian carcinoma cell culture in our study. But LIF alone nevertheless could not increase the FOXP3 expression of activated naïve T cells. Indeed, there should be a complicated interaction among these cytokines derived from ovarian carcinoma cells, which cooperatively induces the expression of FOXP3 in activated naïve T cells and differentiates these cells into regulatory T cells.
It has been reported that LIF is associated with immune tolerance.( 44 , 45 ) However, the mechanism involved remains elusive. Remarkably, the transmembrane protein gp130 is a shared component of the receptor complexes for IL‐6 and LIF, and IL‐6 together with TGF‐β induces the differentiation of pathogenic T(H)17 cells from naïve T cells in mice.( 46 ) Further research is needed to elucidate the potential mechanism of LIF for induction of FOXP3.
Coculture suppression assays are needed to confirm that the FOXP3 expression induced by supernatant of ovarian carcinoma cell culture correlates with regulatory T cell activity. In our study, <10% of the induced naïve T cells expressed FOXP3 in most of our assays, and FOXP3 was not a suitable marker for viable cell isolation. So we isolated CD25+ populations using the autoMACS Separator with CD25 microbeads to enrich the FOXP3+ population. Interestingly, the isolated CD25+ T cells showed a potent suppressive ability of CD4+CD25−CD45RA− T cell proliferation in a dose‐dependent manner.
Our data show that ovarian carcinoma cells are able to induce the expression of FOXP3 and exhibit suppressive ability in activated naïve T cells by producing soluble substances, which may participate in ovarian carcinoma‐induced immune tolerance. Multiple cytokines are involved in the induction of FOXP3 expression, and therapy with neutralization Ab specific to single cytokines may not be effective. Our findings reveal a novel mechanism of immune tolerance induced by ovarian carcinoma cells, suggesting that multiple interventions should be considered for facilitating efficacious antitumor immune responses in patients with ovarian cancer.
Acknowledgments
We thank Wuwen Zhang and Qi Wang for their excellent technical assistance. This work was supported by the Chinese National Natural Science Foundation 30471811, 30672229, and Zhejiang Natural Science Foundation Z205170.
References
- 1. Peoples GE, Schoof DD, Andrews JV et al. T‐cell recognition of ovarian cancer. Surgery 1993; 114 (2): 227–34. [PubMed] [Google Scholar]
- 2. Ioannides CG, Fisk B, Jerome KR et al. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol 1993; 151 (7): 3693–703. [PubMed] [Google Scholar]
- 3. Onizuka S, Tawara I, Shimizu J et al. Tumor rejection by in vivo administration of anti‐CD25 (interleukin‐2 receptor alpha) monoclonal antibody. Cancer Res 1999; 59 (13): 3128–33. [PubMed] [Google Scholar]
- 4. Jones E, Dahm‐Vicker M, Simon AK et al. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun 2002; 2: 1. [PubMed] [Google Scholar]
- 5. Chen L, Huang TG, Meseck M et al. Rejection of metastatic 4T1 breast cancer by attenuation of Treg cells in combination with immune stimulation. Mol Ther 2007; 15: 2194–202. [DOI] [PubMed] [Google Scholar]
- 6. Dannull J, Su Z, Rizzieri D et al. Enhancement of vaccine‐mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115 (12): 3623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu J, Xu D, Liu Z et al. Increased regulatory T cells correlate with CD8 T‐cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007; 132 (7): 2328–39. [DOI] [PubMed] [Google Scholar]
- 8. Curiel TJ, Coukos G, Zou L et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10 (9): 942–9. [DOI] [PubMed] [Google Scholar]
- 9. Griffiths RW, Elkord E, Gilham DE et al. Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother 2007; 56 (11): 1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiraoka N, Onozato K, Kosuge T et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12 (18): 5423–34. [DOI] [PubMed] [Google Scholar]
- 11. Kono K, Kawaida H, Takahashi A et al. CD4+CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 2006; 55 (9): 1064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao Q, Qiu SJ, Fan J et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007; 25 (18): 2586–93. [DOI] [PubMed] [Google Scholar]
- 13. Sato E, Olson SH, Ahn J et al. Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 2005; 102 (51): 18 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Ye DF, Xie X et al. Proportion of CD4+CD25+ regulatory T cell is increased in the patients with ovarian carcinoma. Cancer Invest 2005; 23 (5): 399–403. [PubMed] [Google Scholar]
- 15. Woo EY, Chu CS, Goletz TJ et al. Regulatory CD4+CD25+ T cells in tumors from patients with early‐stage non‐small cell lung cancer and late‐stage ovarian cancer. Cancer Res 2001; 61 (12): 4766–72. [PubMed] [Google Scholar]
- 16. Liyanage UK, Moore TT, Joo HG et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002; 169 (5): 2756–61. [DOI] [PubMed] [Google Scholar]
- 17. Ormandy LA, Hillemann T, Wedemeyer H et al. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005; 65 (6): 2457–64. [DOI] [PubMed] [Google Scholar]
- 18. Miller AM, Lundberg K, Ozenci V et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 2006; 177 (10): 7398–405. [DOI] [PubMed] [Google Scholar]
- 19. Skapenko A, Kalden JR, Lipsky PE et al. The IL‐4 receptor alpha‐chain‐binding cytokines, IL‐4 and IL‐13, induce forkhead box P3‐expressing CD25+CD4+ regulatory T cells from CD25‐CD4+ precursors. J Immunol 2005; 175 (9): 6107–16. [DOI] [PubMed] [Google Scholar]
- 20. Chen W, Jin W, Hardegen N et al. Conversion of peripheral CD4+CD25– naïve T cells to CD4+CD25+ regulatory T cells by TGF‐beta induction of transcription factor Foxp3. J Exp Med 2003; 198 (12): 1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fantini MC, Becker C, Monteleone G et al. Cutting edge: TGF‐beta induces a regulatory phenotype in CD4+CD25– T cells through Foxp3 induction and down‐regulation of Smad7. J Immunol 2004; 172 (9): 5149–53. [DOI] [PubMed] [Google Scholar]
- 22. Yamagiwa S, Gray JD, Hashimoto S et al. A role for TGF‐beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol 2001; 166 (12): 7282–9. [DOI] [PubMed] [Google Scholar]
- 23. Kretschmer K, Apostolou I, Hawiger D et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 2005; 6 (12): 1219–27. [DOI] [PubMed] [Google Scholar]
- 24. Apostolou I, Von Boehmer H. In vivo instruction of suppressor commitment in naïve T cells. J Exp Med 2004; 199 (10): 1401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med 2001; 193 (2): F5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jonuleit H, Schmitt E, Schuler G et al. Induction of interleukin 10‐producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med 2000; 192 (9): 1213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu VC, Wong LY, Jang T et al. Tumor evasion of the immune system by converting CD4+CD25– T cells into CD4+CD25+ T regulatory cells: role of tumor‐derived TGF‐beta. J Immunol 2007; 178 (5): 2883–92. [DOI] [PubMed] [Google Scholar]
- 28. Ghiringhelli F, Puig PE, Roux S et al. Tumor cells convert immature myeloid dendritic cells into TGF‐beta‐secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 2005; 202 (7): 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Valzasina B, Piconese S, Guiducci C et al. Tumor‐induced expansion of regulatory T cells by conversion of CD4+CD25– lymphocytes is thymus and proliferation independent. Cancer Res 2006; 66 (8): 4488–95. [DOI] [PubMed] [Google Scholar]
- 30. Walker MR, Kasprowicz DJ, Gersuk VH et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest 2003; 112 (9): 1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walker MR, Carson BD, Nepom GT et al. De novo generation of antigen‐specific CD4+CD25+ regulatory T cells from human CD4+CD25– cells. Proc Natl Acad Sci USA 2005; 102 (11): 4103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pillai V, Ortega SB, Wang CK et al. Transient regulatory T‐cells: a state attained by all activated human T‐cells. Clin Immunol 2007; 123 (1): 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Allan SE, Crome SQ, Crellin NK et al. Activation‐induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 2007; 19 (4): 345–54. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Ioan‐Facsinay A, Van Der Voort EI et al. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 2007; 37 (1): 129–38. [DOI] [PubMed] [Google Scholar]
- 35. Vukmanovic‐Stejic M, Zhang Y, Cook JE et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo . J Clin Invest 2006; 116 (9): 2423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–61. [DOI] [PubMed] [Google Scholar]
- 37. Khattri R, Cox T, Yasayko SA et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4: 337–42. [DOI] [PubMed] [Google Scholar]
- 38. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–6. [DOI] [PubMed] [Google Scholar]
- 39. Chai JG, Xue SA, Coe D et al. Regulatory T cells, derived from naïve CD4+CD25– T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplantation 2005; 79 (10): 1310–16. [DOI] [PubMed] [Google Scholar]
- 40. Williams LM, Rudensky AY. Maintenance of the Foxp3‐dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 2007; 8 (3): 277–84. [DOI] [PubMed] [Google Scholar]
- 41. Zhou J, Ye F, Chen H et al. The expression of interleukin‐10 in patients with primary ovarian epithelial carcinoma and in ovarian carcinoma cell lines. J Int Med Res 2007; 35 (3): 290–300. [DOI] [PubMed] [Google Scholar]
- 42. Lambeck AJ, Crijns AP, Leffers N et al. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res 2007; 13 (8): 2385–91. [DOI] [PubMed] [Google Scholar]
- 43. Chen LL, Ye F, Lü WG et al. Evaluation of immune inhibitory cytokine profiles in epithelial ovarian carcinoma. J Obstet Gynaecol Res 2009; 35 (2): 212–18. [DOI] [PubMed] [Google Scholar]
- 44. Metcalfe SM, Watson TJ, Shurey S et al. Leukemia inhibitory factor is linked to regulatory transplantation tolerance. Transplantation 2005; 79 (6): 726–30. [DOI] [PubMed] [Google Scholar]
- 45. Zenclussen AC. Regulatory T cells in pregnancy. Springer Semin Immunopathol 2006; 28 (1): 31–9. [DOI] [PubMed] [Google Scholar]
- 46. Bettelli E, Carrier Y, Gao W et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441 (7090): 235–8. [DOI] [PubMed] [Google Scholar]
- 47. Oswald‐Richter K, Grill SM, Shariat N et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T‐cells. PLoS Biol 2004; 2 (7): E198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yagi H, Nomura T, Nakamura K et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol 2004; 16 (11): 1643–56. [DOI] [PubMed] [Google Scholar]
- 49. Kim JY, Kim HJ, Hurt EM et al. Functional and genomic analyses of FOXP3‐transduced Jurkat‐T cells as regulatory T (Treg)‐like cells. Biochem Biophys Res Commun 2007; 362 (1): 44–50. [DOI] [PubMed] [Google Scholar]
- 50. Bennett CL, Christie J, Ramsdell F et al. The immune dysregulation, polyendocrinopathy, enteropathy, X‐linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001; 27 (1): 20–1. [DOI] [PubMed] [Google Scholar]
- 51. Kobayashi I, Shiari R, Yamada M et al. Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX). J Med Genet 2001; 38 (12): 874–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naïve human CD4+FOXP3− T cells by T‐cell receptor stimulation is transforming growth factor‐β‐dependent but does not confer a regulatory phenotype. Blood 2007; 110 (8): 2983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zheng SG, Wang JH, Gray JD et al. Natural and induced CD4+CD25+ cells educate CD4+CD25– cells to develop suppressive activity: the role of IL‐2, TGF‐beta, and IL‐10. J Immunol 2004; 172 (9): 5213–21. [DOI] [PubMed] [Google Scholar]
- 54. Zheng SG, Gray JD, Ohtsuka K et al. Generation ex vivo of TGF‐beta‐producing regulatory T cells from CD4+CD25– precursors. J Immunol 2002; 169 (8): 4183–9. [DOI] [PubMed] [Google Scholar]
- 55. Rao PE, Petrone AL, Ponath PD. Differentiation and expansion of T cells with regulatory function from human peripheral lymphocytes by stimulation in the presence of TGF‐β. J Immunol 2005; 174 (3): 1446–55. [DOI] [PubMed] [Google Scholar]


